Ruying Tanga, Linyuan Wanga, Aimin Lib
a Beijing University of Chinese Medicine, Beijing, China
b New Era Health Industry(Group) Co., Ltd., Beijing, China
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 4
Page No: 729-737
Ruying Tanga, Linyuan Wanga, Aimin Lib
a Beijing University of Chinese Medicine, Beijing, China
b New Era Health Industry(Group) Co., Ltd., Beijing, China
Petko Alov(petko@biophys.bas.bg)
Monika Kosmala(monika.kosmala@p.lodz.pl)
Qian Zhang(zq159519@126.com)
Zhijia Fang(fzj4437549@163.com)
Ruying Tang, Linyuan Wang, Aimin Lib, Antianxiety and anti-depressant effects of Maca (L. meyenii) ethanolic extract on chronic unpredictable mild stress of rats through hypothalamic-pituitary-adrenal axis(2019)SDRP Journal of Food Science & Technology 4(4)
Maca (Lepidium meyenii) is an Andean plant, has been cultivated and used as a traditional medicine for over 2, 000 years. Maca is well documented for anti-inflammation, anti-oxidation and immunoregulatory activities. Chronic stress and depression are lacking clinical available and effective therapeutic agents. The aim of this study was to investigate the anti-depressant function of refined Maca ethanolic extract and discuss the underlying mechanism. 70 Sprague-Dawley rats were randomly divided into 7 groups as blank, chronic-stressed, chronic-stressed + positive control, chronic-stressed + maca crude (2.4g/kg and 1.2g/kg), chronic-stressed + maca ethanolic extract (2.4g/kg and 1.2g/kg). Sucrose preference test, open-field test and elevated plus maze test were adopted as depression-related behavioral tests. Hypothalamic corticotrophin releasing hormone, plasma adrenocorticotropic hormone and cortisol were measured by radioimmunoassay. Sugar consumption of the maca ethanolic extract group was significantly lower compared with chronic-stressed group (p<0.05). Behavioral tests showed the total travel time in the open field and open-arm enter times in the maze increased significantly (p<0.05). Radioimmunoassay showed hypothalamic corticotrophin releasing hormone, plasma adrenocorticotropic hormone and cortisol were decreased by the maca ethanolic extract group. The maca ethanolic extract might have regulatory properties against chronic stressed and might function through a hypothalamic-pituitary-adrenal axis.
Key words: Maca, antianxiety, anti-depression, chronic unpredictable mild stress.
Traditional Chinese medicine (TCM) originated in ancient China and has evolved over thousands of years. TCM encompasses many different practices, including acupuncture, moxibustion, Chinese herbal medicine, Chinese therapeutic massage and so on. Other Asian countries, including Japan and Korea also contribute to TCM practicing systems (Leung et al, 2004; Uchibayashi et al, 2008). Among the various TCM practices, Chinese herbal medicine (CHM) is employed as a complementary health approach by many people all over the world (Linde et al. 2017). Although CHM is extensively used in the East and West nowadays, its scientific evidence of its effectiveness is still limited and are being questioned (Snow et al. 2017). It is difficult for researchers to study CHM in a traditional way because its treatments are often complex and based on ideas that are quite different from modern Western medicine. Thus, modern TCM is focusing on 1. building a suitable model of the indication of specific disease or symptom, to valid the designated CRM; 2. finding the bioactive ingredients of specific CHM, which is already verified using the indicated model.
Maca(Lepidium meyenii Walp.), is a cruciferous plant, though mainly growing in the Peruvian Central Andes, has now been planted widely in the Yunnan-Guizhou Plateau and are being employed as an ingredient of various CRM formulas. Previous studies indicated that maca may have the therapeutics properties of antioxidant, anti-inflammation, and anti-radiation. polysaccharides, amino acids, phenolics, alkaloids and minerals (Li et al, 2018). According to the index of Google patent database, those formulas containing Maca are verified for their effectiveness on treatment of hyperglycemia, prostatic hyperplasia, and to regulate immunity (Sanchez et al, 2018).
Recently, maca are also reported to benefit psychological symptoms in clinical trials, including anxiety, fatigue, and depression (Shin et al, 2010). However, those results are mostly conducted in menopaused women subjects and failed to explain the underlying mechanism. Moreover, the bioactive antianxiety and anti-depressant component of Maca remains unknown.
Chronic restraint stress is responsible to cause anxiety- and depression-like behavior in rodent models and proved for its validity (Chiba et al, 2012). Therefore, we aimed to examine the effectiveness of maca ethanolic extract and the mechanism of its medical benefits using a restraint stressed rodent model.
2.1 Materials and chemicals
The maca powder were obtained from New Era Health Industry Co., Ltd., Beijing, China. The materials were air-dried at room temperature. Other reagents were of analytical grade as commercially available. The ethanolic extraction and macamides of maca was prepared according to a reported publication (Zheng et al, 2000). Briefly, the root of maca was rinsed clean and sliced. The slices were lyophilized, smashed into powder, and filtered through an 80-mesh sieve. Dried maca powder was reflux extracted by 12 volumes of petroleum ether at 50°C for 30 min, and ultrasonically extracted for 15 min. The filtrate was collected by vacuum suction filtration and dried to a powdered extract.
2.2. Animal Maintenance and maca administration
Male Sprague Dawley rats (n = 70) were purchased from SPF Biotechnology Co.,Ltd. Beijing, China) at 5 weeks of age. Animals were housed in a controlled environment under a light–dark cycle (lights on at 0700 and off at 1900). The experimental procedures followed the Guiding Principles for the Care and Use of Animals in the Academic Research Ethical Review Committee of Beijing University of Chinese Medicine. All rats were randomly divided into four groups: control group (CON), depression model group (DM), 2.4g/kg maca ethanolic extract group (MEH), 1.2g/kg maca ethanolic extract group (MEL), 2.4g/kg maca powder group (MH), 1.2g/kg maca powder (ML), and positive control group (XP, Xiaoyao Pill, consists of chai hu (radix bupleuri), dang gui (Angelica sinensis), bai shao (radix paeoniae alba), chao bai zhu (roasted rhizoma atractylodis macrocephalae), fu ling (Wolfiporia extensa), zhi gan cao (radix glycyrrhizae), bo he (mint), and sheng jiang (rhizoma zinjiberis recens).
Rats were maintained on ad libitum diet. Maca powder or maca polysaccharide was dissolved in distilled water and gavaged indicated amount of maca or Xiaoyao pill (2.4g/kg or 1.2g/kg; 0.24g or 0.12g/ml for all maca group, 4.032g/kg, 0.4032g/mL for Xiaoyao pill) per day for 3 weeks before behavioral testing. Control rats received an equal amount of solvent.
2.3. Chronic unpredictable mild stress(CUMS) depression model
The chronic unpredictable mild stress (CUMS) procedure was performed as described previously with minor modifications (). Briefly, the non-stressed control animals were housed in groups of four per cage in standard plastic cages in a separate room and had no contact with the stressed groups. For another three groups, rats were assigned to four per cage accompanied with stress treatment. Animals were exposed to the weekly stress regime consisting of 48 h food deprivation, 24 h water deprivation, 1 min tail pinch (1 cm from the end of the tail), 45° cage tilt, 5 min cold swimming (at 4°C), 2 h physical restraint and overnight illumination. One of these stressor episodes was applied daily to each rat in a random order for a continuous six-week period.
2.4. Open field and elevated plus-maze tests
Rats were transferred to the observation room least 2 h prior to behavioural testing and were there kept in an entirely darkened (by means of black curtains) compartment of the same room. All behavioural observations were carried out during the second half of the dark phase in a darkened room.
Open field. Rats were placed in a corner of a wooden box (56 × 66 cm; comparable to the apparatus described by Prut and Belzung with its floor divided into 9 equal squares by means of red adhesive tape (Prut and Belzung, 2003). The field was bordered by 12-cm side walls. Besides manually recording any crossings (an animal touching a new square with both front paws) and rearings (standing upright on the hind legs), but not head-dippings, separate registrations were done for entries into, and/or passages through, the central square. These were subsequently expressed as a percentage of total crossings. At the end of the 10-min session, rats were removed, any boli counted (defecation score) and the floor of the box was wiped.
Elevated plus-maze. The elevated plus-maze was constructed with the exception of the arm width (12 cm instead of 10 cm). The maze, consisting of two opposing open and two closed arms was elevated 50 cm above the floor. At the start the rat was placed into the center of the maze (facing a closed arm) and any subsequent visit to one of the 4 arms was recorded provided the rat had entered with all 4 paws. After termination of the 10-min trial the number of entries into open arms was calculated as a percentage of total entries (general activity) and this ratio was taken as a measure of the rat's situational fear. Only the number of entries into, but not the time spent in open arms was recorded.
2.5. Sucrose preference test
This test was previously described (Lakkab et al, 2018). Briefly, at the beginning of the test all the groups were singly housed during 48 h in individual cages. Two bottles were available in each cage, one with 200 ml of 32% sucrose (w/v) and the other also with 200 ml of tap water. Preference was measured as follows: (1) sucrose consumption (ml) 200 ml; (2) water consumption (ml) 200 ml; and (3) total liquid, sucrose consumption + water consumption. At the end of 48th hour, the bottles were removed, the consumption was recorded.
2.6. Radioimmunoassay (RIA) protocol
Serum of hippocampus protein was determined by commercial RIA kits (R&D systems, Minneapolis, USA). Briefly, Tissue was homogenized in 2 M acetic acid and immersed in boiling water for 5 min. After centrifuging at 25.000 after 20 min. the supernatant fluid was lyophilized. The residue was reconstituted in RIA buffer solution and aliquots were used for RIA according to manufacturer’s protocol.
2.7. Statistical analysis
The data were expressed as means ± S.D. The significance of difference was evaluated with one-way ANOVA, followed by Student’s t- test to statistically identify differences between the control and treated groups. Significant differences were set at *p<0.05, **p<0.01, and ***p<0.001.
3.1. General results
Body weights were recorded before the experiment (0 d), 7th day, 14th day and on the 21st day (final). As presented in Table 1, significant difference was observed on body weights of the rats between the Con group and DM group since after modeling, indicating a depression condition caused by CUMS in the DM mice. However, the decrease of the body weight caused by CUMS were alleviated by XP administration (positive control), MEH or MEL. Maca crudes, including MH or ML, did not show inhibiting effects of CUMS-induced weight loss.
Table 1. Effects of Maca products on body weight (g)
|
Groups |
0d |
7d |
14d |
21d |
|
Con |
208.80±12.70 |
260.33±15.35 |
311.87±13.57 |
334.75±19.07 |
|
DM |
208.50±15.23 |
224.88±14.21*** |
252.12±24.63*** |
273.87±18.84*** |
|
XP |
208.40±10.75 |
236.88±13.78 |
280.25±7.38△△ |
298.50±11.40△ |
|
MEH |
208.30±12.42 |
235.88±13.22 |
271.87±20.75△ |
297.50±28.68△ |
|
MEL |
208.70±16.04 |
231.88±18.21 |
271.12±18.31△ |
289.25±19.89△ |
|
MH |
208.20±15.74 |
225.77±11.24 |
250.37±7.48 |
268.00±11.40 |
|
ML |
208.60±14.03 |
234.11±17.86 |
254.50±27.20 |
273.87±18.29 |
*p<0.05, **p<0.01, ***p<0.001 compared with Con group. △p<0.05, △△p<0.01, △△△p<0.001 compared with DM group.
3.2 Behavior test results
As presented in Figure. 1A, CUMS induced a decrease of locomotor activities.
Obvious decreases were observed in numbers of step-across (54% preserved) and numbers of standing-up (56% preserved), compared with control groups, indicating inhibitory effects on both horizontal activities (HA) and vertical activities (VA). XP has been reported for its anti-depressant effects and was thus employed as positive control. XP significantly alleviate the decrease of locomotor activities caused by CUMS. XP treated rats retain 90% HA and 85% VA of Con rats. Among other treated groups, only high dose administration of maca ethanolic extract (MEH) helped to restore locomotor activities. MEH treated rats retain 94% HA and 84% VA of Con rats.
As presented in Figure. 1B and 1C, CUMS also induced a decrease on both entries into opened arms and total time stayed still. However, CUMS also induced an increase on total time stayed still in the closed arm, while the total entry time was decreased. These may be attributed to the decreased locomotor activity and the loss of exploration. In the positive control (XP) group, entries into the open arms and total time were both restored. In the other treated groups, only high dose administration of MEH helped to restore the exploration activities.
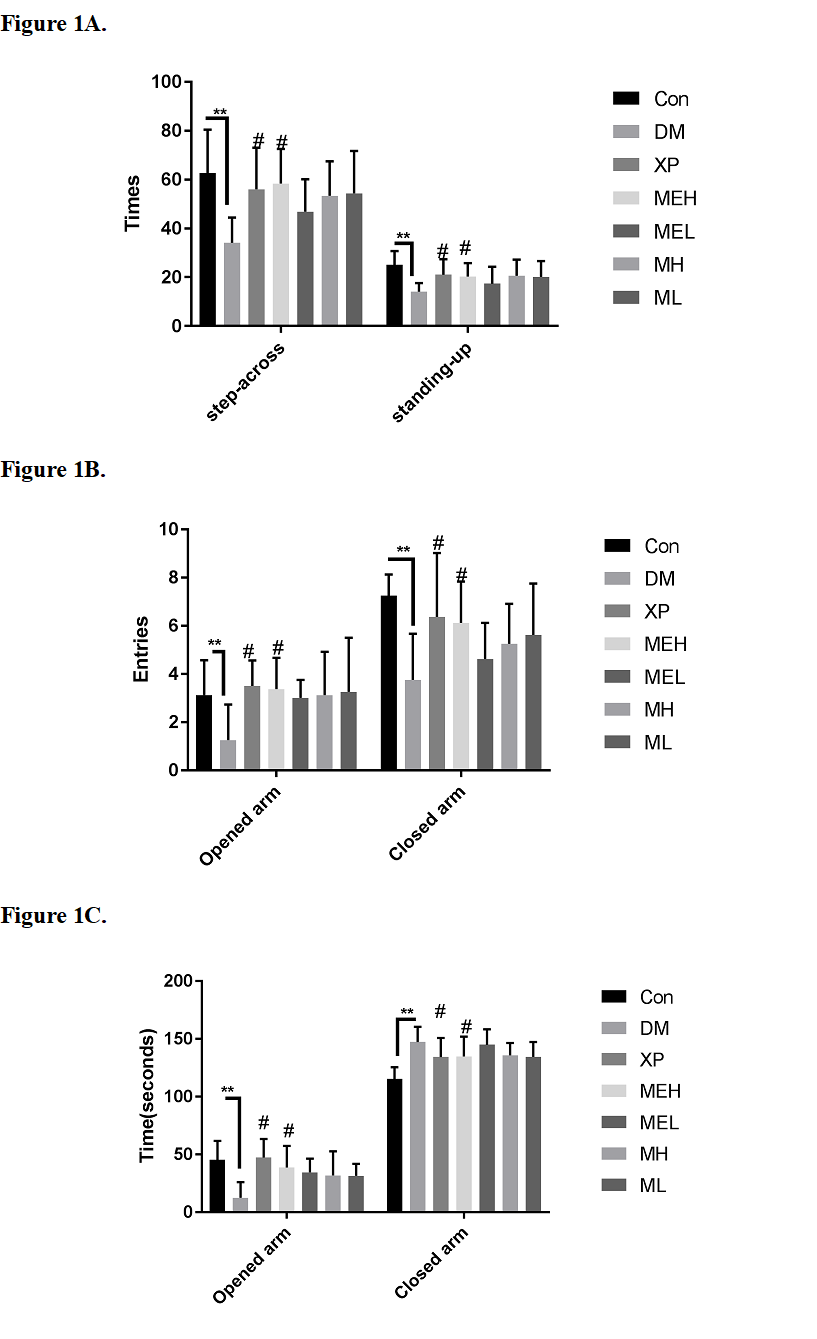
Figure 1. Effects of XP, MEH, MEL, MH and ML on CUMS-induced anxiety and depression behavior. A. Results of open-field test. B. and C. Results of elevate-maze test, B, total entries into closed arms or opened arms; C, total time stayed still in closed arms or opened arms. Con, control group. DM, depression model group. XP, xiaoyao pills. MEH, administration of maca ethanolic extract for 2.4g/kg. MEL, administration of maca ethanolic extract for 1.2g/kg. MH, administration of maca crude for 2.4g/kg. ML, administration of maca crude for 1.2g/kg. Results were expressed as means ± S.D. Statistical significance test for comparison with control (untreated) group was done by Student’s t-test. **, p < 0.01 compared with Con; # p < 0.05 compared with DM.
3.3. Sucrose preference test results
Sucrose preference test was employed to detect the depressant activity of rats. At the beginning of the experiment (the day 0), the sucrose preference had no difference between groups, but significant difference was observed at the end of the experiment (the 21st day). The sugar consumption in normal rats did not change, and that of the
DM rats was significantly reduced. Maca ethanolic extract at doses of 2.4 g/kg and XP at dose of 2.4 g/kg increased intake of sucrose significantly, whereas maca ethanolic extract at dose of 1.2 g/kg and maca crude dose of 2.4 g/kg or 1.2 g/kg had no obvious effect on sucrose consumption. (Table 2).
Table 2. Effects of Maca ethanolic extract on sucrose preference test in CUMS rats (mL).
|
Groups |
0 d |
21st d |
|
Con |
87.62±2.87 |
87.37±2.70 |
|
DM |
86.95±9.65 |
60.87±6.31*** |
|
XP |
87.47±3.12 |
73.22±8.88△ |
|
MEH |
86.37±5.98 |
74.04±11.34△ |
|
MEL |
85.26±4.60 |
66.79±7.46 |
|
MH |
85.51±5.80 |
72.80±16.86△ |
|
ML |
85.06±4.33 |
72.02±17.89△ |
*p<0.05, **p<0.01, ***p<0.001 compared with Con group. △p<0.05, △△p<0.01, △△△p<0.001 compared with DM group.
3.4. Radioimmunoassay results
Corticotropin releasing hormone (CRH), adreno-cortico-tropic-hormone (ACTH) and cortisol (CORT) were measured using commercial ELISA kits. As shown in Figure 2, CRH, ACTH and CORT were both increased by CUMS stimulation, indicating stress-induced hormones were up-tuned by CUMS. However, significant decrease was observed in the positive control (XP) group and high concentration maca extract group (MEH), indicating the alleviation of MEH on CUMS-induced stress-related hormone secretions.
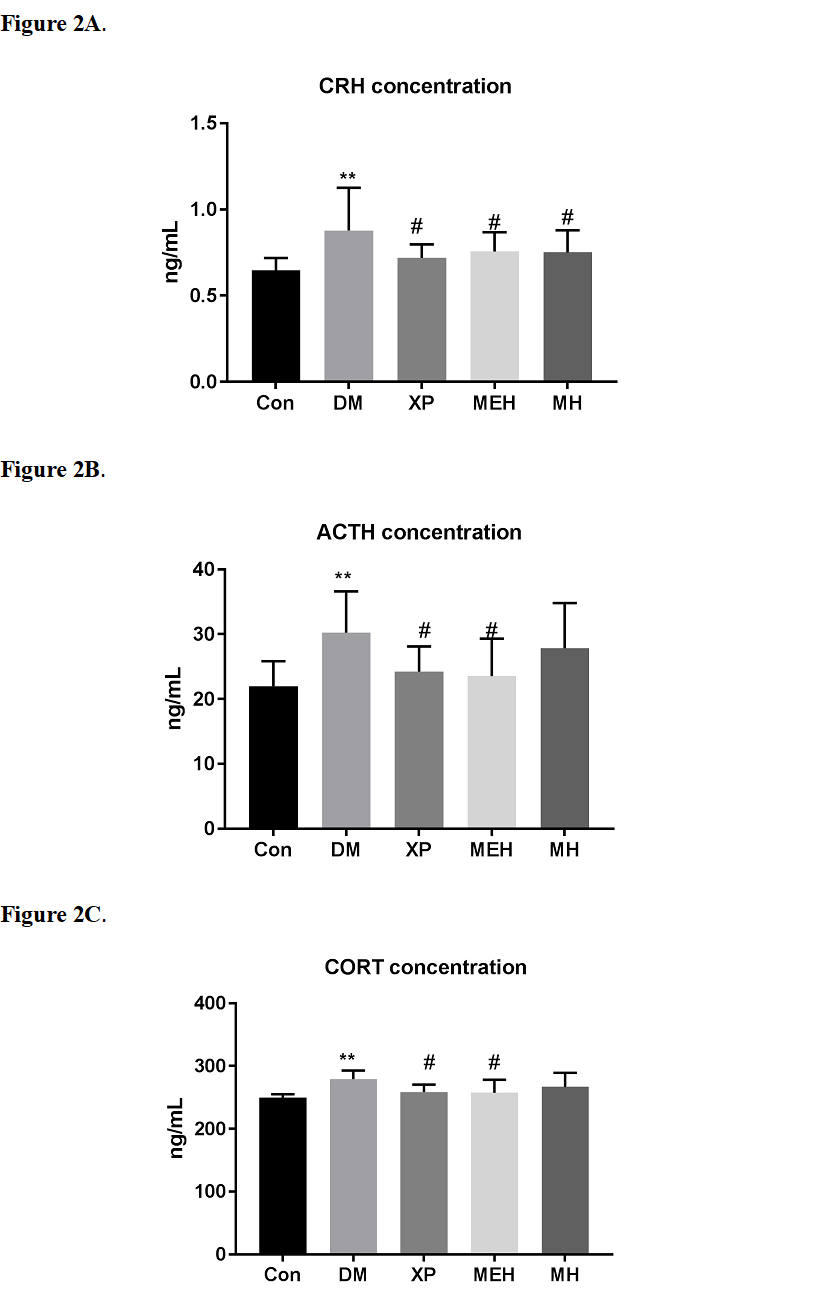
Figure 2. Effects of XP, MEH, MEL, MH and ML on CUMS-induced hormone secretion. A. Results of corticotropin releasing hormone concentration. B. Results of adreno-cortico-tropic-hormone concentration. C. Results of cortisol concentration Con, control group. DM, depression model group. XP, xiaoyao pills. MEH, administration of maca ethanolic extract for 2.4g/kg. MEL, administration of maca ethanolic extract for 1.2g/kg. MH, administration of maca crude for 2.4g/kg. ML, administration of maca crude for 1.2g/kg. CRH, Corticotropin releasing hormone. ACTH, adreno-cortico-tropic-hormone. CORT, cortisol. Results were expressed as means ± S.D. Statistical significance test for comparison with control (untreated) group was done by Student’s t-test. **, p < 0.01 compared with Con; # p < 0.05 compared with DM.
Maca and its extracts were reported for anti-fatigue effects, immunoregulatory effects, sexual behavior-regulatory effects and other biological activities (Li et al; Shin et al; Zheng et al; Gasco et al, 2008). The present study was designed to evaluate the effects of maca extract on antianxiety and anti-depressant in CUMS stimulated rodents. The results indicated that supplementation with ethanolic extract of maca (2.4 g/kg) significantly improved the performance in behavior tests including open-field test, exploration desire in an elevated plus maze test, and preference to sucrose. An important hormonal response system to stress—the hypothalamic–pituitary–adrenal (HPA) axis—may be involved in this process, particularly stress hormones known as CRH, ACTH and primarily cortisol (Zarei et al, 2017; Nilakanthan et al, 2016). To explore the underlying mechanisms, we analyzed the stress-induced hormones in those subjects. Results of our study indicate that the improving effects toward anxiety and depression may be linked with the regulatory effect on those hormones above.
The actions of HPA hormone system normally are tightly regulated to ensure that the body can respond quickly to stressful events and return to a normal state (Nilakanthan et al, 2016). Genetic background and environmental stress are main determinants of HPA axis activity. Among the components of HPA hormone system, CRH is a peptide hormone involved in the stress response (Koob et al, 1993; Witnall, 1993). It is a releasing hormone that belongs to corticotropin-releasing factor family. ACTH is often produced in response to biological stress (along with its precursor CRH from the hypothalamus). Its principal effects are increased production and release of cortisol by the cortex of the adrenal gland (Bale et al, 2000). Deficiency of CRH and ACTH is a sign of secondary adrenal insufficiency or tertiary adrenal insufficiency (Niu, 2018). Conversely, chronically elevated ACTH levels occur in primary adrenal insufficiency (e.g. Addison's disease) when adrenal gland production of cortisol is chronically deficient. Moreover, CRH and ACTH is also related to the circadian rhythm in many organisms and was related to suicide behaviors (Blasiak, 2017; Choi et al, 2018; Helfrich- Förster, 2017). Cortisol is produced by the zona fasciculata of the adrenal cortex within the adrenal gland. It is released in response to stress and low blood-glucose concentration. It functions to increase blood sugar through gluconeogenesis, to suppress the immune system, and to aid in the metabolism of fat, protein, and carbohydrates. It also decreases bone formation (A De Giorgio, 2017; Zorn et al, 2017). Sustained stress can lead to high levels of circulating cortisol, which can create an allostatic load (McEwen et al, 2018). An allostatic load can lead to various physical modifications in the body's regulatory networks (Hough et al, 2017; McEwen and Rasgon, 2018). Changed patterns of serum cortisol levels have been observed in connection with abnormal ACTH levels, mood disorders in numerous studies, fundamentally or clinically (Su et al, 2015, Winters, 1974). To make a brief summarization, the HPA axis plays an important role in the pathogenesis of depression disorder and has been recognized by the academic community. CUMS may stimulate the HPA to over-excite state, and the adrenal gland then secrete corticosterone content to stimulate the body to adapt to the new environment. However, with the HPA function being continuous intensified, serious effects on the physical and mental health of the body may happen and deteriorate the whole-body status. In the present study, we showed that maca ethanolic extract could alleviate depressant and anxiety behaviors and may contribute to improving the HPA axis function through adjusting CRH, ACTH and cortisol profile in CUMS-stimulated rat models. However, the specific functioning compound is unclear. Based on the character of ethanolic extraction, we suppose that macamide may be the functioning compound. A in silico or in vitro study may help to unveil if macamide possess anti-depressant and antianxiety properties. Further studies should focus on the underlying mechanisms as well.
The maca ethanolic extract showed potential regulatory activities against chronic unpredicted mild stress in rats. Evidence showed that the maca ethanolic extract might function as a regulator by adjusting the secretion pattern of CRH, ACTH and CORT, through the hypothalamic-pituitary-adrenal axis.
This study is supported by The National Natural Science Foundation of China (81874350). The authors declare that there is no conflict of interests regarding the publication of this paper. All of the author read and confirmed the final manuscript.
A De Giorgio, Loscalzo, R. M., Ponte, M., Padovan, A. M., Graceffa, G., & Gulotta, F. (2017). An innovative mindfulness and educational care approach in an adult patient affected by gastroesophageal reflux: the IARA model. Journal of Complementary and Integrative Medicine, 14(4). PMid:28731313
View Article PubMed/NCBIBale, T. L., Contarino, A., Smith, G. W., Chan, R., Gold, L. H., Sawchenko, P. E., ... & Lee, K. F. (2000). Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nature genetics, 24(4), 410. PMid:10742108
View Article PubMed/NCBIBlasiak, A., Gundlach, A. L., Hess, G., & Lewandowski, M. H. (2017). Interactions of circadian rhythmicity, stress and orexigenic neuropeptide systems: Implications for food intake control. Frontiers in neuroscience, 11, 127. PMid:28373831
View Article PubMed/NCBIChiba, S., Numakawa, T., Ninomiya, M., Richards, M. C., Wakabayashi, C., & Kunugi, H. (2012). Chronic restraint stress causes anxiety-and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 39(1), 112-119. PMid:22664354
View Article PubMed/NCBIChoi, K. W., Na, E. J., Fava, M., Mischoulon, D., Cho, H., & Jeon, H. J. (2018). Increased adrenocorticotropic hormone (ACTH) levels predict severity of depression after six months of follow-up in outpatients with major depressive disorder. Psychiatry research, 270, 246-252. PMid:30269042
View Article PubMed/NCBIGasco, M., Yucra, S., Rubio, J., & Gonzales, G. F. (2008). Lepidium meyenii (Maca) varieties did not alter female reproductive parameters in adult intact rats. Journal of Complementary and Integrative Medicine, 5(1).
View ArticleHelfrich‐Förster, C. (2017). Interactions between psychosocial stress and the circadian endogenous clock. PsyCh journal, 6(4), 277-289. PMid:29278313
View Article PubMed/NCBIHough, C., Morford, A., Epel, E., Lindqvist, D., Bersani, F. S., Jain, F., ... & Wolkowitz, O. (2017). 1005. Pre-Treatment Allostatic Load and Metabolic Dysregulation Predict Antidepressant Response in Major Depressive Disorder. Biological Psychiatry, 81(10), S406-S407.
View ArticleKoob, G. F., Heinrichs, S. C., Pich, E. M., Menzaghi, F., Baldwin, H., Miczek, K., & Britton, K. T. (1993). The role of corticotropin-releasing factor in behavioural responses to stress. Corticotropin-releasing factor, 172, 277.
View ArticleLeung, K. S. Y., & Chan, K. (2004). Regulatory system of proprietary Chinese medicine in Hong Kong. Journal of Complementary and Integrative Medicine, 1(1).
View ArticleLakkab, I., El Hajaji, H., Lachkar, N., El Bali, B., Lachkar, M., & Ciobica, A. (2018). Phytochemistry, bioactivity: suggestion of Ceratonia siliqua L. as neurodegenerative disease therapy. Journal of Complementary and Integrative Medicine. PMid:29813031
View Article PubMed/NCBILi, Y., Wang, S., Xin, Y., Zheng, M., Xu, F., Xi, X., & Han, C. (2018). Maca Cosmetics: A Review on Constituents, Therapeutics and Advantages. Journal of oleo science, 67(7), 789-800. PMid:29962450
View Article PubMed/NCBIMcEwen, B. S., & Rasgon, N. L. (2018). The Brain and Body on Stress Allostatic Load and Mechanisms for Depression and Dementia. Depression As a Systemic Illness, 14.
View ArticleNilakanthan, S., Metri, K., Raghuram, N., & Hongasandra, N. (2016). Effect of 6 months intense Yoga practice on lipid profile, thyroxine medication and serum TSH level in women suffering from hypothyroidism: A pilot study. Journal of Complementary and Integrative Medicine, 13(2), 189-193. PMid:27054602
View Article PubMed/NCBINiu, Y., Chen, R., Xia, Y., Cai, J., Ying, Z., Lin, Z., ... & Zhou, W. (2018). Fine particulate matter constituents and stress hormones in the hypothalamus-pituitary-adrenal axis. Environment international, 119, 186-192. PMid:29960262
View Article PubMed/NCBISanchez‐Salazar, L., & Gonzales, G. F. (2018). Aqueous extract of yellow maca (Lepidium meyenii) improves sperm count in experimental animals but response depends on hypocotyl size, pH and routes of administration. Andrologia, 50(3), e12929. PMid:29160562
View Article PubMed/NCBIShin, B. C., Lee, M. S., Yang, E. J., Lim, H. S., & Ernst, E. (2010). Maca (L. meyenii) for improving sexual function: a systematic review. BMC Complementary and Alternative Medicine, 10(1), 44. PMid:20691074
View Article PubMed/NCBISnow, J. E., Leach, M. J., & Clare, B. A. (2017). Attitudes, skill and use of evidence-based practice among US Western herbal medicine providers: a national survey. Journal of Complementary and Integrative Medicine, 14(1). PMid:28207415
View Article PubMed/NCBISu, H., Zhou, J., Bao, Y. Q., Mo, Y. F., Zhang, W. T., Zhao, J. G., & Jia, W. P. (2015). Primary empty sella associated with pituitary adenoma diagnosed by inferior petrosal sinus blood sampling. Chinese medical journal, 128(4), 567. PMid:25673467
View Article PubMed/NCBIUchibayashi, R., Yokoyama, Y., Nakajima, Y., Matsunaga, N., Shimazawa, M., & Hara, H. (2008). Antiangiogenic Effects of Chinese Medicines (Hachimijiogan and Kogikujiogan) on Vascular Endothelial Growth Factor-A-Induced Tube Formation in Human Umbilical Vein Endothelial Cells Co-Cultured with Fibroblasts. Journal of Complementary and Integrative Medicine, 5(1).
View ArticleWhitnall, M. H. (1993). Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Progress in neurobiology, 40(5), 573-629. 90035-Q
View ArticleWinters, A. J., OLIVER, C., COLSTON, C., MACDONALD, P. C., & PORTER, J. C. (1974). Plasma ACTH levels in the human fetus and neonate as related to age and parturition. The Journal of Clinical Endocrinology & Metabolism, 39(2), 269-273. PMid:4371228
View Article PubMed/NCBIZarei, S., Shayestehfar, M., Memari, A. H., SeifBarghi, T., & Sobhani, V. (2017). Acupuncture decreases competitive anxiety prior to a competition in young athletes: a randomized controlled trial pilot study. Journal of Complementary and Integrative Medicine, 14(1). PMid:28199216
View Article PubMed/NCBIZheng, B. L., He, K., Kim, C. H., Rogers, L., Shao, Y. U., Huang, Z. Y., ... & Zheng, Q. Y. (2000). Effect of a lipidic extract from Lepidium meyenii on sexual behavior in mice and rats. Urology, 55(4), 598-602. 00549-X
View ArticleZorn, J. V., Schür, R. R., Boks, M. P., Kahn, R. S., Joëls, M., & Vinkers, C. H. (2017). Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology, 77, 25-36. PMid:28012291
View Article PubMed/NCBI