Xue Liangyi
Email: xueliangyi@nbu.edu.cn
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 1
Page No: 174-185
Xue Liangyi
Email: xueliangyi@nbu.edu.cn
Mohammad Aslam Hosain, Xue Liangyi*
School of Marine Science, Ningbo University, Zhejiang 315000, China
Mohammad Aslam Hosain, Xue Liangyi, Impacts of probiotics on feeding technology and its application in aquaculture (2020)Journal of Aquaculture, Fisheries & Fish Science 3(1) P:174-185
Probiotic is a useful microorganism that directly or indirectly used to protect the host animal against pathogens. The bacterial pathogens are becoming more and more resistant to antimicrobial drugs, pesticides, and disinfectants that are used in aquatic disease control and high growth of production. For this reason, the probiotics study in aquaculture is a rising demand to ensure eco-friendly sustainable aquaculture as an alternative to antibiotics. The advantages of such probiotics enhance feed value, enzymatic stimulation in the digestive system, and reverse habitat for pathogens, influence anti-mutagenic activity, and improved immune response. The research of probiotics in gut microbiota of aquatic animals has not characterized adequately, and their impact on the environment is not widely deliberated. The impact on enzyme activity related to the fish metabolism system should be well-identified by further study. The application and the bio-security of probiotics in fish should be properly evaluated. The farmer should have a brief knowledge about the probiotics organism and should be careful during applying to the culture field.
Keywords: Application, aquaculture, bio-security, micro-organism, probiotics
Aquaculture is an important and emerging food-producing sector in the world. The fishery yearbook 2017 has stated that 153 million tones, likely 89 % of total fishery production, was used for human consumption and among them 45 % live and fresh fish was used directly as human consumption [1]. The production is always hindered by the diseases and environmental adverse situation that result in severe financial losses for the farmers. The use of antibiotics has been increased in the past decades in fishery sectors and poultry farming [2] to enhance the efficiency of feeding and disease control [3]. Due to using antibiotics in aquaculture the growth of antimicrobial-resistant pathogens has also increased [4]. There is a great risk that resistant bacteria can transfer from aquaculture species to human. That’s why now we need to develop new feeding techniques to solve this issue. In this case, eco-friendly treatments like probiotics can play an effective role in feeding technology in aquaculture.
The word “probiotics” was first used by Parker, 1974. According to his definition, probiotics are such types of organisms and substances that contribute to gut microbial balance [5]. Probiotics can be emphasized as a nutritious food source and as an eco-friendly biological control mediator [6]. In Fuller’s (1989) definition, the live microbial feed element seems to be beneficial for the host by improving microbial balance in the intestine [7]. Moriarty named probiotics shortly as ‘‘water additives’’ in 1998. Therefore, several terms are generally used to illustrate probiotics such as “beneficial”, “eco-friendly”, or “healthy” bacteria [8].
Aquatic host and microorganism are correlated with each other in their life cycle. This correlation can be treated in a useful way. Bacteria in the aquatic condition influence the activity of gut microbiota as well as vice versa [9]. The microbes that are used in probiotics may help in detoxification of host as well as food digestion in the gut [10, 11]. For this purpose different health and growth regulatory elements such as probiotics, prebiotics, symbiotic and other functional supplements can be applied [12]. Where antibiotics can't work efficiently, microbial involvement can possibly play a functional role to ensure sustainable and eco-friendly alternatives in aquaculture production [13, 14].
A huge number of researches and application has been going on the aquaculture system. The Vibrio alginolyticus isolated probiotics in shrimp hatcheries have been used successfully from earlier [15]. It has resulted that, probiotics treatments can be effective in growth performance, disease control, spawning, gut activity, and hematological parameters in different fishes such as sea bass [16], rainbow trout [17], snook [18, 19], sturgeon [12], and shrimp [20]. This is very important to have brief knowledge about the selection of probionts, use of probiotics, mode of action, safety guideline as well as their characterization [21]. According to the above studies, all these probiotics applications are probably connected with gut or gut microbial function. So, it is cleared that the scientific approach should need to know the details about genomic function with another organ and activity with different enzymes related to the metabolism.
2. Selection and source of probiotics
The selection of probiotics bacteria is very important because the evidence from researchers is very a few that are practically used. It may create an adverse situation for the host due to select inappropriate microbiota. The selection steps should be specific; the adaptation ability of the organism must be correlated with the different host and environmental factors [22]. The mechanisms of probiotics function are very crucial to understand and should have good knowledge about the characterization of potential probiotics which will be used in the farm [23]. Probiotics are generally selected by bio-security considerations of the following methods such as (a) Methods of processing, (b) Method of management of the probiotics and (c) Host body location to apply microorganisms [24].
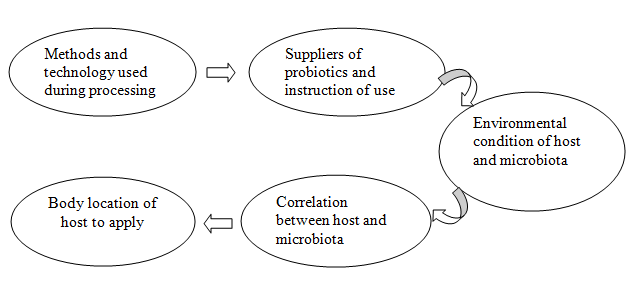
Fig 1: General criteria for selecting probiotics
As a probiotics LAB (lactic acid bacteria) such as Lactobacilli and Bifidobacteria have been broadly used and researched in terrestrial animals and humans [25]. So, it’s so far becoming popular as health-promoting functional foods as well as prophylactic, therapeutic and growth enhancements for the production of aquatic animals and human health. The gut microbiota of human and aquatic animals is dominated by gram-positive or facultative anaerobic bacteria. Bifidobacterium, Streptococcus, Lactobacillu and Streptomyces are the potential probionts among this microbiota [26-29]. LABs are normally found in the fish intestine. This type of bacteria can survive in acidic and bile environment of the intestine helps to convert lactose into lactic acid and thus reduce pH in the gastrointestinal tract [30]. At present, the spore-forming Bacillus sp and Yeast have been used widely because of its adhesion abilities, produce antimicrobial peptides and immune stimulation. The source of the LAB is fermented products like pickled vegetables, buttermilk, kimchi, pancake, soy sauce, etc. Applications of gram-negative facultative anaerobic bacteria in the digestive organ for some crustacean and herbivorous fish have come on succeeding [20]. For marine fish, Pseudomonas and Vibrio are commonly applied in the posterior intestine, and Aeromonas, Plesiomonas, and Enterobacteriaceae are fairly used in freshwater fish. Bacillus sp. as probiotics is popular because of spore-forming and long-lasting shelf life. It’s noticed that some other types of commercial probiotics feeds also found in the market which does not emphasize too much on the different publishers.
3.How do probiotics work?
The application of probiotics in aquafarming is modern technology. This actually begun by applying in finfish juveniles but recently it is applied to shrimp larvae. The bacteria taken from the surrounding environment are commonly ingested with the feed or mixed with drinking water in the filter feeder case [32]. There are various mechanisms performed in the probiotics process in the pond. One research showed that probiotics application reduce hatching time seven days/months and 21 days/year [33]. The properties of bacteria in ambient water are very crucial in this technology. Bacillus PC465 secluded from the intestine of Fenneropenaeus chinensis that works against WSSV (white spot syndrome virus) and improves the health condition and resistance in Litopenaeus vannamei [34]. Probiotic creates a biosecure environment and acts as transport stress resistance [35]. It creates an adverse habitat to the pathogens of the host animal. Useful bacteria take the place of harmful bacteria, furthermore de-colonized the pathogens and eventually destroy those [36]. Mechanisms action of different probiotics is potentially different such as (a) Making a bio-security shell on the gut, young larvae, and egg surface, (b) Increase metabolism of cells like enzyme or vitamins, (c) Formation of the colony and peptide bonding and (d) Stimulation of the immune system.
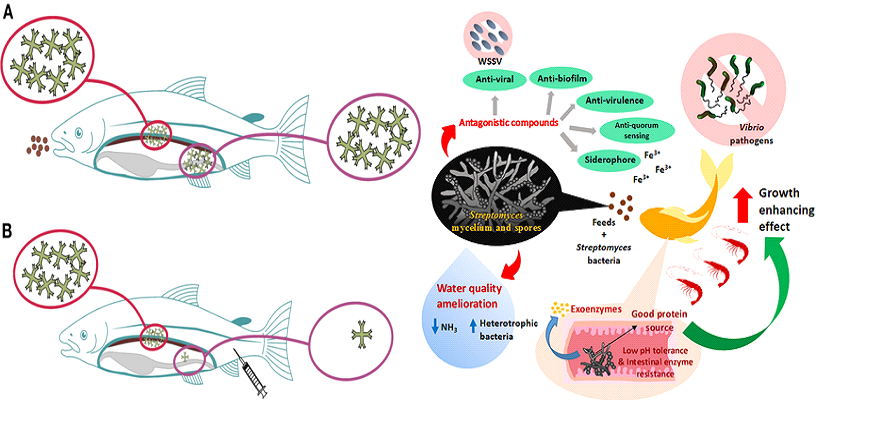
Fig 2: Probiotics mechanism (Tan LT-H et al., 2016) [31].
3.1. Competitive exclusion of harmful bacteria
Competitive exclusion (CE) includes the addition of a harmless bacteria culture of single or various strains to the gut intestinal territory of animals in order to reduce the pathogenic bacteria colony. Good bacteria always compete with bad bacteria for nutrients that are hosted by cell surfaces. This is actually discouraging to the harmful bacteria. The useful bacteria can change pH in the gut that reduces the growth of pathogens. So the lower number of pathogens indicates the lower cause of diseases. Competitive exclusion is now a phenomenon to prevent or reduces the competing bacterial colony and creates a challenging environment in the same position on the gut by a recognized microorganism. A study on Nile tilapia treated with 1 × 106 and 1 × 104 CFU g−1 of B. amyloliquefaciens for 30 days resulted in higher resistance against harmful Y. ruckeri or C. perfringens [37]. Obtaining a sustainable, pleasant and controlled microorganism is the goal of probiotics feeds that is designed in competitive exclusion. Prevention of replication of pathogenic microbiota and decolonization by making competition during taking nutrients, produce adverse habitat on the mucosa [8].
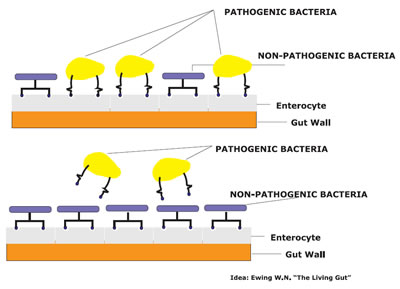
Fig 3: Interaction between pathogenic and non-pathogenic bacteria in the mucosa [From Ewing W.N].
For making a bond with microorganism, various types of tactics such as electrostatic interactions, lipoteichoic acids, hydrophobic, passive forces, static forces and structure of adhesion are applied [38]. Adhesion and colonization in the gut help to protect against pathogens through competition for concerning tissue and nutrients.
3.2. Creation of adverse habitat of pathogenic bacteria
Probiotics feeds can make an adverse habitat for the pathogens in the system. It is known that different species of bacteria can’t be a friend to each other; they always act as antagonism [39]. That’s why probiotics microbiota can play a major role to destroy potential pathogens. The chemical antagonistic compounds created by probionents are toxic or destructive to the other pathogens habitat. The presence of pro-bacteria creates an antibacterial environment in the host’s intestine as well as in culture water. This prevents the proliferation of active pathogenic microbes and even eliminates these. The structure and antibacterial compound activity should be more clear and up to date on the present situation. Moreover, researchers should demonstrate that the antimicrobial compound whatever produced in live conditions. If the production of probiotics is only for the situation demand but no specific future planning, the pathogen eventually developed their resistance against the probionts. Then probiotics would be ineffective like antibiotic treatment [40]. So the risk assessment and sustainability against pathogenic organism is very important to assure.
3.3. Stimulation of immunity against pathogenic microorganisms
The stimulation of immune response is done for host bio-security applying various biological functions by probionents to kill pathogens. In a word, it can be said that “protection against pathogens’’. It is found similarity in fish and higher vertebrate’s immune system and both have two essential components (a) The innate, natural or nonspecific security system that is formed by a series of a cellular and humoral mechanism and improve immunity and resistance, e.g. common carp [41], angelfish [42], olive flounder [43] and grass carp [44]. (b) The adaptive acquired or specific immune system can be defined by the hormonal immune response through the production of antibodies and by the cellular immune response which is mediated by T-lymphocytes, capable to react with antigens. The normal microbes in the ecosystem affect the fish’s immune system that is actually very important to disease control by enhancing physical barriers, hormonal and cellular components. Research in rainbow trout infection, host-isolated probiotics Enterococcus casseliflavus increases resistance against Streptococcus through immune modulation [45]. Cytokines are an essential element of the adaptive and natural immune response, particularly interleukin-lb [46], interferon, tumor necrosis factor-a, transforming growth factor-b and several chemokines influence natural immunity [47]. Probiotics can also influence the unidentified immune response and enzymatic activity [48]. Bacillus sp. can activate cellular and hormonal immune security in tiger shrimp that protect against disease [49]. That actually increases phagocytosis and antibacterial function which influences the growth and reduces the early mortality of postlarvae and influences the immune response in shrimp.
3.4. Antiviral Agent
Viruses are one of the great threats to aquaculture. It normally causes random mortality of post larvae. The biochemical substances extract from marine algae and bacterial extra-cellular agents inactive the viruses functioning. But it’s mechanism for antiviral effect is not even cleared. A study showed that the strains of Vibrio sp., Pseudomonas sp., Aeromonas sp., and coryneform agents isolated from salmonid acted as an antiviral function against affected hematopoietic necrosis virus with above 50% plaque reduction [50]. Moraxella species is a marine bacterium that affects the activity of poliovirus with plaque reduction percentage is about 62-99%. This was isolated from P. monodon hatchery [51].
4. Commercial Preparations
Day by day, the interest for probiotics such as an eco-friendly option is expanding experimentally and scientifically. The worldwide market for probiotics elements, supplements, and feedings is highly demandable. Recently commercial value of probiotics that include at least one live microorganism is rising. Probiotics can be utilized as food additives to the culture farm directly or blended with fish feeds. Aside from laboratory preparation arrangement of microscopic organisms, some commercially accessible items are currently found. At first, Assessments of commercial items focused on a microbial preparation named ‘Biostart’ that is isolated from Bacillus sp [52]. In 1998 it was first utilized in cultured catfish to test the effects of inoculums absorption. Moriarty (1998) emphasized that the utilization of commercial probiotics strains of Vibrio sp. expanded the quality and reasonability of pond culture of shrimp [53]. In the meantime, it is tested the function of Bacillus toyoi and Enterococcus faecium which was present in Carnival LBC and Toyocerin separately to diminish the European eel mortality rate that ensured more prominent efficiency with Enterococcus faecium [54]. This was utilized to supplement the food strengthen of Nile tilapia getting a significant increase in efficiency [55]. The lactic-acid creating microorganisms have been the focal point of much interest. The human probiotics, Lactobacillus rhamnosus ATCC was utilized as a part of rainbow trout for 51 days to diminish the death rate by Aeromonas salmonicida, to notice the reaction of fish pathogens. "Furunculosis" is one of the major fish diseases. Mortality was lessened from 52.6 to 18.9% when 109 cells g−1 were controlled with probiotics fish feed [56]. Experiment with Penaeus vannamei demonstrated that probiotics poly-culture expands survival, encourages transformation, and increases the final production of cultivated shrimp [15]. A study has shown that, poly-culture of bacteria (L. acidophilus, B. subtilis, and C. butyricum) and yeast (S. cerevisiae, B. licheniformis) upgraded unidentified immune expression parameters of tilapia [57] such as, lysozyme action, movement on neutrophils, and plasmatic action, bringing about change of protection from Edwardsiella tarda disease [58]. Additional investigations of probiotics demonstrated their ability to stimulate the activity of microbiota in the higher vertebrate colon [59]. Some commercial aquaculture probiotics such as mannans, glucans, and yucca improve the quality of the product. Recently, the advanced process technique of probiotics has been developed like immobilization and microencapsulation to ensure superlative quality. Right now, commercial items are found as a powder or liquid type in the box or poly packet. The production is carried out in general batch to batch cultures because of the complex mechanism of ongoing systems [60]. Usually, the techniques such as emulsion, shower drying, expulsion, and attachment to starch, have been utilized for micro-encapsulation of probiotics. Concentrated on the application to aquaculture have effectively exemplified cells of Shewanella putrefaciens in calcium alginate, showing the survival of typified probiotics cells through the intestinal tract in fish. The capacity of transport, dehydration temperature and osmoregulation parameters are crucial to assure the suitability of micro-organisms [61]. It is important that the product must ensure a healthy and bio-security benefit to the host. It is essential to ensure suitable conditions for probiotics organisms during storage and application on the host intestine. The user should be followed the packet labeling properly to ensure the best probiotics storage and safety of cultured species.
5. Probiotics applications for sustainable aquaculture
The requirement for feasible aquaculture modern research is required into the utilization of probiotics fed on aquatic animals. Initially, it was focused on their utilization as growth agents and health improvement but now the investigation has been going on multiplication or stress resilience.
5.1. Growth enhancer agent
Probiotics have been utilized as a part of aquaculture to build the growth development of farming species [62]. Actually, it isn't known whether these items enhance the hunger, or enhance absorbability. A few people are biased to imagine that it could be the two variables. Besides, it is vital to decide if probiotics really taste useful for aquaculture species [63]. It is indicated, probiotics microorganisms can colonize the gastrointestinal tract because they can do higher multiplication than the rate of removal. This likewise relies upon variables, for example, hydrobiont species, body temperature, compound levels, hereditary protection, and water quality. The effect of probiotics on phytoplankton influences the food chain by photosynthetic activities. Inside gatherings of microalgae utilized as a part of aquaculture are recognized focal diatoms as Chaetoceros sp., which is decent live food it may confinements because of the side effects of their healthy prerequisites. Rotifers are one of the essential feed for hatchlings of most refined amphibian species. For example, rotifer B. plicatilis and LABs (Lactococcus casei and Lactobacillus lactis) blend are used to feed the nauplii of marine shrimp to acquire the best outcome [64]. The Bacillus sp. probiotics, isolated from the fish gut into their diet has been studied and it’s found a positive approach in increasing size and weight of fishes. The utilization of probiotics as development promoters of palatable fishes has been accounted for the eating regimen of Nile tilapia [57]. In another study showed that unrefined protein and irregular fish lipid are significantly influenced by probiotics Streptococcus strain [65].
5.2. Application in fish/shrimp pathogen control
Probiotics microorganisms can discharge synthetic substances with a bacteriostatic effect on pathogenic microscopic organisms [66] that live in the digestive organ of the host, subsequently constituting an obstruction against the multiplication of entrepreneurial pathogens. The creation of antimicrobials, bacteriocins, siderophores, proteins, hydrogen peroxide, and modification of the intestinal pH are important to kill pathogens [67]. Some researchers demonstrated that suitable probiotics expanded nonspecific invulnerable reaction in salmon and trout fish. In this case, leukocytes number was more prominent than live cells [68]. Bacillus cereus, Paenibacillus polymyxa, and Pseudomonas sp. as bio-control are acted against pathogens from different Vibrio species [69]. Gastrointestinal tract straining probiotic of Amphiprion percula has been utilized to kill some of the pathogens like A.hydrophila and V. alginolyticus. It has been showed that probiotics application in live animals, the main goal is the stock density that permits the generation of antimicrobial metabolites in this way. Anti-infection agents were utilized in aquaculture for disease control up to the harvest. In any case, this creates different issues like anti-toxin environment in living tissues and adverse condition for the gut microbiota of host, which influence host bio-security [70]. At present, it is requested for characteristic items, free of added substances like anti-toxins. Besides, there is an inclination for counteracting ailments as opposed to treating them. Along these lines, the utilization of probiotics referred as an effective treatment for pathogens.
5.3. Change nutrient during digestion
Probiotics can play a vital role in the digestive system in fish. Probiotics strains combine extracellular enzymes like amylases, proteases, and lipases. In addition, it can stimulate vitamins, unsaturated fats, and amino acids by making efficient food supplement with probiotics. In this sense, probiotics have been utilized as a part of edible fish feed in hatchlings of European bass [71]. The probiotics from yeast Debaryomyces hansenii can generate spermine and spermidine, two polyamines engaged with the deviation and development of the gastrointestinal tract in warm-blooded organisms. Furthermore, yeast produces trypsin and amylase that guide absorption in sea-bass hatchlings. Concentrates in the juvenile of dentex demonstrated that feed added with 0.5 grams of B. cereus strain expanded fish growth [72]. In white shrimp different strains of Bacillus sp. have been utilized as probiotics feed to increase absorbability of the dry issue, unrefined protein, and phosphorus. Results demonstrated higher growth when the eating routine is supplemented with 50 grams of probiotics [73].
5.4. Change of water quality
To improve culture farm water quality probiotics may be an eco-friendly option. In a few experiments, water quality was recorded amid the expansion of probiotics strains, particularly of Bacillus sp. Most likely since this bacterial gathering is more effective than gram-negative in changing natural issues to CO2. It is recommended that keeping up irregular amounts of probiotics in ponds, fish ranchers can limit the gathering of natural carbon along with the developing time and this can adjust the creation of phytoplankton [39]. The assumption is not yet confirmed in tests basis on the development of shrimps or catfish. In this way enhancing water quality is limited, with the exclusion of nitrification [67]. In other studies shown for tilapia generation in recycling frameworks, groupings of aggregate ammonia (NH4 + NH3) expanded from 4.73 to 14.87mgL−1 / 21-day experiment, in this case, nitrite expanded from 3.75 to 9.77mgL−1. Because of the high convergences of delivered nitrogen mix and the harmful bad-smelling salts, the utilization of probiotics is suggested that it may enhance water quality by using B. subtilis and B. licheniformis in 17 weeks. Evaluation of water parameters confirmed a satisfactory quality range for fish development: 5.7– 6.3mgL−1 for break up oxygen, 0.36– 0.42mgL−1 for NH3 concentration, and pH in the area of 6.3 and 8.2 [55]. The probiotics used in culture farms can help to improve the water quality by balancing the nitrogen and pH levels of water.
5.5. Stress reliever
Stress in fish’s life cycle hampered the total production. The culture species may be weakened and dislike to take food, this is called food phobia. In this situation, for probiotics in culture farm can relieve these types of stress [74]. Research has been accounted for zebra fish (Danio rerio), general dejection on the combination of muscle protein initiated to enlarge stress resistance by utilizing probiotics [75]. One of the fastest formal reports on this area examined the supplementation of Lactobacillus delbrueckii in the eating regimen of E. sea bass, from 25- 59 days. In growing stage hormone cortisol was quantized in fish tissue as a stress marker since it is directly engaged with the host reaction to stress [76]. Another approach to evaluate stress in fish the treated group of probiotics indicated more prominent resilience in the stress test than the control group [77]. The outcomes got so far raise the possibility of good production of fish within time with probiotics treatment, for ordinary aquacultures rehearses that make stress in the animal during transport, change in water temperature, and irregular controls. The probiotic outcomes demonstrated the high possibility of stress prevention in aquatic animals.
5.6. Effect on reproduction of aquatic species
The quality of cultured fish species depends on broodstock quality. Rearing the broodstock it’s highly required nutritious feeds containing lipids, proteins, unsaturated fats and vitamins. Besides, the relationships of these elements influence multiplication in production, e.g. probiotics may play an important role to improve food value and hatchlings in ornamental fish [78, 79]. The utilization of these natural fish items frequently doesn't give sufficient levels of supplements required by broodstock fishes. Sometimes pathogens may transfer to the host including parasites, microscopic organisms, and infections. Researchers has shown that the total creation of egg per female and the relative fertility were assessed their outcomes indicated significant deference between the control and probiotics-treated group [79]. So, probiotics as a feed additive or water purifier effective investigation is required before applying in aquaculture.
6. Safety regulation, advantages, and disadvantages of probiotics
It is very important to apply the correct and quality probiotics to the culture farm. Quality probiotics can ensure proper growth and give bio-security in the farming. But if there is a problem with the selection and application of probiotics it may create adverse effects, and the risky species may grow out and make them more resistant against the host. This depends on the selection of probiotics. During selecting probiotics a farmer should know the molecular function of it. Effects of common antibiotics such as quinolone, macrolide, tetracycline and subsequent confirmation of drug resistance genes should be well-analysis before applying probiotics. During feed formulation the ratio should be perfect otherwise it may harm the culture farm. Modern technology can be useful to know it’s used and molecular function. So specific labeling with specification should be followed before application. GMP (good management practice) and GLP (good laboratory practice) should be followed during the production of probiotics. The quality probiotics may ensure disease control and sustainable healthier condition of the gut area and the immune system of fish. At present, the application field of probiotics has been applying to the confined level but it should be more affordable. Eco-friendly bacteria can be more beneficial for the aquaculture community to disease control and health improvement. The evidence of probionts efficiency in aquaculture should be more available as possible. The production of probiotics should be increased through effective research. This is a very pleasant approach that FAO has now concern about probiotics using policy for improvement of aquatic environmental quality. Here, we list the advantages and disadvantages of probiotics (table 1).
Table 1: Advantages and disadvantages of probiotics
|
Advantages |
Disadvantages |
|
1. Hindrance of pathogens in the gut or elsewhere 2. Enhanced digestibility and microbial adjustment 3. Provide nutrition supplied for aquatic animals 4. Host immune response stimulation to disease 5. Improve water quality and ensure a friendly environment. 6. Reduced blue-green algae load and cleaned the bottom of the pond. 7. Water exchange needed less that reduces water pumping costs. 8. Reduced green vibrio counts and reduced overall vibrio loads 9. Reduce ammonia level in water insuring healthier production and increased profits. |
1. Improper application in host sometime may be negatively treated. 2. Probiotics work slowly than antibiotics 3. Application in the gut for fish is studied but no more results found in other organs. 4. It cannot reach and maintain itself in the location where the effect is to be applied. 5. Mislabeling and mishandling may cause an adverse condition for the environment. 6. Misguidance may resist the new pathogens and will be risky for the human being. 7. Advance technology required to prepare probiotics which are the main challenge [80].
|
The selection of probiotics is essential and this is also a great challenge for the culture species how they adopt this. A good mechanism for applying in the cultured field can be useful in different situations. Recent efforts of research in probiotics showing the positive result in cultured fields. That's why more information should be noted on the host and microbe interactions in life. The technology should be more developed for monitoring tools. For example, a brief understanding of the chemical composition and activity of the native microorganism and their microbial cultures of molecular knowledge is required. The efficiency of competition between species or strains should be more analyzed. Probiotics are beneficial microorganisms for the host though some of these benefits still have to be confirmed using clinically apply. There is however still a problem of preservation of these cultures microbes both in storage and in the gastrointestinal tract. The research for modern technology that can protect and retain the feasibility of probiotics fish cultures is needed. There are also concerns about the negative impacts of probiotics on receptive consumers but there is insufficient data to support the raised concerns. Probiotics and symbiotic can be alternative mechanisms for increasing the levels of beneficial microorganisms in the gut intestine. Given the health issues of fisheries associated with probiotics, it can be an innovative approach for the future aquatic species.
FAO (2019). FAO yearbook. Fishery and Aquaculture Statistics 2017/FAO
Penaloza-Vazquez et al, (2019). Isolation and characterization of Bacillus spp. strains as potential probiotics for poultry. Canadian Journal of Microbiology 4166, 65(10) 762-774 PMid:31393167
View Article PubMed/NCBICabello FC. (2006). Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol. 8, 1137-1144. PMid:16817922
View Article PubMed/NCBIXu, H. M., Rong, Y. J., Zhao, M. X. et al., (2014). Antibacterial activity of the lipopetides produced by Bacillus amyloliquefaciens M1 against multidrug-resistant Vibrio spp. isolated from diseased marine animals. Appl Microbiol Biotechnol 98, 127-136. PMid:24132666
View Article PubMed/NCBIParker RB (1974). Probiotics, the other half of the antibiotics story. Anim Nutr Health; 29: 4-8.
Ahmad, I., Babitha Rani, A.M., Verma, A.K. et al., (2017). Biofloc technology: an emerging avenue in aquatic animal healthcare and nutrition. Aquacult Int 25: 1215.
View ArticleFuller R. (1989). Probiotic in man and animals. J Appl Bacteriol. 66:365-378.
View ArticleMoriarty DJW (1998). Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture. 164: 351-358 00199-9
View ArticleMoriarty DJW (1990). Interactions of microorganisms and aquatic animals, particularly the nutritional role of the gut flora. In: Lesel R, ed. Microbiology in Poecilotherms. 217-222.
Merrifield, D. L., and Carnevali, O. (2014). Probiotic Modulation of the Gut Microbiota of Fish. Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics.
View ArticleLi, T. et al. (2017). Bacterial signatures of "Red-Operculum" disease in the gut of Crucian carp (Carassius auratus). Microb Ecol 74, 510-521. PMid:28364130
View Article PubMed/NCBIHoseinifar, S. H., Ringø, E., Shenavar Masouleh, A. et al., (2016). Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: a review. Rev. Aquacult. 8, 89-102.
View ArticleCamila Sayes et al., (2017). Probiotic Bacteria as a Healthy Alternative for Fish Aquaculture. Journal: Antibiotic Use in Animals 51-3751-1, 978-953.
Mingmongkolchai, S., and Panbangred, W. (2018). Bacillus probiotics: an alternative to antibiotics for livestock production. Appl. Microbiol. 124, 1334−1346. PMid:29316021
View Article PubMed/NCBIWang Y. B., Xu Z. R., and Xia M. S. (2005). The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds," Fisheries Science, 71 (5), 1036- 1041.
View ArticleAdorian T J , Jamali H , Farsani H G , et al., (2018). Effects of Probiotic Bacteria Bacillus on Growth Performance, Digestive Enzyme Activity, and Hematological Parameters of Asian Sea Bass, Lates calcarifer (Bloch). Probiotics and Antimicrobial Proteins; 12602-018-9393
Jamali H , Moghadam H , Pariche N , et al. (2015). Effects of probiotic bacteria on survival, growth and body composition of rainbow trout (Oncorhynchus mykiss) larvae fed diets with various fish meal. 海岸生命医学杂志(英文版), 3(2):91-97
Rhody, N. R., Puchulutegui, C., Taggart, J. B. et al., (2014). Parental contribution and spawning performance in captive common snook Centropomus undecimalis broodstock. Aquaculture 432, 144-153.
View ArticleTarnecki, A. M. & Rhody, N. R. (2017). Microbiota of common snook Centropomus undecimalis larvae exhibiting high mortality. Aquac Res 48, 5693-5698.
View ArticleFerreira, G. S., Bolívar, N. C., Pereira, S.A. et al., (2015). Microbial biofloc as source of probiotic bacteria for the culture of Litopenaeus vannamei. Aquaculture 448, 273-279.
View ArticleWanka K M , Damerau T , Costas B , et al. (2018). Isolation and characterization of native probiotics for fish farming. BMC Microbiology, 18(1). PMid:30236057
View Article PubMed/NCBIHuis Veld JHJ, Havenaar R, Marteau PH. (1994). Establishing a scientific basis for probiotic R&D. Tibtech.; 12, 6-8. 90004-3
View ArticleKarthikeyan N., Elango A., Kumaresan G. et al., (2013). Augmentation of Probiotic Viability in Ice Cream Using Microencapsulation Technique; IJAVST, 2320-3595
Oelschlarger TA. (2010). Mechanisms of probiotic actions e a review. Int J Med Microbiol., 300, 57-62. PMid:19783474
View Article PubMed/NCBIHagi T, Tanaka D, Iwamura Y, et al., (2004). Diversity and seasonal changes in lactic acid bacteria in the intestinal tract of cultured freshwater fish. Aquaculture, 234, 335-346.
View ArticleVan Doan, H., Hoseinifar, S. H., Dawood, M. A. O. et al., (2017). Effects of Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum on mucosal, serum immunology and growth performance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immun. 70 (Supplement C):87-94. PMid:28882795
View Article PubMed/NCBITan, L. T., Chan, K. -G., Lee, L. -H. et al., (2016). Streptomyces bacteria as potential probiotics in aquaculture. Front. Microbiol. 7:79 PMid:26903962
View Article PubMed/NCBIDuc LH, Hong HA, Barbosa TM et al., (2004). Characterization of Bacillus probiotics available for human use. Appl Environ Microbiol. 70(4), 2161-2171. PMid:15066809
View Article PubMed/NCBIRoss RP, Desmond C, Fitzgerald GF et al., (2005). Overcoming the technological hurdles in the development of probiotic foods. Appl Microbiol. 98, 1410-1417. PMid:15916653
View Article PubMed/NCBIRingo E, Gatesoupe FJ. (1998). Lactic acid bacteria in fish: a review. Aquaculture. 160, 177-203 00299-8
View ArticleTan LT-H, Chan K-G, Lee L-H et al., (2016). Streptomyces Bacteria as Potential Probiotics in Aquaculture. Front. Microbiol. 7: 79.doi:10.3389/fmicb.2016.00079
View ArticleSudhansu S. Mishra (2017). Present Status of Fish Disease Management in Freshwater Aquaculture in India: State-of-the-Art-Review.: 10.24966/5523/100003
Ali A. (2000). Probiotic in Fish Farming-Evaluation of a Candidate Bacterial Mixture. Umea, Senegal: Sveriges Lantbruks Universitet.
Chai, P. C., Song, X. L., Chen, G. F. et al., (2016). Dietary supplementation of probiotic Bacillus PC465 isolated from the gut of Fenneropenaeus chinensis improves the health status and resistance of Litopenaeus vannamei against white spot syndrome virus. Fish Shellfish Immun. 54, 602-611. PMid:27177431
View Article PubMed/NCBITarnecki, Andrea M., Wafapoor, Marzie Phillips et al., (2019). Benefits of a Bacillus probiotic to larval fish survival and transport stress resistance. Scientific Reports 4892 (9)-1; 2045-2322. PMid:30894554
View Article PubMed/NCBIMoubareck C., Gavini F., Vaugien L. et al., (2005). Antimicrobial susceptibility of Bifidobacteria. J Antimicrob Chemother. 55, 38-44. PMid:15574479
View Article PubMed/NCBISelim, K. M., and Reda, R. M. (2015). Improvement of immunity and disease resistance in the Nile tilapia, Oreochromis niloticus, by dietary supplementation with Bacillus amyloliquefaciens. Fish Shellfish Immun. 44, 496-503 PMid:25783002
View Article PubMed/NCBISalyers AA, White DD. (2002), Bacterial Pathogenesis, a Molecular Approach. Washington D. C: ASM Press.
Balc'azar J. L., Blas I. D., Ruiz-Z I. et al., (2006). "The role of probiotics in aquaculture," Veterinary Microbiology, 114 (3-4), 173-186. PMid:16490324
View Article PubMed/NCBILedesma Rosas P., Le'on-Rubio J. M., Alarc'on F. J. et al., (2012), Calcium alginate capsules for oral administration of fish probiotic bacteria: assessment of optimal conditions for encapsulation, Aquaculture Research, 43, 106-116.
View ArticleModanloo, M., Soltanian, S., Akhlaghi, M. et al., (2017). The effects of single or combined administration of galactooligosaccharide and Pediococcus acidilactici on cutaneous mucus immune parameters, humoral immune responses and immune related genes expression in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immun. 70, 391-397. PMid:28917489
View Article PubMed/NCBIAzimirad, M., Meshkini, S., Ahmadifard, N. et al., (2016). The effects of feeding with synbiotic (Pediococcus acidilactici and fructooligosaccharide) enriched adult Artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish (Pterophyllum scalare). Fish Shellfish Immun. 54, 516-522. PMid:27150050
View Article PubMed/NCBIBeck, B. R., Kim, D., Jeon, J. et al., (2015). The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immun. 42, 177-183. PMid:25449382
View Article PubMed/NCBIWu, Z., Jiang, C., Ling, F. et al., (2015). Effects of dietary supplementation of intestinal autochthonous bacteria on the innate immunity and disease resistance of grass carp (Ctenopharyngodon idellus). Aquaculture 438, 105-114.
View ArticleSafari, R., Adel, M., Lazado, C. C. et al., (2016). Host-derived probiotics Enterococcus casseliflavus improves resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss) via immunomodulation. Fish Shellfish Immun. 52, 198-205. PMid:26997202
View Article PubMed/NCBIChristine Beecher, Maire'ad Daly, Donagh P Berry, et al., (2009). Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1b and IL-8 gene expression PMid:19445831
View Article PubMed/NCBIGomez GD, Balcazar JL. (2008). A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol. 52, 145-154. PMid:18081845
View Article PubMed/NCBIEshaghzadeh, H., Hoseinifar, S. H., Vahabzadeh, H. et al., (2015). The effects of dietary insulin on growth performances, survival and digestive enzyme activities of common carp (Cyprinus carpio) fry. Aquacult Nutr. 21, 242-247.
View ArticleBalcazar JL. (2003). Evaluation of Probiotic Bacterial Strains in Litopenaeus Vannamei: Final Report. Guayaquil, Ecuador: National Center for Marine and Aquaculture Research
Girones R, Jofre JT, Bosch A. (1989) Isolation of marine bacteria with antiviral properties. Can J Microbiol. 35, 1015-1021. PMid:2558789
View Article PubMed/NCBIDirekbusarakom S, Yoshimizu M, Ezura Y et al., (1998). Vibrio spp. the dominant flora in shrimp hatchery against some fish pathogenic viruses. J Mar Biotechnol. 6, 266-267.
Queiroz J. F. and Boyd C. E. (1998), "Effects of a bacterial inoculum in channel catfish ponds," Journal of the World Aquaculture Society, 29 (1), 67-73.
View ArticleAli Frzanfar (2009). The use of probiotics in shrimp aquaculture: Iranian Fisheries Research Organization (IFRO), 297.
Chang C. I. and Liu W. Y. (2002). An evaluation of two probiotic bacterial strains, Enterococcus faecium SF68 and Bacillus toyoi, for reducing edwardsiellosis in cultured European eel, Anguilla anguilla L," Journal of Fish Diseases, 25 (5), 311-315.
View ArticleHaroun E., Goda A., and Kabir M., (2006). Effect of dietary probiotic Biogen supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.), Aquaculture Research, 37 (14), 1473-1480.
View ArticleNikoskelainen S., Ouwehand A., Salminen S. et al., (2001). Protection of rainbow trout (Oncorhynchus mykiss) from furunculosis by Lactobacillus rhamnosus, Aquaculture. 198 (3-4) 229-236. 00593-2
View ArticleHan, B., Long, W. Q., He, J. Y. et al., (2015). Effects of dietary Bacillus licheniformis on growth performance, immunological parameters, intestinal morphology and resistance of juvenile Nile tilapia (Oreochromis niloticus) to challenge infections. Fish Shellfish Immun. 46, 225-231. PMid:26108035
View Article PubMed/NCBITaoka Y., Maeda H., Jo J. Y. et al., (2006). Use of live and dead probiotic cells in tilapia Oreochromis niloticus, Fisheries Science, 72 (4), 755-766.
View ArticleTuohy K.M., Probert H.M., Smejkal C.W et al., (2003). Using probiotics and prebiotics to improve gut health, Drug Discovery Today, 8 (15), 692-700. 02746-6
View ArticleSoccol C., Porto L., Rigon M. et al., (2010), The potential of probiotics: a review, Food Technology and Biotechnology, 48 (4), 413-434
Muller J.A., Ross R. P., Fitzgeralk G. F et al., (2009). Manufacture of Probiotic Bacteria, Prebiotics and Probiotics Science and Technology, 125-759.
View ArticleRohyati, I. S. (2015). Improved of growth rate of abalone haliotis asinine fed pudding probiotic-enriched protein. Procedia Environ. Sci. 23, 315-322.
View ArticleIrianto A. and Austin B. (2002). Probiotics in aquaculture, Journal of Fish Diseases, 25 (11), 633-642,
View ArticlePlanas M., V'azquez J., Marqu'es J. et al., (2004). Enhancement of rotifer (Brachionus plicatilis) growth by using terrestrial lactic acid bacteria, Aquaculture, 240 (1-4), 313-329.
View ArticleLara F., Olvera N., Guzm M. et al., (2003). Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus), Aquaculture, 216 (1-4) 193-201. 00277-6
View ArticleDe Schryver, P. & Vadstein, O. (2014). Ecological theory as a foundation to control pathogenic invasion in aquaculture. ISME J, 8, 2360-8. PMid:24892581
View Article PubMed/NCBIVerschuere L., Rombaut G., Sorgeloos P. et al., (2000). "Probiotic bacteria as biological control agents in aquaculture," Microbiology and Molecular Biology Reviews, 64 (4), 655-671. PMid:11104813
View Article PubMed/NCBIRobertson P. A. W., O'Dowd C., Burrells C. et al., (2000). Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss, Walbaum),"Aquaculture, 185 (3-4), 235-243. 00349-X
View ArticleRavi V., Musthafa K. S., Jegathammbal G. et al., (2007). Screening and evaluation of probiotics as a biocontrol agent against pathogenic Vibrios in marine aquaculture, Letters in Applied Microbiology, 45 (2), 219-223. PMid:17651222
View Article PubMed/NCBINakano T. (2007) Microorganism. En dietary supplements for the health and quality of cultured fish, CAB International, London, UK.
Tovar D., Zambonino J., Cahu C. et al., (2002). Effect of live yeast incorporation in compound diet on digestive enzyme activity in sea bass (Dicentrarchus labrax) larvae, Aquaculture, 204 (1-2), 113-123. 00650-0
View ArticleHidalgo M. C., Skalli A., Abellan E., et al.,(2006). Dietary intake of probiotics and maslinic acid in juvenile dentex (Dentex dentex L.): Effects on growth performance, survival and liver proteolytic activities. Aquaculture Nutrition, 12 (4) 256-266.
View ArticleZiaei S., Habibi M., Azari G. et al., (2006). The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus, Aquaculture, 252 ( 2-4), 516-524.
View ArticleMohapatra S , Chakraborty T , Kumar V , et al., (2012). Aquaculture and stress management: A review of probiotic intervention. Anim Physiol a Anim Nutr, 97(3). PMid:22512693
View Article PubMed/NCBIVianello S., Brazzoduro L., Dalla V. et al., (2005). Myostatin expression during development and chronic stress in zebrafish (Danio rerio), Journal of Endocrinology, 176 (1) 47-59. PMid:12525249
View Article PubMed/NCBICarnevali O., De Vivo L., Sulpizio R. et al., (2006). "Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax, L.), with particular attention to IGF1, myostatin and cortisol gene expression," Aquaculture, 258, (1-4), 430-438.
View ArticleCastex M., Lemaire P., Wabete N. et al., (2009). Effect of dietary probiotic Pediococcus acidilactici on antioxidant defense and oxidative stress status of shrimp Litopenaeus stylirostris, Aquaculture, 294 (3-4), 306-313.
View ArticleGhosh S., Sinha A., and Sahu C., (2007). "Effect of probiotic on reproductive performance in female live bearing ornamental fish," Aquaculture Research, 38, (5), 518-526.
View ArticleAbasali H. and Mohamad S. (2010), Effect of dietary supplementation with probiotic on reproductive performance of female live bearing ornamental fish, Research Journal of Animal Sciences, 4 (4), 103-107.
View ArticleWang Y. B., Li J. R., and Lin J. (2008). Probiotics in aquaculture: challenges and outlook," Aquaculture, 281, (1), 1-4.
View Article