T.M Salami
E-mail : major_brain2005@yahoo.co.uk
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 1
Page No: 553-562
T.M Salami
E-mail : major_brain2005@yahoo.co.uk
Salami, Taofeek Mayowa a, Xiaoxiao Jianga, Pan, Weichun b,*
aCollege of Chemistry and Materials Engineering, Wenzhou University, Chashan University Town, Wenzhou, Zhejiang, China 325035. major_brain2005@yahoo.co.uk (T.M Salami)
a,*College of Chemistry and Materials Engineering, Wenzhou University, Chashan University Town, Wenzhou, Zhejiang, China 325035.ywf@wzu.edu.cn (W. Yu)
bThe School of Food Science and Biotechnology, Zhejiang Gongshang University, Xiasha Gaojiao Garden, Xuezheng Street 18,Hangzhou, China, 310018. panweichun1@yahoo.com(W. Pan)
Weichun Pan, LIQUID CHROMATOGRAPHIC ANALYSIS OF FREE AMINO ACIDS IN AMERICAN COCKROACH (Periplaneta americana)(2018)SDRP Journal of Food Science & Technology 4(1)
Insects could serve as an alternative amino acids source with less environmental impact and low production cost. In this study, phenylisothiocianate (PITC) pre–column derivatization and high-pressure liquid chromatographic (HPLC) method combined with solid phase extraction (SPE) was developed and used to quantify free amino acids present in American cockroach. Powdered cockroach sample were extracted in water at 90 oC for 20 min, pre-purified on silica-based cationic exchange (SCX) cartridges, derivatized and analyzed for the free amino acid compositions. The free amino acids derivatives were separated on Agilent Zorbax Extend-C18 (4.6 x 250 mm, 5 μm) HPLC column at 33 ± 0.1 oC with diode array detection (DAD) set at 254 nm. A total analysis time of 30 min was used to separate 15 amino acids including nine essential and six non-essential ones with gradient elution at a flow rate of 1.0 mL/min. The results showed linear calibration range from 50 to 150 pmol/uL for all the free amino acids. The sensitivity expressed as limits of quantification (LOQ) and limit of detection (LOD); repeatability and reproducibility precisions expressed as relative standard deviation (RSDs) percentages for all amino acids characterized by the following ranges:2.6 to 35.5 pmol/μL; 0.9 to 11.7 pmol/μL; 1.0 to 10.5 %; 6.8% to 51.9%. The optimized and validated analytical method was applied for the determination of free amino acids in American cockroach. The free amino acids concentrations ranged from His, 0.43 mg/g to Pro, 311.2 mg/g and the recovery ranged from Thr, 82.9 % to Glu and Gly, 100 %. The free amino acids concentrations were in the order Pro > Thr > Leu > Glu > Ile > Lys > Met > Val > Asp > Ser > Try > Gly > Arg > His. The concentration of Pro was 5 – 100 folds higher than other free amino acids and representing 50.64% of the total free amino acid. The free amino acids were found to constitute 61.5% of the total nutrients in Asmerican cockroach, indicating its promising source of amino acid and protein for food and feed.
Keyword: Free amino acids, phenylisothiocianate, pre-column derivatization, limits of quantification, limit of detection, solid phase extraction.
Insects have been human traditional food from time immemorial and its satisfactory nutritional values in comparison with that of animal has increased its interest for food and feed alternative [1]. The nutritional value of food largely depends on the quality of proteins it contains which is to a great extent determined by the amino acid composition [2]. Insects as an important source of proteins have been extensively reported in literatures [2,3,4,5,6,7,8,9,10,11]. The low production cost, short life cycles, high reproduction rates [12], traditionally and medicinally proven nutrient compositions [2,13,14,15] make insect industries fast growing as a new source of cheap pharmaceutical raw materials, feedstock for biodiesel production [12] amongst other importance such as source of animal feed and human food [2,14,16].
American cockroach; Periplaneta americana (Linnaeus) which has been stigmatized for years is the largest of the common peridomestic cockroach and the strongest insect groups used as kind of traditional Chinese medicine for treatment of many diseases including gastroenteritis and bone tuberculosis [10]. Its medicinal uses are well documented in Chinese medical encyclopedia, such as Ben Cao Gang Mu and Shen Nong Ben Cao Jing [17]. The extract of roasted American cockroach with water is popularly used by Arunachal Pradesh tribe of India to cure asthma [11]. Modern medical researchers have shown that its extracts have anti-virus, anti-tumor, improving immunity, promoting tissue repair, anti-inflammation, anti–ulcer, analgesia and protecting liver effect [10] with a recorded success of the extract development into drugs that are now marketable [17]. The nutrient composition showed that American cockroach contains various amino acids and peptides [18], vitamin A, C and D [2,11], proteins, oils and fat [18,19,20], octopamine, tachykinin [14], chitin and diphenols [10,21]. Its elemental analysis revealed its richness in trace elements especially Zn, Mg, Cu and K, which may be related to improving body's immunity [2,11,15,21].
However, water–soluble substances (chitins and diphenols) present in American cockroach can co-elute with amino acids thereby, affecting derivatization process and amino acids separation by HPLC resulting in a low–resolution chromatogram. To correct this, it is very necessary to pre–purify the American cockroach extract using a suitable extraction method. The most widely used method for amino acids analysis is solid-phase extraction (SPE) on an account of its simplicity, rapid, economical, and sensitivity nature [22]. Other methodologies include Liquid–liquid extraction (LLE), solid–phase micro extraction (SPME), liquid–phase micro extraction (LPME), and supercritical fluid extraction (SFE) [22]. Therefore, SPE was chosen as pre-purification method prior to derivatization and HPLC-DAD analysis.
Over the years, there have been several methods developed to determine the amino acids components of samples. Concisely, the amino acid components of food proteins are mainly determined by reversed phase high performance liquid chromatography (RP–HPLC) [23]. In order to effectively determine amino acids, however, it is first necessary to chemically modify them (derivatization), which usually involves converting them into derivatives that absorb or fluoresce in the ultraviolet-visible (UV-vis) wavelength range [23]. The derivatization may be done before injection of the sample into HPLC (pre–column) or after injection (post–column). Several reagents include but are not limited to ninhydrin [24], phenylisothiocyanate (PITC) [25], o-phthaldialdehyde (OPA) [26], 9-fluorenylmethyl chloroformate (FMOC-Cl) [27], 4-dimethylaminoazobenzene-4-sulfonyl chloride (dabsyl chloride) [28], 2,4-dinitrofluorobenzene (DNFB) [29,30], 6-aminoquinolyl-N-hydroxysuccinimidylcarbamate (AQC) [31] among others have been developed for this purpose with each method having its drawback such as sample preparation, pH effect, low reactivity with secondary amines, chromatographic difficulty in separating residual amino sugars [31], use of excess reagent that must be extracted prior to chromatography, which often results in hydrolysis and loss of the amino acids adducts, reliability and accurate [23]. These methods require specific and unique protocols for preparation of samples prior to amino acid analysis which is cumbersome and require longer time especially hydrolysis as reported in many studies ranging from 16–24 hours, derivatization, and gradient elution. In addition, literature revealed that OPA derivatization has been the major method used for the analysis of insect amino acids [2,7,16,24]. Hence, the need to develop easy and rapid HPLC method is important. Because of the ability of PITC to reacts with both primary and secondary amino acids and also generates a single, stable sulfur amino carbonyl derivative of benzene (phenylthiocarbamyl) [PTC], PITC derivatization method was employed in this study. Although, its major drawback is longer time sample preparation and low-sensitivity which may be the reason for it’s often nonuse by researchers compared with other derivatization methods. However, sensitivity problem was overcome by using large amount of cockroach sample during sample preparation.
However, to the best of our knowledge, this is the first study documenting the use of PITC pre-column derivatization and HPLC–DAD combined with SPE for the quantification of American cockroach free amino acids. Therefore, the overall goal of this investigation was to establish sensitive HPLC–DAD method for the analysis of free amino acids in American cockroach with PITC pre–column derivatization and HPLC combined with SPE.
2.1. Reagents and chemicals
Amino acid standard mixtures at a concentration of 2.5 mM dissolved in 0.1N HCl was obtained from Thermo Scientific (Rockford, IL, USA). Triethylamine (TEA) and phenylisothiocyanate (PITC) were purchase from Aladdin Industrial Corporation (Shanghai, China). HPLC–grade acetonitrile (CH3CN) and methanol (CH3OH) (J&K Scientific, China), Glacial acetic acid, Sodium ethanoate, and Disodium hydrogen phosphate were obtained from Sinopharm Chemical Reagent, China. The Megabond Elut C18 and SCX cartridges (6mL, 500mg and 10 mL, 500 mg respectively) were purchased from Agilent Technologies, Inc. (USA). All reagents were analytical grade, unless otherwise noted. All aqueous solutions were prepared with ultrapure water purified.
2.2. Sample collection and treatment
American cockroach of different stages of development and different sexes (n = 500) were supplied by Teng Fei cockroach farm located in Anhui Province, China. The sexes were not separated. The insects were taxonomically identified as Periplaneta americana (Linnaeus). In the laboratory, the freeze-dried sample were washed thoroughly with distilled water, oven–dried (50 °C), grounded into powder in pestle and mortar and prepared for further analyses.
2.3. Sample preparation and pre treatment
Powdered cockroach samples (1.0 g) were accurately weighed and extracted in 10 mL water at 90 oC for 20 min. The mixture was allowed to cool and filtered thoroughly using two filter membranes (0.45μm) which are superimposed. The filtrate was made up to 10 mL with water and vortex. Using accurately measure 1mL extract, the extraction of the amino acids on SCX cartridges which had been conditioned with 25 mL MeOH and equilibrated with 10 mL H2O was accomplished. After the filtrate was bound to the cartridge, it was eluted with 5 mL 40% CH3CN and the corresponding elute was collected, evaporated to dryness in a rotary vacuum evaporator (Buchi, Switzerland) and stored at 4 oC until analysis.
2.4. Preparation of amino acids standards
The amino acids standards contain Ala, Arg, Asp, Gly, Glu, His, Ile, Leu, Lys, Met, Phe, Pro, Ser, Thr, Tyr, and Val. The standard mixture solution was prepared at five concentration levels (50, 75, 100, 125, and 150 pmol/μL) in H2O:CH3CN (4:1 v/v). The working standard solutions were vortexed and then centrifuged at 10, 000 r/min for 5 min at 4 oC for adequate homogenization. All amino acid stock standard mixtures and amino acids working standards were stored at 4oC until use.
2.5. Instrumentation
A digital OHAUS pH meter (ST3100/F) was used for pH adjustment. The amount of solute was measured using a PTT–A2000 USA.HZI & HUAZHI high precision balance (Huazhi, China). Quantitative liquid transfers were performed with a micropipette (Thermo Scientific, USA). All analysis were performed on Agilent 1260 Infinity Binary LC system (Agilent Technologies, Waldbronn, Germany) equipped with a degassing unit, a quaternary pump, sampling port with 50µL high–pressure coloured syringe, and infinity diode array detector (G4212B). The column temperature was controlled by Julabo model F34 column heater module and a Julabo model ED temperature control module. The system was linked to a Lenovo desktop for recording chromatograms, using ChemStation edition software (Waldbronn, Germany).
2.6. PITC precolumn derivatization
Derivatization procedure was carried out according to reported method [23] with some modifications. In summary, the dried fractions of sample were dissolved in 1.0 mL H2O:CH3CN (4:1 v/v) and filtered through a 0.22 μm Millipak filter. Then, pre-column derivatization was performed on both standards and samples by adding 30µL of a mixture of 7:1:1 MeOH:TEA:PITC (v/v) separately to each of 50µL amino acid standards and aliquot cockroach sample. They were vortex mixed and the reaction to produce amino acids derivatives {phenylthiocarbamyl (PTC)} was allowed to complete for 30 min at room temperature. Samples were then dried in oven at 65 oC and stored at 4 oC till further analysis. Before HPLC analysis, the amino acids derivatives were reconstituted in buffer (250 μL of 5 mM Na2HPO4 pH 7.4 containing 5% CH3CN) and filter through 0.22μm Millipak filter. Samples were reconstituted one at a time due to the PTC amino acid sensitivity to light and ambient temperature. Twenty microliters of sample were injected and analyzed with an HPLC-DAD system.
2.7. Analysis by high-performance liquid chromatography
The analytical column used was Agilent Zorbax Extend-C18 (4.6 x 250 mm, 5 μm) HPLC column with diode array detection (DAD) set at 254 nm. The column temperature was maintained at 33oC ± 0.1oC, mobile phase consist of A (CH3CN:H2O/60:40 v/v) and B (0.14 M CH3COONa + 0.05% TEA titrated with glacial acetic acid to pH 6.5). Separation was accomplished using a gradient elution (Table 1) as follows: Initially, A was set at 10 % which was run to 17.6 % in 5 min, then 17.6 % to 20% A from 5 to 5.1 min, then 20 % to 65% A from 5.1 to 25.95 min followed by an increase to 100 % A from 25.95 to 30 min. The column was washed with 100 % A for 5 min, and then returned to 10% A from 35 to 36 min. For every analysis, 5 min washing of the column was used in other to wash off accumulated non-target analytes and obtain consistent retention times. The flow rate was set at 1.0 mL/min.
2.8. Peak identification and quantitation
Peak area was used as detector response, and qualitative analysis was based on the comparison between the peak retention time (tR) of the standard amino acids and those in the sample and was confirmed by standard addition while quantitative analysis was based on external standard method using calibration curve fitted by linear regression analysis.
Table 1: The elution gradient for HPLC-DAD Analysis
|
Time (min) |
Solvent A (%) |
Solvent B (%) |
|
0.0 |
10.0 |
90.0 |
|
5.0 |
17.6 |
82.4 |
|
5.1 |
20.0 |
80.0 |
|
25.95 |
65.0 |
35.0 |
|
30.0 |
100.0 |
0.0 |
|
35.0 |
100.0 |
0.0 |
|
36.0 |
10.0 |
90.0 |
3.1. Optimization of chromatographic analysis
In order to achieve optimum chromatographic conditions for the analysis of free amino acids, variables including mobile phase composition, wavelength and mobile phase flow–rate were optimized. Under established conditions using Agilent Zorbax Extend-C18 (4.6 x 250 mm, 5 μm), a chromatogram of standard amino acids at injection volume 20 μL and concentration 50 pmol/μL was detected using gradient elution (10% - 65% in 25.95 min) and aqueous mobile phase (CH3COONa) at pH 6.5. The chromatogram was presented in Fig 1 (a).
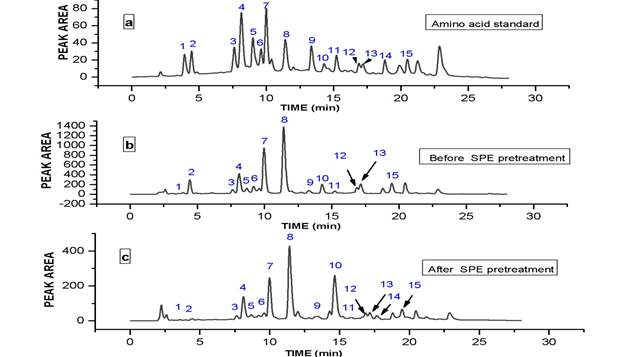
Fig. 1. (a) HPLC-DAD Chromatogram of 15 PTC-amino acid standards. Peaks: 1, Asp; 2, Glu; 3, Ser; 4, Gly; 5, His; 6, Arg; 7, Thr; 8, Pro; 9, Try; 10, Val; 11, Met; 12, Ile; 13, Leu; 14, Phe, 15, Lys. (b) HPLC-DAD Chromatogram of PTC-Cockroach free amino acids after SPE clean-up. (c) HPLC-DAD Chromatogram of PTC-Cockroach free amino acids before SPE clean-up study.
3.1.1. Solvent selection for free amino acids preparation
The preparation of standard amino acids involves the use of different solvents including water [23, 25, 30], acetic acid (2.0 M), 0.1 M hydrochloric acid [27], buffer solution such as borate buffer at pH 9.5 [27]. In this study, a mixture of water and acetonitrile [H2O:CH3CN 4:1 v/v] was screened along with some of the reported solvents for the amino acids preparation as presented in Fig 2(a). The result showed the amino acids concentrations in H2O:CH3CN (4:1 (v/v) > water > borate buffer. Except Gly, Arg, Try, and Val showing relatively low concentration values in the mixture of water and acetonitrile [H2O:CH3CN (4:1 (v/v)], other amino acids obtained with this solvent were higher than those obtained using water or borate buffer solution as preparatory solvent for amino acids. Hence, the use of mixture of water and acetonitrile [H2O:CH3CN (4:1 (v/v)] in further analysis in this study.
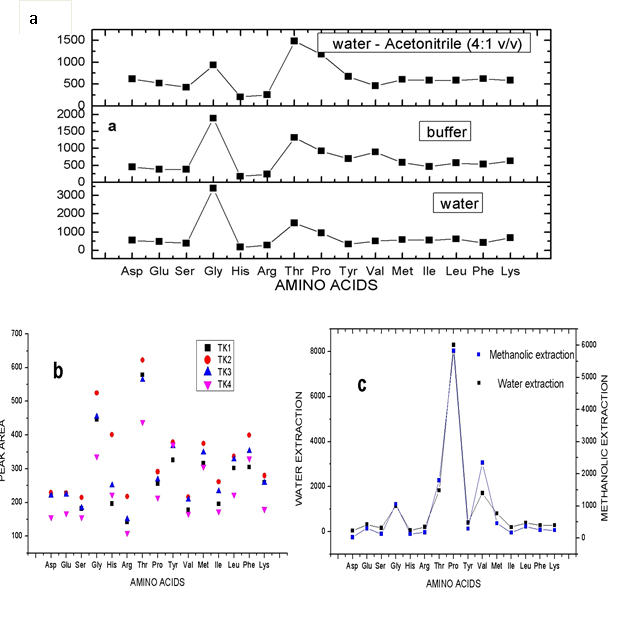
Figure. 2 (a) Screening of different preparatory solvents for amino acids
(b) Elution strength of different solvents on the retained amino acid in the SPE.
(c) Different extraction methods for amino acids.
3.2 Optimization of solid phase extraction (SPE)
3.2.1. Eluent type
In SPE, there are several factors with which retention and elution of an analyte could be altered for extraction recovery, hence the need for SPE optimization. To optimize SPE, two different SPE cartridges (Megabond Elut C18 and SCX) were investigated for the ability to retain amino acids. The SCX SPE cartridge retained more of the analyte than C18. The SCX SPE cartridge affords a higher level of hydrophilicity than C18 cartridge, which enable the amino acids to be more retained. This was then followed by screening solvents of different strength for their ability to produce optimum elution of the retained amino acids from the cartridge. After conditioning columns with 25 mL of MeOH followed by equilibration with 10 mL deionized water, 1 mL 150 pmol/μL amino acid standard was added and allowed to flow through under the action of gravity. The retained analyte was eluted with different solvents namely: 20%, 40%, 60%, acetonitrile, and 10% ethanol labeled as TK1, TK2, TK3, and TK4 respectively and were handled as previously described. The results [Fig 2b] revealed that TK2 and TK3 were both efficient for the elution of the retained free amino acids but TK2 optimally eluted the retained free amino acids as compared to other eluents. In comparison of TK2 and TK3, the recoveries using TK2 were higher than that of TK3.
3.2.2 Study of extraction methods
In order to produce optimum extraction of the free amino acids present in the American cockroach sample, two different extraction methods namely: water extraction at 90 oC and methanolic extraction were studied. Accurately weighed 1.0 g of processed American cockroach samples were separately weighed and extracted differently. Extraction using water involves extracting the sample in 10 mL ultrapure water at 90 oC in water bath for 20 min and allowed to cool. The methanolic extraction involves vortexing 20 mL of mixture of methanol, water and acetic acid (MeOH:H2O:CH3COOH (90:9:1 v/v) with 1.0 g cockroach sample for 20 min. Both were then filtered using superimposed filter membranes (0.45 μm membrane filter for water extract and 0.22 μm nylon membrane filter for methanolic extract). Water extract was diluted with ultrapure water to a total volume of 10 mL while methanolic extract was dried in rotary evaporator and later resuspended in 10 mL ultrapure water. The samples were then handled as earlier discussed in sample preparation and treatment section. Except Gly, Thr, and Val, the result presented in Fig 2(c) showed that extraction using water produced higher extraction efficiency compared to methanolic extraction. Therefore, in this study, extraction using water at 90 oC for 20 min was selected and used for cockroach free amino acids.
3.3. SPE pre-treatment
Sample was severally injected before and also after SPE pre-treatment and the chromatograms presented in Fig 1(b) and (c). The chromatograms revealed that the matrix interferences leading to low quantification of amino acids: Glu, Gly, His, Arg, Ile, Leu, and especially Thr, and Pro was highly minimized after the SPE pre-treatment given higher and accurate quantification of the free amino acids. Thr, and Pro were three times higher in concentrations after application of SPE. For Phe, negative effect of SPE was much more evident as it was finally not detected after the application of SPE. Moreover, Val concentration was slightly reduced while for other free amino acids quantified such as Asp, Ser, Try, Met and Lys, SPE pre-treatment did not show significant or any additional benefit. The recovery study of spiked American cockroach showed a good recovery with SPE pre-treatment (Table 2).
Table 2: Sensitivity, Linear ranges, Repeatability, Reproducibility and Recovery
|
Amino acid (AA) |
Sensitivity (pmol/μL) |
Linearity (pmol/μL) |
RSDa (%) (n=6) |
RSDb (%) (n=6) |
Recovery (%) |
||
|
|
LOD |
LOQ |
|
|
|
SPE |
HPLC-DAD |
|
|
|
|
|
|
|
|
|
|
Asp |
5.2 |
15.9 |
50 – 150 |
5.0 |
6.9 |
98.0 |
93.6 |
|
Glu |
3.4 |
10.4 |
50 – 150 |
4.7 |
6.9 |
100.0 |
98.2 |
|
Ser |
2.6 |
7.9 |
50 – 150 |
5.3 |
7.2 |
96.0 |
96.4 |
|
Gly |
11.7 |
35.5 |
50 – 150 |
5.8 |
7.2 |
100.0 |
99.7 |
|
His |
6.6 |
20.0 |
50 – 150 |
9.2 |
7.9 |
95.2 |
90.0 |
|
Arg |
6.2 |
18.8 |
50 – 150 |
6.5 |
8.7 |
89.1 |
82.0 |
|
Thr |
5.1 |
15.6 |
50 – 150 |
6.6 |
9.7 |
82.9 |
85.0 |
|
Pro |
7.2 |
21.7 |
50 – 150 |
3.1 |
6.8 |
84.8 |
82.5 |
|
Tyr |
2.4 |
7.2 |
50 – 150 |
4.9 |
9.8 |
85.2 |
78.8 |
|
Val |
9.5 |
28.9 |
50 – 150 |
9.2 |
14.2 |
96.5 |
86.9 |
|
Met |
1.4 |
4.2 |
50 – 150 |
5.1 |
14.3 |
89.5 |
83.1 |
|
Ile |
0.9 |
2.6 |
50 – 150 |
10.5 |
51.9 |
99.9 |
95.0 |
|
Leu |
4.1 |
12.5 |
50 – 150 |
6.9 |
8.6 |
94.6 |
93.9 |
|
Phe |
3.2 |
9.7 |
50 – 150 |
8.9 |
41.4 |
ND |
ND |
|
Lys |
2.9 |
8.7 |
50– 150 |
1.0 |
14.3 |
88.4 |
88.2 |
RSDa = Relative standard deviation for samples at six injections in the same day
RSDb = Relative standard deviation for samples at six injections in different same day
ND = Not detected
3.4. Method validation
In the validation of the analytical method for the quantification of free amino acids in American cockroach, the following parameters were determined and results presented in Table 2: recovery (SPE and HPLC-DAD), sensitivity, linearity, repeatability and reproducibility.
3.4.1. Recovery Test
Two different recoveries were carried out. Firstly, SPE recoveries using the best elution solvents (TK2) from the above studied. The extraction recoveries in percentages were calculated as the ratio of the peak area in the chromatogram of the eluted amino acid fraction to those in the chromatogram of amino acid standard solution as shown in Table 2. The second recoveries involve validating the entire analytical procedures used for the analysis. This was done by conducting a spike recovery test on the samples with different concentrations of amino acid standards. In summary, portions of powdered American cockroach sample equivalent to 1.0 g were spiked with different concentrations of amino acid standards ranging from 25 and 150 pmol/μL. For each concentration used, triplicate spiking was prepared, and the total samples analyzed was twelve. These samples were handled as explained in the sample preparation section followed by HPLC-DAD analysis. The recoveries of the analyte were carried out in triplicate while injections were done six times for each spiked sample. The concentrations were calculated using regression equation from the calibration curves for each free amino acid. The recoveries of free amino acids from American cockroach were then evaluated as follows [(amount found − mean value)/amount added] × 100% and the results presented in Table 2.
3.4.2 Performance characteristics of the method
The limit of detection (LOD) and the limit of quantification (LOQ) were determined from the calibration curves of the amino acid standards. The LOD and LOQ were calculated as three and ten times the ratio of standard deviation (SD) of the response to slope (S) of the calibration curve respectively. The HPLC-DAD method was tested for linearity (Table 2) of the DAD-detector response by analyzing the working standard solutions of amino acids at different concentrations. Based on the replicate analysis of standards over a range of 1–150 pmol/μL, the column and detector performance showed good retention and selectivity of the analyte in the concentration range 50–150 pmol/μL. Calibration curves were then plotted using the integrated peak areas versus concentrations (pmol/μL) of the amino acid derivatives. For method repeatability, amino acid working standard solutions at different prepared concentrations (50, 75, and 100 pmol/μL) were repeatedly injected six times on the same day while for reproducibility, freshly prepared solutions at aforementioned concentration levels were analyzed at different days (24 h interval) and results were statistically evaluated and presented in terms of % RSD (Table 2).
3.5. Identification and quantification of free amino acids in American cockroach
The optimized and validated method developed was applied for the determination of free amino acids in American cockroach. The amount of each amino acid as presented in Table 3 was quantitatively estimated using the established calibration curve. The chromatograms obtained before SPE and after SPE application followed by HPLC–DAD determination were shown in Fig 1 (b) and (c). Pro, Thr, Leu, Glu, Ile, and Lys were the major free amino acids found in abundance followed by Met, Val, Asp, Ser, Tyr, Gly, and Arg that were relatively abundant, His was the least in terms of concentration. Before SPE was used to purify the sample, Phe was detected in the American cockroach extract but later not detected after the application of SPE. Seasonal changes have a profound effect on the free amino acid concentrations of several insects. Free amino acid concentrations usually increase during cold weather in response to cold-acclimation especially Pro [32] which have been reported to serves as reserve energy source and also flight muscle fuel in a number of insects [33]. This was in agreement with the period of sample collection. Others amino acids that increase in concentrations during cold weather are Val, Ile, Glu and Thr [33]. On the other hand, His showed a reciprocal trend [34]. During winter, the extremely high concentration of Pro could be linked to Pro biosynthesis from accumulated Glu, Arg, and His leading to low concentrations of Glu, Arg, and His [34]. Pro and Thr concentrations were three times higher after the application of SPE as shown in Fig 1 (b) and (c). The result of this study showed that the concentration of Pro exceeded those of all other free amino acids in American cockroach. The levels were 5– 100 folds higher than other free amino acids. Pro alone represented 50.64% of the total free amino acid concentrations in American cockroach. The recoveries of the free amino acids from American cockroach ranged from Thr 82.9% – Glu and Gly 100%, and RSDs ranged Lys (1.0 %) – Ile (10.5 %). The method is sensitive enough to accurately evaluate the free amino acids in American cockroach. The total free amino acids obtained from accurately measured 1.0g powdered samples of American cockroach prepared was 614.52 mg/g (Table 3), representing 61.5% of the total nutrient present in American cockroach. The free amino acids contents of cockroach species (Blattedea) ranged from 43.90 to 65.60% depending on the stage of development (larvae, pupae and adults), feed given, environment, and sex [2]. The main components of edible insects are proteins [2], hence the results of this study agreed with the statement. Comparing the amino acid requirements for adults published by WHO [35] in mg/g proteins, it is evident that American cockroach meets the many essential amino acid requirements for adults while still possessing larger amounts of non-essential amino acids.
Table 3: Concentration of free amino acids in American cockroach using developed method
| Free amino acids | Concentration (mg/g) (n = 6) | ||||
| Mean ± SD | Maximum | Minimum | |||
|
Asp |
5.5 ± 0.08 |
5.73 |
5.20 |
||
|
Glu |
52.0 ± 0.69 |
55.96 |
49.55 |
||
|
Ser |
4.64 ± 0.10 |
5.42 |
4.49 |
||
|
Gly |
4.10 ± 0.15 |
4.44 |
3.83 |
||
|
His |
0.43 ± 0.06 |
0.57 |
0.39 |
||
|
Arg |
3.40 ± 0.07 |
3.68 |
3.39 |
||
|
Thr |
65.92 ± 1.01 |
70.71 |
63.03 |
||
|
Pro |
311.2 ± 0.55 |
330.75 |
291.52 |
||
|
Tyr |
4.48 ± 0.12 |
4.57 |
4.36 |
||
|
Val |
9.95 ± 0.26 |
11.39 |
8.98 |
||
|
Met |
11.26 ± 0.44 |
12.88 |
10.06 |
||
|
Ile |
45.21 ± 1.00 |
48.46 |
42.84 |
||
|
Leu |
58.14 ± 1.8 |
61.25 |
56.21 |
||
|
Phe |
ND |
ND |
ND |
||
|
Lys |
38.29 ± 0.60 |
40.91 |
36.98 |
||
|
Total |
614.52 |
|
|
||
ND = Not detected
An efficient and sensitive HPLC-DAD method has been developed that can accurately identify and quantitate 15 free amino acids. The method selectivity and recovery were satisfactory; an indication that matrix interferences from other constituents present in extracts of American cockroach were minimal. The effect of seasonal changes revealed high concentration of some free amino acids in American cockroach during cold weather. This provides guidance for future industrial production of amino acids especially those at higher concentration.
This work was supported by Zhejiang Provincial Natural
Chakravorty J, Ghosh S, Meyer- Rochow VB (2013) Comparative survey of entomophagy and entomotherapeutic practices in six tribes of eastern Arunachal Pradesh (India). Journal of Ethnobiology and Ethnomedcine, 9:50 PMid:23866996 PMCid:PMC3750809
View Article PubMed/NCBILadron de Guevara, O., P. Padilla, L. Garcia, J. M. M. Pino, and J. Ramos-Elorduy (1995) Amino acid determination in some edible Mexican insects. Amino Acids, 9(2):161–173. PMid:24178816
PubMed/NCBISingh OT, Chakravorty J, Nabom S, Kato D (2007) Species diversity and occurrence of edible insects with special reference to coleopterans of Arunachal Pradesh. J NatCon, 19:159–166.
Durst PB, Shono K. (2008) Edible forest insects: exploring new horizons and traditional practices In: Durst PB, Johnson DV, Leslie RN, Shono K, Editors. In: Edible Forest Insects: Humans Bite Back. pp.1–4. Food and Agricultural Organization of the United Nations Regional Office for Asia and the Pacific.
Chakravorty J, Ghosh S, Meyer-Rochow VB (2011) Practices of entomophagy and entomotherapy by members of the Nyishi and Galo tribes, two ethnic groups of the state of Arunachal Pradesh (North-East India). Journal of Ethnobiology and Ethnomedcine, 7:5. PMid:21235790 PMCid:PMC3031207
View Article PubMed/NCBIC. Azagoh, F. Ducept, R. Garcia, L. Rakotozafy, M.-E Cuvelier, S. Keller, R. Lewandowski and S. Mezdour (2016) Extraction and physicochemical characterization of Tenebrio molitor proteins, Food Research International, 88: 24–31. PMid:28847399
View Article PubMed/NCBIRumpold, BA & Schlüter, OK (2013) Nutritional composition and safety aspects of edible insects. Molecular Nutrition and Food Research, 57, 802–823. PMid:23471778
View Article PubMed/NCBIHuis AV, Itterbeeck JV, Klunder H, Mertens E, Halloran A, Muir G, Vantomme P (2013) Edible insects: future prospects for food and feed security. FAO Forestry Paper no 171, Rome, 187 pp. ISBN 978-92-5-107595-1 [print], E-ISBN 978-92-5-107596-8 [PDF]
Harinder P.S. Makkar, Gilles Tran, Valérie Heuzé, Sylvie Giger-Reverdin, Michel Lessire, François Lebas and Philippe Ankers (2016) Seaweeds for livestock diets: A review, Animal Feed Science and Technology, 212, 1 – 17.
View ArticleJiang Ling-yun, Liu Xi, Xia Cong-Long, Chen Ke-xin, He Shun-Zhen, Liu Guang-Ming (2012) Research advance on chemical constituents and anti-tumor effects of Periplaneta americana L. Medicinal plant, 3(11): 95 –97, 102.
Rumki H. Ch. Sangma, Ram, Pal, D. R. Singh (2016) Edible insects in Northeast India. Bioprosoecting of indigenous Bioresources of North-East India. 253–267.
Hoang Chinh Nguyen, Shih-Hsiang Liang, Shang-Sian Chen, Chia-Hung Su, Jhih-Huei Lin and Chien-Chung Chien (2018) Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology, Energy Conversion and Management, 158, 168 – 175.
View ArticleJulieta Ramos-Elorduy Blásquez, José Manuel Pino Moreno, Víctor Hugo Martínez Camacho (2012) Could grasshoppers be a nutritive meal? Food and Nutrition Sciences, 3, 164-175.
View ArticleReinhard Predelac, Roland Kellnerb, Juergen Rapusc, Heinz Penzlinc, Gerd Gadean (1997) Isolation and structural elucidation of eight kinins from the retrocerebral complex of the American cockroach, Periplaneta americana. Regulatory Peptides, 71 (3):199-205. 01029-X
View ArticleSandra G.F. Bukkens (2010) The nutritional value of edible insects. 287 – 319 pp.
Sarah McCusker, Preston R. Buff, Zengshou Yu, and Andrea J. Fascetti (2014) Amino acid content of selected plant, algae and insect species: a search for alternative protein sources for use in pet foods. Journal of Nutritional Science, 3: e39, 1–5.
Sheng L, Shiming Z, Qiangqiang J, Dongwei Y, Chonghua R, Kang L, Suning L, Yingying C, Haigang Z, Yanghui C, Gangqi F, Daqi L, Xiaoming Z, Jianzhen Z, Qiaoyun Y, Yongliang F, Xiaoqiang Y, Qili F, & Shuai Z (2018) The genomic and functional landscapes of developmental plasticity in the American cockroach. Nature Communications, 9:1008. PMid:29559629 PMCid:PMC5861062
View Article PubMed/NCBIF.S Agbidye, T.I. Ofuya and S.O Akindele (2009) Marketability and Nutritional Qualities of Some Edible Forest Insects in Benue State, Nigeria. Pakistan Journal of Nutrition, 8: 917-922.
View ArticleLeon L. Pierre (1962) Synthesis of ascorbic acid by the normal fat body of the cockroach, Leucophaea madarae (F.), and by its symbionts. Nature, 193: 904 – 905.
View ArticleOguri E, Steele JE (2003) A novel function of cockroach (Periplaneta americana) hypertrehalosemic hormone: translocation of lipid from hemolymph to fat body. General and Comparative Endocrinology, 132 (1): 46–54. 00029-7
View ArticleKarl J. Kramer, Allyson M. Christensen, Thomas D. Morgan, Jacob Schaefer, Thomas H. Czapla and Theodore L. Hopkins (1991) Analysis of cockroach oothecae and exuviae by solid-state 13C-NMR spectroscopy, Insect Biochemistry, 21, 2, (149).
Wang L, Xu RJ, Hu B, Li W, Sun Y, Tu YY, Zeng XX. (2010) Analysis of free amino acids in Chinese teas and flower of tea plant by high performance liquid chromatography combined with solid-phase extraction. Food Chemistry, 123, 1259-1266.
View ArticleGonzalez-Castro, M.J., M.J Lopez-Hernandez, J. Simial-Lozano, and M.J. Oruna-Concha (1997) Determination of amino acid in green beans by derivatization with phenylisothiocianate and high performance liquid chromatography with ultraviolet detection, Journal of Chromatographic Science, 35: 181–185.
View ArticleBednarov M, Borkovcov M, Komprda T. (2014) Purine derivate content and amino acid profile in larval stages of three edible insects. Journal of the Science of Food and Agriculture, 94(1): 71–76. PMid:23633284
View Article PubMed/NCBIZhihong Shi, Hui Li, Zhimin Li, Junda Hu, Hongyi Zhang (2013) Pre-column derivatization RP-HPLC Determination of Amino Acids in Asparagi Radix before and after Heating Process. IERI Procedia (5) 351–356.
Zhaolai Dai, Zhenlong Wu, Sichao Jia and Guoyao Wu (2014) Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection, Journal of Chromatography B, 964, (116).
View ArticleLopez-Cervantes J, Sánchez-Machado DI, Rosas-Rodríguez JA (2006) Analysis of free amino acids in fermented shrimp waste by high-performance liquid chromatography. Journal of Chromatography A, 1105(1–2) 106–110. PMid:16439254
View Article PubMed/NCBIGuoqiang Chen, Yun Wang, Weiqi Song, Bo Zhao and Yuling Dou (2012) Rapid and selective quantification of l-theanine in ready-to-drink teas from Chinese market using SPE and UPLC-UV, Food Chemistry, 135, 2, (402).
Chen L, Chen Q, Zhang Z, Wan X (2009) A novel colorimetric determination of free amino acids content in tea infusions with 2,4-dinitrofluorobenzene. Journal of Food Composition and Analysis, 22(2):137–141.
View ArticleZhang, XL, Zhao T, Cheng T, Liu XY, Zhang HX (2012) Rapid resolution liquid chromatography (RRLC) analysis of amino acids using pre-column derivatization, Journal of Chromatography B, 906: 91– 95. PMid:22981597
View Article PubMed/NCBIPalace GP, Fitzpatrick R, Tran KV, Phoebe HC, Norton K (1999) Determination of amino acids in diverse polymeric matrices using HPLC, with emphasis on agars and agaroses. Biochimica et Biophysica Acta (BBA) - General Subjects, 1472, 3,(509).
D. Renault, A. Bouchereau, Y.R. Delettre, F. Hervant and P. Vernon (2006) Changes in free amino acids in Alphitobius diaperinus (Coleoptera: Tenebrionidae) during thermal and food stress, Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 143, 3,(279).
Vladimir Koštál, David Renault and Jan Rozsypal (2011) Seasonal changes of free amino acids and thermal hysteresis in overwintering heteropteran insect, Pyrrhocoris apterus, Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 160, 2, (245).
Lalouette L, Kostal V, Colinet H, Gagneul D, Renault D (2007) Cold exposure and associated metabolic changes in adult tropical beetles exposed to fluctuating thermal regimes. FEBS Journal 274: 759–1767. PMid:17331186
View Article PubMed/NCBIProtein and amino acid requirements in human nutrition (2007) Report of a joint FAO/WHO/UNU expert consultation (WHO Technical Report Series 935).
