Jean-Louis Marty
Email: jlmarty@univ-perp.fr; Tel.: +33 6 16 814591
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 5 ISSUE: 2
Page No: 49-65
Jean-Louis Marty
Email: jlmarty@univ-perp.fr; Tel.: +33 6 16 814591
Magda Márcia Beckera,b, Gaëlle Catanantea, Ines Marmolc, Maria Jesus Rodríguez-Yoldic, Rupesh K. Mishrad, Sergio Barbosae, Oscar Nuneze,f,g, Gilvanda Silva Nunesb and Jean-Louis Martya*
a BAE-LBBM, University of Perpignan Via Domitia, 52 avenue Paul Alduy, 66860 Perpignan Cedex, France.
b Program in Biodiversity and Biotechnology, Bionorte, Federal University of Maranhão (UFMA), Portugueses Avenue, 1966, Bacanga, 65080-805, São Luís, Maranhão, Brazil.
c University of Zaragoza, Dept. of Pharmacology and Physiology, Veterinary Faculty, C/ Miguel Servet 177, 50013 Zaragoza, CIBERobn (ISCIII), IIS Aragón, IA2, Zaragoza, Spain.
d Amity Institute of Biotechnology, Amity University Rajasthan, Jaipur 303002, India
e University of Barcelona, Department of Chemical Engineering and Analytical Chemistry, Martí i Franques̀ 1-11, E 08028, Barcelona, Spain.
f Research Institute in Food Nutrition and Food Safety, University of Barcelona. Av. Prat de la Riba 171, Edifici Recerca (Gaudí), E-08901 Santa Coloma de Gramanet, Barcelona, Spain.
g Serra Hunter Program, Generalitat de Catalunya, Barcelona, Spain
MARTY Jean Louis, Phenolic Composition, Antioxidant Capacity and Antiproliferative Activity of Ten Exotic Amazonian fruit(2020)Journal of Food Science & Technology 5(2)
The socioeconomic, cultural, therapeutic, and nutritional potential of Amazonian fruits has been limited due to the scarce information about its phytochemical profile. The aim of this study was to determine the total phenolic content profiles by Folin colorimetric miniaturized assay, the identification of phenolic compounds by UHPLC-HRMS, the antioxidant capacity by DPPH, ABTS, and NBT assays, as well as, the antiproliferative activity by sulforhodamine B assay of extracts obtained from ten native fruits to the Amazon region, some of these consumed especially by indigenous population. A strong positive correlation between the content of total phenolic compounds and antioxidant capacity was demonstrated. The antiproliferative activities against Caco-2 cell line didn‘t necessarily be associated with a high antioxidant capacity and the total phenolic concentration, possibly, qualitative and quantitative differences in phenolic composition of these fruits influenced the antiproliferative activities. This research presented, for the first time, important characteristics of Amazon exotic fruits.
Keywords: Amazonian fruits, antioxidant capacity; antiproliferative activity; phenolic profiles
Amazon region is recognized for harboring the greatest biodiversity on the planet [1]. In this planet’s richest ecosystem, the fruit trees have gained attention due to the hundreds native edible fruit, the relevance of wild fruit due the highest nutritional content, the strong appeal through the exotic flavor, color, and texture, but, above all, the status of a functional food due to this bioactive composition and its possible therapeutic effect [2].
The strong bioactive activity in fruit is related with the substantial amounts on antioxidants compounds, especially the phenolic compounds that attribute them beneficial effects on human health through the reduction of the oxidative stress by the antiradical activities [3-5].
Epidemiological studies have repeatedly shown a strong relationship between the consumption of antioxidants compounds rich fruit and the protection against several chronic human diseases, as well as their beneficial bioactive activities related such as pro-apoptotic, antiaging, anticarcinogenic, overall inhibiting the cell proliferation processes [6-8].
The phenolic groups in polyphenols can accept an electron to form relatively stable phenoxyl radicals, thereby disrupting chain oxidation reactions in cellular components [9]. The presence of reducing polyphenols and their metabolites in plasma may increase plasma antioxidant capacity by their effects upon concentrations of other reducing agents (sparing effects of polyphenols on other endogenous antioxidants), or by their effect on the absorption of pro-oxidative food components, such as iron [9,10].
An improved information of the chemical composition and biological properties of sub explored edible fruit could help to define and valorize those with a greater nutraceutical potential to suggest to consumers.
The aim of this study was to evaluate the phenolic compounds profiles, the antioxidant capacity, and the antiproliferative activity of extracts obtained from ten native fruits to the Amazon region, some of these consumed especially by the indigenous population.
2.1. Reagents and equipment
Ascorbic acid, gallic acid, Folin-Ciocalteu, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), Nitrotetrazolium Blue chloride (NBT), hypoxanthine (HX), K2S2O, xanthine oxidase (XOD) from bovine milk, were purchased from Sigma-Aldrich Corp (Darmstadt, Germany).
Fifty-three polyphenolic standards belonging to different families (phenolic acids, benzoic acids, cinnamic acids, phenolic aldehydes, phenolic terpenes, flavones, flavanols, proanthocyanidins, and stilbenes) were employed, all purchased from Sigma (Steinhein, Germany). LC-MS grade water, methanol, acetonitrile, formic acid (98-100%), and acetone were also purchased from Sigma-Aldrich, and hydrochloric acid (98%) was from Merck (Seelze, Germany). Stock standard solutions of all polyphenols (∼1000 mg/L) were prepared in LC-MS-grade methanol in amber-glass vials.
Deionized water (Milli-Q Millipore 18.2MΩ cm-1) was used in all experiments.
Spectrophotometric analyses were carried out in a Multiskan Ex Primary using an Ascent Software Version 2.6. Chromatographic analysis were carried out on an Accela UHPLC system (Thermo Fisher Scientific, San José, CA, USA), coupled to a Q-Exactive Orbitrap HRMS system (Thermo Fisher Scientific) plus a heated electrospray ionization source (HESI-II) operated in negative ionization mode, according to the method proposed by Barbosa at al (2018). A C18 column (150 × 2.1 mm, 2.7 μm partially porous particle size) provided by Supelco (Bellefonte, PA, USA) was used for the compounds separation.
2.2. Sample Collection
The native fruits at the complete physiological maturity stage were collected in the following Brazilian states: Amazonas, Maranhão, and Roraima (Figure 1). The common mane, Latin name, family name and informations about the edible portions are presented in the Table 1. The traditionally fruit edible portions were lyophilized (Liotop, L101) and stored in the dark prior to extraction procedure.
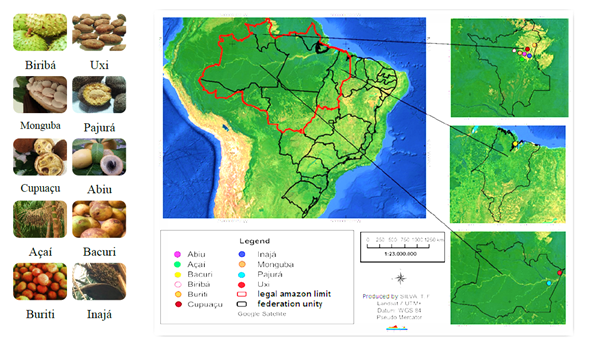
Figure 1. Map with the geographical location of the collection sites and the 10 fruit analyzed. Source: elaborated by Tatiane Silva and organized by author.
Table 1 . Agronomic information, classification and the portion analysed of the Amazonian fruits studied.
|
Common name |
Scientific name |
Family |
Portion analysed |
||
|
Abiu |
Pouteria caimito |
Sapotaceae |
pulp |
|
|
|
Açaí |
Euterpe oleracea |
Arecaceae |
pulp |
|
|
|
Bacuri |
Platonia insignis |
Clusiaceae |
pulp |
|
|
|
Biribá |
Rollinia orthopetala |
Annonaceae |
pulp |
|
|
|
Buriti |
Mauritia flexuosa |
Arecaceae |
pulp |
|
|
|
Cupuaçu |
Theobroma grandiflorum |
Sterculiaceae |
pulp |
|
|
|
Inajá |
Maximiliana maripa |
Arecaceae |
pulp |
|
|
|
Monguba |
Pachira aquática |
Malvaceae |
seed |
|
|
|
Pajurá |
Couepia bracteosa |
Chrysobalanaceae |
pulp |
|
|
|
Uxi |
Saccoglotis uxi |
Humiriaceae |
pulp |
|
|
2.3. Antioxidant Potential
Shaking and ultrasound-assisted extraction procedures were previously tested in order to obtain higher inhibitions by DPPH assay, as well as the influence of time, temperature, amount of lyophilized samples and extraction solvent was evaluated. Thus, the following optimized extraction procedure was employed: 100 mg/mL of the lyophilized samples were shaked for 1 h in a sample mixer (HulaMixer, Invitrogen Dynal AS, Life Technologies) at ~4°C and protected from light. The supernatant was collected after centrifugation for 10 min at 5000 rpm (Hettich, Rotina 380 R).
All lyophilized samples were tested using DPPH, ABTS, NBT, and Folin miniaturized assays to calculate the 50% signal inhibition concentration (IC50 in mg/mL that means the concentrations of samples required to scavenge 50% of free radicals) and expressed in ascorbic acid equivalents.
All the assays were performed in triplicate in a microplate reader. DPPH, ABTS, NBT methods were performed according to Becker et al. [66], using the same solvents.
2.4. Cytotoxicity on Caco-2 cell line
Human colorectal adenocarcinoma Caco-2 cell line (TC7 clone) was kindly provided by Dr. Edith Brot-Laroche (Université Pierre et Marie Curie-Paris 6, UMR S 872, Les Cordeliers, France). Cells were maintained in a humidified atmosphere of 5% CO2 at 37ºC. Cells (passages 20-50) were grown in Dulbecco’s Modified Eagles medium (DMEM) (Gibco Invitrogen, Paisley, UK) supplemented with 20% fetal bovine serum, 1% non-essential amino acids, 1% penicillin (1000 U/mL), 1% streptomycin (1000 μg/mL) and 1% amphotericin (250 μg/mL). Cell medium was replaced every three days and cells were passaged once a week with 0.25% trypsin-1 mM EDTA. Cell confluence (80%) was confirmed by microscopic observance. Assays were performed 24h post-seeding to avoid cell differentiation.
Caco-2 cells were seeded in 96-well plates at 4x103 cells/well density and incubated overnight under above mentioned conditions. For treatment, lyophilized fruits were diluted in cell culture medium without fetal bovine serum to the required concentrations: 62.5, 125, 250, 500 and 1000 μg/mL. Cell culture medium was then replaced with fresh medium containing lyophilized fruits and cells were incubated during 72h. Mock-treated cells were used as negative control. Thereafter, cell proliferation was measured using the sulforhodamine B assay as described by Jiménez et al. [28]. Absorbance was measured with a scanning multiwell spectrophotometer (Biotex Sinergy ht Siafrtd, Vermont, USA) at 540/620 nm. Experiments were conducted in quadruplicate wells and repeated at least three times. The effect on cell proliferation was expressed as percentage of the control and calculated as % control and IC50 value.
2.5. Phenolic Composition Profile
2.5.1. Folin-Ciocalteu assay
Total phenolic compounds content was determined using Folin-Ciocalteu colorimetric method with some modifications [67] in three different extracts: pH=7.4 PBS, pure ethanol, and 1:1 water/ethanol mixture, according to 2.3 item. The determination of phenol content was carried out mixing 125 μL 10 %(w/v) Folin-Ciocalteu solution (FCS), 25 μL of the fruit extract, and 100 μL 7.8 %(w/v) Na2CO3. The blank was determined in absence of the fruit extract and adding 25 μL of the solvent. The absorbance increasing, measured after 1 h at darkness, 400 rpm and 37°C was registered at λ = 750 nm. A calibration curve of gallic acid in a range from 1 to 15 µg/mL was constructed following the AOX reagents sequence addition, obtaining at 15 µg/mL an absorbance around 1.0 ± 0.1 unit at 750 nm. Absorbance measurements were performed in triplicate in a microplate reader, and the results were expressed as mg of gallic acid equivalents (GAE)/100 g of dry samples.
2.5.2. Polyphenols and phenolic acids chromatographic analysis
0.1 g of sample was extracted by sonication during 20 minutes at room temperature using 10 mL of acetone:water:hydrochloric acid (70:29.9:0.1 v/v/v) solution, followed by centrifugation (3500 rpm, 15 min). The supernatant was then collected, filtered (0.45 μm nylon filters, Whatman, Clifton, NJ, USA) and kept at -4°C until analysis.
In order to obtain the separation and identification of a greater variety of phenolic compounds, two methods of elution were employed, denominated Method 1 and 2, the mobile phase of Method 1 being the mixture of a 0.1% aqueous solution of CH2O2 (solvent A) and the mobile phase of Method 2 is a solution of C2H3N also containing 0.1% CH2O2 (solvent B). Elution of the compounds was carried out according to the following polarity gradient: 0-1 min, 10% isocratic conditions of A; 1 to 20 min, linear gradient from 10% to 95% A; 20 to 23 min, isocratic step to 95% A; 23 to 24 min back to the initial conditions at 10% A; and from 24 to 30 min, 10% isocratic conditions of A to rebalance the column. The same elution procedure was used for solvent B (Method 2). The mobile phase flow rate was 300 μL/min; injection volume (full loop mode) of 10 μL; nitrogen was used as a sheath gas, sweep gas, and auxiliary gas at flow rates of 60, 0, and 10 au (arbitrary units), respectively. HESI-II heater temperature at 350 °C and capillary voltage at - 2.5 kV were applied. Instrument capillary temperature was set at 320 °C, and an SLens RF level of 50 V was used. The Q-Exactive Orbitrap HRMS system was tuned and calibrated using commercially available Thermo Fisher calibration solution every 3 days. The HRMS instrument was operated in full MS scan mode with a m/z range from 100 to 1500 at a mass resolution of 70 000 full width at half-maximum (FWHM) at m/z 200, with an automatic gain control (AGC) target (the number of ions to fill the C-Trap) of 1.0E6 with a maximum injection time (IT) of 200 ms. Full MS scan mode was followed by a data-dependent scan operated product ion scan mode and applying for the fragmentation stepped normalized collision energies (NCE) of 17.5, 35, and 52.5 eV. Product ion spectra with an isolation window of 0.5 m/z and a fixed first mass of m/z 50 were registered. At this stage, a mass resolution of 17 500 FWHM at m/z 200, with an AGC target at 2.0e5, and a maximum IT of 200 ms were employed. Data-dependent scan was triggered with an intensity threshold of 1.0E5.
2.6. Statistical Analysis
The Origin Pro 8.6 software was used to perform the analysis of variance (ANOVA) in order to compare results, being accepted significance at ? < 0.05. The data were previously self-scaled, before being submitted to Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) because of the variety response, that is, they differ in the order of magnitude, thus assigning the same weight to all variables. The Euclidean distance was used to obtain the HCA dendrogram. The chemical analyzes were performed in triplicate, for the construction of the 9x30 data matrix (nine variables and ten samples, in triplicate).
3.1. Antioxidant Potential
The different methods in the literature for the antioxidant capacity determination of biological systems involve different radicals/oxidant sources, consequently more than one chemical mechanism [11]. Additionally, the antioxidants chemical diversity allows the different behavior to eliminate the unpaired condition of the free radical or to chelate metals. In view of this, no single assay accurately reflects the mechanism of action of all radical sources or all antioxidants in a complex system [12], consequently, more than one antioxidant capacity method must be used for comparing the mode of action of crude or pure compounds [11,13].
In this work, DPPH, ABTS, and NBT methods were chosen based upon differing reaction mechanisms, with one utilizing the single electron transfer (SET), and the other the ability to hydrogen atom transfer (HAT) and/or SET mechanism utilizing the DPPH• and ABTS•+ synthetic radicals [12]. Ethanol and/or aqueous buffer were adopted in the samples antioxidants extractions after considering safety in handling, the human consumption in some food products such as beverages, wine, and liquors, as well as, the reference as a good solvent for antioxidant extraction [14].
Overall, the samples exhibited relevant antioxidant qualities and small amount of the extracts were enough to inhibit at 50% of the activity of its respectively free radicals (Table 2).
Table 2. The Antioxidant capacity by IC50 (mg/mL) of the different Amazonian fruits extracts determined by the miniaturized spectrophotometric assays and the cytotoxicity on Caco-2 cell line by IC50 (µg/mL). Vitamin C (Vit. C) has been added as a positive control. Cisplatin has been added as positive control in anti-proliferative assays.
|
Samples |
DPPH |
ABTS |
NBT |
Cytotoxicity |
||
|
EtOH |
EtOH |
PBS |
PBS:EtOH |
K-PBS |
||
|
Abiu |
9.77±0.61a,b |
2.26±0.05a |
0.31±0.01a |
0.16±0.01a,b |
0.43±0.07a |
> 1000 |
|
Açaí |
9.77±0.06a,b |
0.81±0.03b |
0.89±0.01b |
0.60±0.01 |
0.50±0.01a |
272±32b |
|
Bacuri |
6.97±0.25a |
0.36±0.06c |
0.64±0.01 |
0.40±0.01c |
6.77±0.33b |
393±41d |
|
Biribá |
5.36±0.07a |
0.58±0.01b,c |
1.71±0.12c |
0.51±0.02 |
7.62±0.20b |
233±20b,c |
|
Buriti |
79.74±6.90 |
13.37±0.22 |
1.49±0.02 |
0.90±0.01 |
7.34±0.19b |
349±34a,d |
|
Cupuaçu |
27.59±0.10c |
9.15±0.17 |
0.82±0.01b |
0.77±0.03d |
5.31±0.29 |
>1000 |
|
Inajá |
13.54±0.66a,b |
2.45±0.11a |
0.40±0.03a |
0.38±0.03c |
0.53±0.02a |
230±14c |
|
Monguba |
20.70±0.63c |
4.96±0.10 |
0.35±0.01a |
0.11±0.01a |
0.35±0.01a |
213±49 |
|
Pajurá |
10.58±0.41a,b |
1.97±0.04 |
1.65±0.03c |
0.17±0.01b |
6.67±0.26b |
< 125 |
|
Uxi |
106.14±12.44 |
3.65±0.01 |
1.15±0.12 |
0.82±0.03d |
16.07±1.23 |
353±31a,d |
|
Vit. C |
62.26±2.13 |
-- |
-- |
-- |
|
250±14 |
|
Cisplatin |
|
|
|
|
|
11.10±1.53 |
Means followed by the same letter in the columns do not differ significantly from each other by the Tukey test at the 5% probability level
A lower IC50 value which corresponds to a higher antioxidant power was obtained in the biribá, and bacuri ethanolic extracts against the DPPH• (<6.97 mg/mL) and ABTS•+ (<0.58 mg/mL) radicals, followed by açaí, pajurá, abiu, and inajá. Therefore, it can be deduced that the hydrophobic antioxidant content is higher compared to hydrophilic antioxidants in such extracts. DPPH IC50 values were higher when compared with the ABTS, even with the same solvent extraction, proving the need for higher amounts of samples to inhibit this radical. This occurs due to the DPPH higher stability and lower sensitivity to ABTS [10, 11], the presence of compounds which containing ABTS•+ radical scavenging capacity, not showing it against [15], difference in incubation time to reach a steady state, ratio of sample quantity to radical concentrations, among others [16], may have contributed to these results.
Abiu, açaí, inajá and monguba aqueous buffer extracts exhibited higher antioxidant capacity against O2•– radicals (IC50 not exceeding 0.53 mg/mL, including açaí sample), and ABTS•+ radicals (IC50 <0.40 mg/mL), probably due to the higher hydrophilic antioxidant amount. In the biological point of view, NBT assay represents a more concise tool allowing investigators to assess antioxidant capacity against a specific, biologically relevant free radical. O2•– as an oxygen-derived species that are potentially cytotoxic and causes damage to DNA. Such chemical species have been usually related to a number of disorders such as Parkinson’s disease and cancer [17-20]. Thus, abiu, açaí, inajá, and monguba can be considered fruits with great pharmaceutical and industrial potential.
Abiu, monguba, and pajurá binary extracts showed the higher antioxidant capacity (IC50 <0.17 mg/mL) among all extracts and assays. In fact, the binary mixture solvent (1:1) showed to be the better antioxidants extractor in comparison to an unique solvent (buffer or pure ethanol), perhaps due to the polarity of the compounds [15,21].
The reference literature reports some relatively recent investigations on Amazonian fruits [22-24]. A comparison between the results showed here and these ones is complex because it involves fruits/vegetables obtained under different conditions (climate, soil, and moisture), and taxonomies variety (species, varieties, and cultivar). Moreover, laboratory procedures such as sample preparation, extraction, wavelength, standard antioxidants solutions and mode for calculation of the antioxidant capacity, have been quietly different during analysis.
Neves et al. [22] evaluated the antioxidant capacity by DPPH method of eight lyophilized species of Amazonian fruits, among them açaí, inajá, and uxi. The authors used methanol as extraction solvent and trolox as standard antioxidant. In despite the difference between the reaction time (25 min) and wavelength applied (517 nm), previous tests performed in this work showed no statistical difference (p < 0.05) between the results obtained with 20 min, this was confirmed by the end-point (20 and 30 min) most frequently used during DPPH reaction [25]. Also, previous tests performed here at 490 and 560 nm resulted in no statistical difference (p < 0.05) between the results, confirming the range of the absorption effective band of DPPH ethanolic solution, corroborating thus with Marinova et al. [25].
Canuto et al. [23] determined in fifteen wet samples of fruit pulps from Amazonia the physical and chemical characteristics, levels of bioactive compounds (ascorbic acid, total phenolics), and the antioxidant capacity by ABTS method after ultrasound system extraction. Different solvents were used during the procedure making difficult to measure the real antioxidant capacity, as well as, to make comparisons. The authors expressed the results in µmol/L trolox equivalents, and an increasing antioxidant capacity sequence could be stablished: açaí>buriti>abiu=bacuri=cupuaçu.
In general, the series of the in vitro tests of all Amazonian fruits exhibited significant antioxidant capacity by acting as donators of proton or electron in the DPPH, ABTS assays and possessed O2•– scavenging properties. This study yielded new data on the antioxidant activity of native fruits in the Amazon region.
3.2 Antiproliferative effect on Caco-2 cells
Given the current trend to obtain cytotoxic compounds derived from natural products with therapeutic purposes, along with the positive correlation between the consumption of fruits and vegetables and a decreased risk of colorectal cancer [26], the antiproliferative effect of Amazonian fruit fractions were evaluated on human colorectal adenocarcinoma Caco-2 cell line. Cells were incubated with an increaseing range of concentrations (0-1000 µg/mL) of each fruit extract for 72h and the IC50 value (Table 1) was obtained with the well-established sulforhodamine B assay.
Biribá, inajá, monguba, and pajurá extracts presented the lowest IC50 values, showing thus a great ability to induce a decrease in cell proliferation of Caco-2 cells. On the other hand, abiu and cupuaçu extracts did not cause a significant reduction on cell viability, even at the highest concentration tested (IC50>1000 µg/mL). Finally, bacuri, buriti and uxi displayed moderate antiproliferative activity when compared to the rest of the Amazonian fruits previously mentioned.
Caco-2 cells have been well characterized and used in numerous anticancer studies, allowing for direct comparison of efficacies between studies. In this line, positive antiproliferative tests on Caco-2 cell line by employing different plant extracts have been done [27]. Jiménez et al [28] achieved an IC50 value of 250 μg/mL with rosehip extracts from Rosa canina, and then Gascón et al [29] found that Pinus pinea and Pinus pinaster bark extracts displayed IC50 values of 40 and 30 μg/mL, respectively. In this work, the obtained results suggest that the anticancer effect of biribá, inajá and monguba could be comparable to that from rosehip extracts, whereas pajurá might be comparable with pine bark instead. Moreover, the antiproliferative effect of such fruits could not be limited to colorectal cancer and further evaluation on different cancer cell lines should be performed in the future. A recent study developed by Shalom and Cock [30], demonstrated the potential of the ethyl acetate, methanolic, and aqueous T. ferdinandiana fruit and leaf extracts to block proliferation of some cancer cells, including Caco-2 cell line. The Caco-2 cell IC50 results in aqueous extracts were higher than 1000 µg/mL to fruit, and leaf (1520 and 1230 µg/mL, respectively), which shows that the majority of evaluated fruits show a higher anticancer effect.
Focusing on the antiproliferative effect of exotic fruits, Silva et al. [31] evaluated the anticancer activity of hydroalcoholic extracts of açaí toward a panel of cancer cell lines, including Caco-2. Authors noticed no significant changes in cell viability after 24 and 48h incubation with neither 10, 20 nor 40 μg/mL of the extracts. Since we have obtained an IC50 value of 272±32 μg/mL after 72h incubation, it is not surprising that at such low concentrations and short incubation time they did not observe any decrease in cell viability. Regarding to the rest of the evaluated Amazonian fruit extracts, to the best of our knowledge this is the first time that they have been tested toward Caco-2 cells due to their anticancer potential.
This preliminary screening showed that the Amazonian fruit extracts are promising natural sources of bioactive compounds with antiproliferative effect against colorectal cancer Caco-2 cell line. Although the mechanism of action of the studied fruits remains unclear, some studies have reported that some antioxidant phytocompounds displayed cytotoxic effects toward a panel of cancer cell lines [32-35]. Therefore, a likely correlation between antioxidant capacity and antiproliferative activity must be considered. Moreover, since phenolic compounds are usually the main responsible of the antioxidant properties of fruits and vegetables [36], the observed antiproliferative effect, as well as the antioxidant capacity previously showed, could be related to the presence of these compounds as has been previously reported [28].
3.3. Phenolic Composition Profile
In order to identify and quantify the phenolic compounds responsible for the antioxidant capacity and the cytotoxic activity observed, the content of total phenolic compounds was determined (Table 3).
Table 3. Total phenolic compounds expressed as mg equivalents of gallic acid/100 g of dry samples.
|
Samples |
Phenolic compound (mg GAE/100 g) |
||
|
EtOH |
EtOH:K-PBS |
K-PBS |
|
|
Abiu |
134.4±9.1a |
779.0±56.7a |
333.5±17.3a |
|
Açaí |
95.7±7.6b |
353.2±24.6b,c |
214.8±7.5b |
|
Bacuri |
331.5±4.5 |
531.8±16.7d |
328.3±24.9a |
|
Biribá |
62.1±2.5c |
427.0±30.9b,e |
139.3±13.7c,d |
|
Buriti |
28.1±1.1d |
188.6±7.9f |
170.4±7.3d |
|
Cupuaçu |
111.3±5.1b,e |
302.9±22.2c |
241.9±3.5b |
|
Inajá |
121.7±4.3a,e |
476.9±18.4d,e |
351.7±7.1a |
|
Monguba |
105.0±10.5b |
751.1±13.2a |
324.1±13.2a |
|
Pajurá |
69.9±1.7c |
800.6±24.0a |
140.0±0.2c,d |
|
Uxi |
44.1±2.9d |
157.8±6.3f |
107.5±0.4c |
Means followed by the same letter in the columns do not differ significantly from each other by the Tukey test at the 5% probability level.
In all samples, the phenolic compounds extraction was dependent on the solvent used and its polarity. In the same way, the antioxidant capacities of the studied fruit extracts also showed a strong relationship to the employed solvent.
In fact, crude phenolic extracts contain complex mixtures of some classes of phenols, which are selectively soluble in the different solvents. In this sense, solvent polarity plays a key role in increasing phenol solubility [37]. Organic solvent-water mixtures have been reported as more efficient in extracting phenolic compounds than their respective pure organic solvents [38].
Based on the present experimental results, it was predicted that in all samples, the binary extraction allowed to obtain higher concentration of phenolic compounds, followed by the K-PBS buffer, and pure ethanol extractors. The probable explanation for this behavior is the better solvation of antioxidant compounds present in fruits, such as phenolic compounds, as a result of hydrogen bonds interactions between the polar sites of the antioxidant molecules and the solvent. Ethanol was less efficient in the extraction of phenolic compounds, probably because of the presence of the ethyl radical that is longer, resulting in a lower solvation of antioxidant molecules [38].
Bacuri extracts exhibited important phenolic content in different polarities, displaying diverse phenolic composition. Abiu, monguba, and pajurá fruits showed higher concentration in binary extraction, whereas abiu, bacuri, inajá, and monguba in K-PBS buffer extraction. On the other hand, all polarities extract of buriti and uxi fruits presented the lower phenolic content.
Following the example of Vasco et al. (2008) [39] who classified the phenolic content measured, other studies have stablished some range valuation categories [24, 40, 41]. In this view, the results obtained for the better extraction (etOH:K-PBS) could be classified into three categories: low (<300 mg GAE/100g), moderate (300-600 mg GAE/100g), and high (>600 mg GAE/100g) phenolic content on dry matter.
The richest fruit in phenolic compounds were abiu, monguba, and pajurá indicating that these fruits are excellent phenolic sources which are well known for possessing anti-atherosclerotic, anti-inflammatory, antitumor, antithrombotic, anti-osteoporosis and antiviral activities [42]. Açaí, bacuri, biribá, cupuaçu, and inajá fruits can be classified as having moderate phenolic content, whereas buriti, and uxi fruits, low quantities.
Despite the different extraction procedure and solvents used, as well as the environmental conditions which reflect strongly on the fruit phenolic composition, recently studies yielded close findings for total phenol contents in Brazilian abiu fruit from Rondônia, BR (172.3 mg GAE/100g fresh weight) [43] and from Roraima, BR (900.2 mg GAE/100g dry weight) [44] in comparison to our results (220.2 mg GAE/100g fresh weight or 779 mg GAE/100g dry weight). In another study, involving traditionally consumed palm fruits from Amapá, BR, the results after extraction steps involving acetone, methanol and water solvents, were higher to buriti (118±2 mg GAE/100g fresh weight) and lower to inajá (45±2 mg GAE/100g fresh weight) [45], considering our results in fresh weight 83.15 and 155.0 mg GAE/100g, respectively.
The knowledge of the content of phenolic compounds in fruits is important because it reflects the mechanism of adaptation and resistance of the plant to the environment and influences the flavor and the technological characteristics of the food, as well as the nutritive and functional potential of these fruits [46].
The chromatographic profile (Table 4) of the Amazonian fruits extracts were determined although the detection and identification of several known polyphenols.
Table 4. Chromatographic profile of the phenolic extracts, which were identified based on the retention times of the corresponding standards, using different methods.
|
Phenolic compounds |
AB |
AÇ |
BA |
BI |
BU |
CU |
IN |
PA |
MO |
UX |
|||||||||||
|
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
||
|
1 |
(-)-Epicatechin |
X |
X |
X |
|
X |
|
X |
X |
X |
X |
X |
X |
X |
|
X |
X |
X |
|
X |
X |
|
2 |
2,5-dihydroxybenzoic acid |
X |
|
X |
|
X |
X |
X |
X |
X |
|
|
|
X |
X |
X |
|
X |
|
X |
|
|
3 |
3,4-dihydroxybenzaldehyd |
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
|
4 |
4-hydroxybenzoic acid |
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
X |
|
|
5 |
4-o-caffeoylquinic acid |
X |
|
|
|
|
|
X |
X |
X |
X |
|
|
X |
X |
X |
X |
X |
|
X |
X |
|
6 |
Arbutin |
|
|
X |
X |
|
|
|
|
|
|
X |
|
X |
|
X |
|
|
|
X |
|
|
7 |
Asiatic acid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
X |
|
|
X |
|
|
8 |
Caffeic acid |
X |
X |
X |
|
X |
X |
|
|
X |
X |
X |
|
X |
|
X |
X |
X |
X |
X |
X |
|
9 |
Carnosic acid |
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
Chlorogenic acid |
X |
|
|
|
|
|
X |
X |
X |
X |
|
|
X |
X |
X |
X |
X |
|
X |
X |
|
11 |
Cirsimaritin |
|
|
X |
|
|
|
X |
X |
|
|
|
|
|
|
|
|
X |
X |
X |
|
|
12 |
D-(-)- quinic acid |
X |
X |
X |
|
|
|
X |
X |
X |
|
|
|
X |
|
X |
X |
X |
X |
X |
X |
|
13 |
Ellagic acid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
X |
|
14 |
Ethyl gallate |
|
|
X |
X |
|
|
X |
X |
|
|
X |
|
X |
X |
|
|
|
|
X |
X |
|
15 |
Ferulic acid |
X |
X |
X |
|
X |
|
X |
X |
X |
X |
X |
|
X |
X |
X |
X |
X |
X |
X |
X |
|
16 |
Gallic acid |
X |
|
|
|
X |
|
|
|
X |
X |
|
|
|
|
X |
X |
X |
|
X |
|
|
17 |
Genkwanin |
|
|
X |
|
|
|
|
|
X |
|
|
|
|
|
X |
X |
|
|
|
|
|
18 |
Hesperidin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
X |
|
|
X |
X |
|
19 |
Homoplantaginin |
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
X |
X |
X |
X |
X |
|
20 |
Homovanillic acid |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
|
|
X |
X |
|
21 |
Kaempferol |
X |
X |
X |
|
X |
|
X |
|
X |
X |
X |
|
|
|
X |
X |
X |
|
X |
X |
|
22 |
Morin hydrate |
|
X |
X |
|
|
|
|
|
X |
X |
X |
X |
X |
X |
X |
|
X |
X |
X |
X |
|
23 |
Nepetin-7-glucoside |
X |
|
|
|
|
|
|
|
X |
X |
|
|
|
X |
|
|
X |
|
|
|
|
24 |
P-coumaric acid |
X |
X |
X |
|
X |
|
X |
X |
|
|
X |
X |
X |
|
|
X |
X |
|
X |
|
|
25 |
Quercetin |
|
X |
X |
|
|
|
|
|
X |
X |
X |
X |
X |
X |
X |
|
X |
X |
X |
X |
|
26 |
Quercitrin hydrate |
|
|
X |
|
|
|
|
|
X |
X |
|
|
|
|
X |
|
X |
X |
X |
X |
|
27 |
Rosmanol |
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
28 |
Rosmarinic acid |
|
|
|
|
|
|
|
|
|
|
|
|
X |
X |
|
|
X |
|
|
|
|
29 |
Rutin hydrate |
X |
X |
X |
|
|
|
X |
X |
X |
X |
X |
|
|
X |
X |
X |
X |
X |
|
|
|
30 |
Sinapic acid |
|
|
X |
|
|
|
X |
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
31 |
Syringaldehyd |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
|
|
X |
X |
|
32 |
Syringic acid |
|
|
X |
X |
|
|
X |
X |
|
|
X |
|
X |
X |
|
|
|
|
X |
X |
|
33 |
Taxifolin |
|
|
X |
|
|
|
|
|
X |
X |
|
|
|
|
X |
X |
X |
|
X |
X |
|
34 |
Trans-cinnamic acid |
|
|
X |
|
|
|
X |
X |
|
|
X |
|
X |
X |
|
|
|
|
X |
|
|
35 |
Umbeliferon |
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
X |
|
36 |
Vanillic acid |
|
|
X |
|
X |
|
X |
X |
X |
X |
|
|
X |
|
X |
X |
|
|
X |
|
|
37 |
Vanillin |
X |
|
X |
|
X |
X |
X |
|
X |
X |
|
X |
X |
X |
X |
X |
|
|
X |
|
|
38 |
Veratric acid |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
|
|
X |
X |
|
TOTAL |
18 |
12 |
28 |
6 |
16 |
6 |
21 |
16 |
23 |
18 |
17 |
8 |
23 |
16 |
25 |
20 |
22 |
9 |
30 |
20 |
|
The results of the qualitative chromatographic profile evidenced a great diversity in phenolic substances, wherein 38 phenolic compounds could be separated and identified.
Comparing the evaluated methods, it can be affirmed that the chromatographic conditions established in Method 1 allowed the separation and identification of a larger number of phenolic compounds, being higher in the açaí and uxi samples (28, and 30 phenolic compounds, respectively). However, some compounds were only possible to identify using the Method 2 (morin hydrate and quercetin in abiu; vanillin in cupuaçu; nepetin-7-glucoside and rutin hydrate in inajá; homoplantaginin and p-coumaric acid in pajurá; umbelliferon in uxi sample).
Among the Amazonian fruits evaluated in this study, inajá, biribá, and monguba were selected to be used in phenolic compounds quantitative determination (Table 5) by HPLC based on that samples showed the highest antiproliferative activity against Caco-2 cell lines, as well as high/moderate total phenolic compounds contents.
Table 5. Quantification of polyphenols and phenolic acids in Amazonian fruits extracts (mg/kg sample on a dry basis) by HPLC.
|
Phenolic compound |
Subclass |
Chemical formula |
Biribá |
Inajá |
Monguba |
|
simple phenols – phenolic acids |
|||||
|
2,5-dihydroxybenzoic acid |
Hydroxybenzoic acids |
C7H6O4 |
0.5 |
0.5 |
0.6 |
|
3,4-dihydroxybenzaldehyde/4- hydroxybenzoic acid |
Hydroxybenzaldehydes /Hydroxybenzoic acids |
C7H6O3 |
0.5 |
0.9 |
0.1 |
|
Caffeic acid |
Hydroxycinnamic acids |
C9H8O4 |
1.5 |
3.6 |
52.9 |
|
Chlorogenic acid |
Hydroxycinnamic acids |
C16H18O9 |
4.8 |
10.8 |
215.2 |
|
Ethyl gallate |
Hydroxybenzoic acids |
C9H10O5 |
46.2 |
Nd |
Nd |
|
Ferulic acid |
Hydroxycinnamic acids |
C10H10O4 |
Nd |
2.1 |
11.5 |
|
Gallic acid |
Hydroxybenzoic acids |
C7H6O5 |
Nd |
Nd |
1.9 |
|
Homogentisic acid |
Hydroxyphenylacetic acids |
C8H8O4 |
Nd |
0.4 |
0.5 |
|
Homovanillic acid |
Hydroxyphenylacetic acids |
C9H10O4 |
414.3 |
Nd |
Nd |
|
p-Coumaric acid |
Hydroxycinnamic acids |
C9H8O3 |
0.3 |
1.4 |
11.0 |
|
Quinic acid |
Phenolic acid |
C7H12O6 |
1031.6 |
34.2 |
571.9 |
|
Rosmarinic acid |
Hydroxycinnamic acids |
C18H16O8 |
Nd |
4.1 |
Nd |
|
Sinapic acid |
Hydroxycinnamic acids |
C11H12O5 |
Nd |
13.9 |
3.0 |
|
polyphenols – flavonoids |
|||||
|
(-)-Epigallocatechin gallate |
Flavanols |
C22H18O11 |
Nd |
181.9 |
Nd |
|
(-)-Epicatechin |
Flavanols |
C15H14O6 |
52.1 |
83.1 |
76.2 |
|
Procyanidin-C1 |
Flavanols |
C45H38O18 |
172.9 |
176.8 |
196.3 |
|
Fisetin |
Flavonols |
C15H10O6 |
Nd |
55.9 |
9.6 |
|
Kaempferol |
Flavonols |
C15H10O6 |
Nd |
0.2 |
0.1 |
|
Morin hydrate |
Flavonols |
C15H10O7 |
Nd |
84.6 |
83.0 |
|
Quercetin |
Flavonols |
C15H10O7 |
Nd |
4.4 |
10.0 |
|
Rutin hydrate |
Flavonols |
C27H30O16 |
4.6 |
0.9 |
21.7 |
|
Homoplantaginin |
Flavonoid glycoside |
C22H22O11 |
Nd |
0.2 |
0.4 |
|
Nepetin-7-glucoside |
Flavones |
|
Nd |
45.1 |
65.1 |
|
Procyanidin-A2 |
proanthocyanidins (condensed tannins) |
C30H24O12 |
Nd |
6.5 |
29.8 |
|
polyphenols – stilbenes |
|||||
|
Polydatin |
Stilbene |
C20H22O8 |
Nd |
0.3 |
1.2 |
|
Resveratrol |
Stilbene |
C14H12O3 |
Nd |
1.3 |
Nd |
|
other phenolic compounds |
|||||
|
Vanillin |
Hydroxybenzaldehydes |
C8H8O3 |
Nd |
24.2 |
Nd |
|
Arbutin |
Chalcone |
C12H16O7 |
Nd |
0.9 |
Nd |
Eleven phenolic compounds were quantified in biribá pulp, wherein quinic acids stand out due to its higher concentration (414, and 1032 mg/kg, respectively) as well as its significant biological activities. In the human body, quinic acid has been characterized as a pro-metabolite that leads to the induction of efficacious levels of nicotinamide and tryptophan in the gastrointestinal tract, being a source of those essential metabolic ingredients [47]. Besides that, quinic acid has potent broad-spectrum antioxidant, anti-inflammatory, hepatoprotective, and several other medicinal properties [48-51]. Regarding homovanillic acid, higher content was also obtained in bibibá fruit. Homovanillic acid is a major catecholamine metabolite occurring in human biofluids, and it was reported to exhibit antioxidant capacity [52]. In psychiatry and neuroscience, brain and cerebrospinal fluid levels are measured as a marker of metabolic stress caused by 2-deoxy-D-glucose [53].
Greater variability in phenolic compounds was observed in the inajá pulp, including 25 compounds which belong for the classes of flavonoids, stilbenes, and phenolic acids and have been identified in this plant for the first time. Among the phenolic compounds found in inajá pulp, an important (-)-epigallocatechin gallate (EGCg) concentration was verified. Such compound presents important medicinal properties, including beneficial effects in studies of Parkinson's and Alzheimer's diseases, stroke, obesity, diabetes, inhibition of cancer proliferation, cancer chemoprevention, and antioxidant activity [54,55]. EGCg is present in a limited number of plant-based foods and beverages. Arts et al. [56] determined the levels of some catechin, including EGCg in 24 types of fruits, 27 types of vegetables and legumes, some staple foods, and processed foods commonly consumed in the Netherlands, but none of the foods contained EGCg. In a recent research, 10 sugar apple (Annona squamosa L.) cultivar peels from Thailand were evaluated. The content of antioxidants was notably lower (ranging from 0.4 to 32 mg/kg) [57] than these obtained to the inajá pulp (181.9 mg/kg), and higher to the biribá pulp (<LOD) which belongs to the same botanic family. The inajá pulp showed a different stilbenes profile highlighting the resveratrol content, which inhibits the formation of free radicals and has antimutagenic activity [58].
After analysis of monguba seeds, 21 phenolic compounds were quantified, among other, higher levels in the phenolic acids, such as 2,5-dihydroxybenzoic acid, caffeic acid, ferulic acid gallic acid, homogentisic acid, p-coumaric acid, and especially chlorogenic acid. Chlorogenic acid is compound derived from caffeic acid and it exhibits several beneficial biological properties, including antibacterial, antiphlogistic, antiviral, and inhibitory effects on carcinogenesis in the large intestine and liver [59,60]. The chlorogenic acid values found in our study were higher than those contents reported for Gordon et al. [61] in açaí pulp (0.2 to 16.4 μg/g dry basis).
3.4. Multivariate statistical analysis
The principal component analysis (PCA) was applied in order to characterize the ten Amazonian fruits according to their biological activity. Nine variables were measured in in such 10 matrices, in triplicate, being the phenolic content after the extraction in ethanol (Folin etOH), aqueous buffer (Folin K-PBS), and the both mixed 1:1 (Folin K-PBS:etOH), the antioxidant capacity by the different assays and its solvent extraction (DPPH, NBT, ABTS etOH, ABTS PBS, and ABTS PBS:etOH), as well as the results of cancer cell viability (ANTIPROLIFERATIVE).
The cumulative percentage of the total variance explained by the first two components was 70.35 %. A bidimensional plot was designed (Figure 1). The distribution of the varieties along PC1 and PC2 showed that samples could be divided into three main groups: group A, in which are included inajá, monguba, açaí, bacuri, and abiu fruits positioned near to the central bidimensional plot; group B, which comprises biribá and pajurá; and group C, corresponding to buriti, uxi, and cupuaçu fruits. The results obtained by the PCA were confirmed through dendrogram obtained by the HCA (Figure 2), that presents three clusters formation.
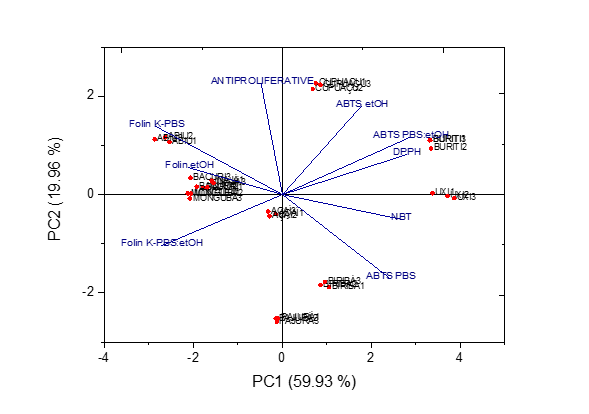
Figure 2. PCA scatter diagram: distribution of 10 fruit, in triplicate, along principal components 1 (PC1) and 2 (PC2) using 9 variables.
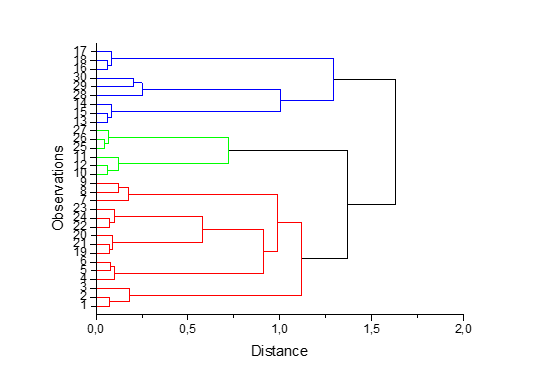
Figure 3. Dendrogram representing the similarity relations using the Euclidean distance.
Group A included fruit that differed from the others due to their higher concentrations of phenolic compounds, better antioxidant capacity (lower IC50), as well as an important capability to inhibit the growth of the cancer cells (lower IC50). The group B (biribá, and pajurá) distinguished from A, and C due to their high antiproliferative effects, and the moderate antioxidant capacity and the phenolic compounds content. Indeed, a strong correlation between the total concentration of phenolic compounds and the antioxidant capacity was observed, such as r: -0.904 to Folin (K-PBS) and ABTS (PBS), r: -0.976 to Folin (K-PBS:etOH) and ABTS (PBS:etOH), r: -0.738 to Folin (K-PBS) and NBT (K-PBS). The correlation results were negative due to the different way to express the results, which was used IC50 to antioxidant capacity, while mg GAE/100g concentration to phenolic content. The analysis suggested that the phenolic content was accountable for the antioxidant capacity of the fruit extracts. The free hydroxyl groups in phenolics are reported to be the main responsible for antioxidant capacity [62,63], followed by other influence factors such as the character of substituents (carboxyl or acetyl group) and their position in relation to the hydroxyl groups.
On the other hand, the distinctive behavior shown by the group C, that is, the biological activity discrimination was caused especially by the lower phenolic contents, antioxidant capacities, and less important antiproliferative effects.
Cytotoxicity on Caco-2 cell line induced by fruit extracts showed no correlation with neither antioxidant capacity nor total phenolic content. Although a high concentration of an individual or a specific group of phenolic compounds could be correlated with a strong induction of high antiproliferative activity, this assertion should not be necessarily associated with a great antioxidant capacity and the total phenolic concentration, which, indeed, it depends on synergistic effects of the extracted phenolics, as well as, of the oxidant source used in the antioxidant capacity determination. Qualitative and quantitative differences in phenolic composition between the fruits possibly influenced the antiproliferative activities. Gascón et al. [29] evaluated isolated components of pine bark extracts, namely procyanidins B1 and B2, and evidenced a significative lower antiproliferative activity in comparison to whole extracts.
Considering the specific role of individual phenols in carcinogenesis that has been and still is very unclear due to controversial results, Salucci et al. [64] investigated the antioxidant capacity, the cytotoxicity, the cellular uptake, and the effect on Caco-2 cell cycle of some phenolic compounds. Epicatechin, epigallocatechin gallate, gallic acid and quercetin-3-glucoside showed a significant antioxidant effect, but only epigallocatechin gallate or gallic acid was able to interfere with the cell cycle in Caco-2 cell lines. The results suggested the antioxidant activity of flavonoids was not related to the inhibition of cellular growth. Once again it is evident that components with antioxidative characteristics cannot act alone on the effect of certain diseases, in general the synergistic combination of different compounds around this aim is necessary.
In this sense, the fruit extracts tested are crude and complex mixtures with antioxidant activity and that containing a large number of phenolic compounds, that can allow multiple mechanisms simultaneously within cells, such as the inhibition of Caco-2 cell line proliferation. The antioxidant capacity and antiproliferative activities derived from the synergy between phenolic compounds acting in concert in whole foods may be considered one of the main therapeutically exploitable polyphenolic actions [65]. Furthermore, a deeper research in order to determine the mechanism of action of fruit extracts as well as the kind of cell death triggered must be performed. The results derived from this research might influence the therapeutic strategies towards consumption of the evaluated functional foods.
In this work were evaluated ten Amazonian fruits regarding their chemical composition, as well as their antioxidant and antiproliferative capacities. Inajá, monguba, açaí, bacuri and abiu fruits showed higher concentrations of phenolic compounds, antioxidant capacity, as well as an important capability to inhibit the growth of Caco-2 cell line, while biribá, and pajurá fruits exhibited higher antiproliferative effects and moderate antioxidant capacity and phenolic compounds content.
A strong positive correlation between the content of total phenolic compounds and antioxidant capacity was evidenced. The antiproliferative activities against Caco-2 cell line didn’t necessarily be associated with a high antioxidant capacity and the total phenolic concentration.
The main quantified phenolic compounds in biribá, inajá, and monguba were part of the flavonoids, stilbenes, and phenolic acids group. These fruits showed one or more potential phenolic compounds with anti-cancer activity, such as EGCg, resveratrol, chlorogenic acid, morin hydrate, and gallic acid. This discovery of the anticancer potential of the Amazonian fruits studied may help in the development of drugs and may have therapeutic effects in the treatment of colon cancer. However, further studies are needed to confirm the Caco-2 cell death by an apoptotic pathway, to identify the specific compound(s) responsible for the cytotoxic activity, as well as the molecular target in the cell.
The profiles phytochemical, antioxidant capacity, and the antiproliferative activity for most Amazonian species are still scarce, and this limits the valuation of their nutritional content and industrial purposes. The results shown here represent a great contribution, since that evaluated fruits have shown promise to the rational pharmaceutical and industrial exploitation.
This work was supported by the CAPES/Doctoral Sandwich Program Abroad [Case nº 88881.133624/2016-01]; and the FAPEMA/Scholarship Abroad [Case nº BEST-EXT-07273/16].
Neugarten R., Ceotto P., Acero N., Coutinho B., Flores-Gutierrez R., Hierholzer M., Kasecker T., Koenig K., Ledezma J.C., Pinheiro R., Turner W., Wright T.M., Amado N., Bernard C., Encomenderos I., Martinez C., Marhe S., Isabel Martinez M., Mendoza E., Peralvo M., Ruiz C., Troëng S. Mapping essential natural capital in amazonia: identifying important places for biodiversity and ecosystem services. Conservation International. 2015.
Clement C.R.; Cornelius, J.P.; Pinedo-Panduro M.H.; Yuyama K. Native Fruit Tree Improvement in Amazonia: An Overview. In indigenous fruit trees in the tropics: domestication, utilization and commercialization. Akinnifesi F.K., Leakey R.R.B., Ajayi O.C., Sileshi G., Tchoundjeu Z., Matakala P., Kwesiga F.R., Eds. CAB International: Wallingford, UK, 2007, p. 100-119.
View ArticleXu D-P., Li Y., Meng X., Zhou, T., Zhou Y., Zheng J., Zhang J-J., Li H-B. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96, p. 1-32. PMid:28067795
View Article PubMed/NCBIDziało M., Mierziak J., Korzun U., Preisner M., Szopa J., Kulma A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160, p. 1-41. PMid:26901191
View Article PubMed/NCBIWalter M., Marchesan E. Phenolic compounds and antioxidant activity of rice. Braz. arch. biol. technol. 2011, 54, 2, p. 371-377.
View ArticleOmbra M.N., D'Acierno A., Nazzaro F., Riccardi R., Spigno P., Zaccardelli M., Pane C., Maione M., Fratianni F. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of Southern Italy before and after cooking. Oxid. Med. Cell. Longev. 2016, p. 1-2. PMid:28105248
View Article PubMed/NCBIBouayed J., Bohn T. Exogenous antioxidantsDouble-edged swords in cellular redox state. Oxid. Med. Cell. Longev. 2010, 3, 4, p. 228-237. PMid:20972369
View Article PubMed/NCBIPisoschi A.M., Negulescu G.P. Methods for total antioxidant activity determination: a review. Biochem & Anal Biochem. 2011, 1, 1, p. 1-10.
Pandey K.B, Rizvi S.I., Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009, 2, 5, p. 270-278. PMid:20716914
View Article PubMed/NCBIScalbert A., Manach C., Morand C., Remesy C. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005, 45, p. 287-306. PMid:16047496
View Article PubMed/NCBIShalaby E.A., Shanab S.M., Antioxidant compounds, assays of determination and mode of action. Afr J Pharm Pharmacol. 2013, 7, p. 528-39.
View ArticlePrior R.L., Wu X., Schaich K.J. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, p. 429-4302. PMid:15884874
View Article PubMed/NCBIMoharram H.A., Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Fd. Sci. & Technol. 2014, 11, 1, p. 31-42.
View ArticleSultana B., Anwar F., Przybylski R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007, 104, 3, p. 1106-1114.
View ArticleWang J., Sun B., Cao Y., Tian Y., Li X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 2, p. 804-810.
View ArticleCerretani L., Bendini A. Rapid Assays to Evaluate the Antioxidant Capacity of Phenols in Virgin Olive Oil. In: Olives and Olive Oil in Health and Disease Prevention, OXFORD, Academic Press, 2010, p. 625-635.
View ArticleVanella A., DiGiacomo C., Sorrenti V., Russo A., Castorina C., Campisi A., Renis M., Perez-Polo J. Free radical scavenger depletion in post-ischemic reperfusion brain damage. Neurochem Res. 1993, 18, 1337-1340. PMid:8272198
View Article PubMed/NCBIKontos H.A., Wei E.P. Auperoxide production in experimental brain injury. J. Neurosurg. 1986, 64, p. 803-807. PMid:3009736
View Article PubMed/NCBIBirnboim H.C. DNA strand breaks in human leukocytes induced by superoxide anion, hydrogen peroxide and tumor promoters are repaired slowly compared to breaks induced by ionizing radiation. Carcinogenesis. 1986, 7, p. 1511-1517. PMid:3017600
View Article PubMed/NCBIKhodade V.S., Sharath-Chandra, M., Banerjee A., Lahiri S., Pulipeta M., Rangarajan R., Chakrapani H., Bioreductively activated reactive oxygen species (ROS) generators as MRSA Inhibitors. ACS Med. Chem. Lett. 2014, 5, p. 777-781. PMid:25050164
View Article PubMed/NCBIZhang, Z-S.; Li, D.; Wang L-J.; Ozkan, N.; Chen, X. D.; Mao, Z-H.; Yang, H-Z. Optimization of ethanol-water extraction of lignans from flaxseed. Sep Purif Technol, 2007, 57, 1, p. 17-24.
View ArticleNeves L.C., Campos A.J., Benedett, R.M., Tosin J.M., Chagas E.A. Characterization of the antioxidant capacity of natives fruits from the Brazilian Amazon region. Rev. Bras. Fruticultura. 2012, 34, 4, p. 1165-1173.
View ArticleCanuto G.A.B., Xavier A.A.O., Neves L.C., Benassi M., Physical and chemical characterization of fruit pulps from amazonia and their correlation to free radical scavenger activity. Rev. Bras. Fruticultura. 2010, 32, p. 1196-1205.
View ArticleRufino M.S., Alves R.E., Brito E.S., Pérez-Jiménez J., Saura-Calixto F., Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 nontraditional tropical fruits from Brazil. Food Chem. 2010, 121, p. 996-1002.
View ArticleMarinova G., Batchvarov V. Evaluation of the methods for determination of the free radical scavenging activity by dpph. Bulg. J. Agric. Sci. 2011, 17, 1, p. 11-24.
Gallaher D.D, Trudo S.P. Nutrition and colon cancer. In: Coulston AM, Boushey CJ, Ferruzzi M, eds. Nutrition in prevention and treatment of disease. London: Academic Press. 2013, p. 697-715.
View ArticleMármol I., Sánchez-De-Diego C., Pradilla D.A., Cerrada E., Rodriguez Yoldi M.J. Colorectal carcinoma: a general overview and future perspectives in colorectal Cancer. Int J Mol Sci. 2017, 18, 197, p. 1-39. PMid:28106826
View Article PubMed/NCBIJiménez S., Gascón S., Luquin A., Laguna M., Ancin-Azpilicueta C., Rodríguez-Yoldi M.J. Rosa canina extracts have antiproliferative and antioxidant effects on Caco-2 human colon cancer. PLoS ONE, 2016, 11, 7, p. 1-14. PMid:27467555
View Article PubMed/NCBIGascón S., Jiménez-Moreno N, Jiménez S., Queroa J., Rodríguez-Yoldia M.J., Ancín-Azpilicueta C. Nutraceutical composition of three pine bark extracts and their antiproliferative effect on Caco-2 cells. J Funct Foods. 2018, 48, p. 420-429.
View ArticleShalom J., Cock I.E. Terminalia ferdinandiana Exell. Fruit and leaf extracts inhibit proliferation and induce apoptosis in selected human cancer cell lines. Nutr Cancer. 2018, p. 1-5. PMid:29641917
View Article PubMed/NCBISilva D.F., Vidal F.C.B, Santos D., Costa M.C.P, Morgado-Díaz J.A., Nascimento M.D.S.B., Moura R.S. Cytotoxic effects of Euterpe oleracea Mart. in malignant cell lines. BMC Complement Altern Med., 2014, 14, 175, p. 1-9. PMid:24886139 PMCid:PMC4047259
View Article PubMed/NCBIJain P., Pareek A., Ratan Y., Sharma S., Paliwal S. Free radicals and dietary antioxidants: a potential review. Int J Pharm Sci Rev Res. 2013, 18, 1, p. 34-48.
Sharoni Y., Danilenko M., Levy J. Molecular mechanism for the anticancer activity of the carotenoid lycopene. Drug Develop Res. 2000, 50, p. 448-456. (200007/08)50:3/4<448::AID-DDR28>3.0.CO;2-U
View ArticleNovi A.M. Regression of aflatoxin B1-induced hepatocellular carcinomas by reduced glutathione. Science. 1981, 212, p. 541-542. PMid:6782675
View Article PubMed/NCBISindhi V., Gupta V., Sharma K., Bhatnagar S., Kumari R., Dhaka N. Potential applications of antioxidants - A review. J Pharm Res. 2013, 7, p. 828-835.
View ArticleHervert-Hernández D., García O.P., Rosado J.L., Goñi I. The contribution of fruits and vegetables to dietary intake of polyphenols and antioxidant capacity in a Mexican rural diet: Importance of fruit and vegetable variety. Food Res Int. 2011, 44, p. 1182-1189.
View ArticleNaczk M., Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006, 41, 1523-1542. PMid:16753277
View Article PubMed/NCBIBoeing J.S., Barizão É.O., Silva B.C., Montanher P.F., Almeida V.C., Visentainer J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem Cent J. 2014, 8, 1, p. 48. PMid:25246942
View Article PubMed/NCBIVasco C., Ruales J., Kamal-Eldin A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, p. 816-823.
View ArticleMaen A., Rayan P., Matthews B., Cock I.E. High performance liquid chromatography-mass spectrometry analysis of high antioxidant australian fruits with antiproliferative activity against cancer cells. Phcog Mag. 2016, 12, p. 181-194. PMid:27279705
View Article PubMed/NCBISouza V.R., De Pereira P.A.P., Queiroz F., Borges S.V., Carneiro J. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012, 134, p. 381-386,
View ArticleNijveldt R.J., Van Nood E., Van Hoorm, D.E., Boelens P.G., Van Norren K., Van Leeuwen P.A. Flavonoids: a review of probable mechanisms of action and potential application. Am J Clin Nutr. 2001, 74, 4, p. 418-425. PMid:11566638
View Article PubMed/NCBIVirgolin L.B., Seixas R.S.F., Janzantti N.S. Composition, content of bioactive compounds, and antioxidant activity of fruit pulps from the Brazilian Amazon biome. Pesqui Agropecu Bras. 2017, 52, 10, p. 933-941.
View ArticleMontero I., Chagas E.A., Melo Filho A.A., Saravia S.A., Santos R.C., Chagas P.C., Duarte E.D.R.S. Evaluation of Total Phenolic Compounds and Antioxidant Activity in Amazonian fruit. Chem. Eng. Trans. 2018, 64, p. 649-654.
Santos M.F.G., Mamede R.V.S., Rufino M.S.M., Brito E.S.B., Alves R.E. Amazonian native palm fruits as sources of antioxidant bioactive compounds. Antioxidants. 2015, 4, p. 591-602. PMid:26783846
View Article PubMed/NCBIRocha W.S., Lopes R.M., Silva D.B., Vieira R.F., Silva J.P., Agostini-Costa T.S. Compostos fenólicos totais e taninos condensados em frutas nativas do cerrado. Rev. Bras. Fruticultura. 2011, 33, 4, p. 1215-1221.
View ArticlePero R.W, Lund H., Leanderson T. Antioxidant Metabolism Induced by Quinic Acid. Increased Urinary Excretion of Tryptophan and Nicotinamide. Phytotherapy Res. 2009, 23, 3, p. 335-346. PMid:18844285
View Article PubMed/NCBISoh Y, Kim J.A., Sohn N.W., Lee K.R., Kim S.Y., Protective effects of quinic acid derivatives on tetrahydropapaverolineinduced cell death in C6 glioma cells. Biol Pharm Bull. 2003, 26, 6, p. 803-807. PMid:12808290
View Article PubMed/NCBIPero R.W., Lund H. In vivo treatment of humans with quinic acid enhances dna repair and reduces the influence of lifestyle factors on risk to disease. IJBB. 2009, 5, 3, p. 293- 305.
Xiang T., Xiong Q.B., Ketut A.I., Tezuka Y., Nagaoka T., Wu L.J., Kadota S., Studies on the hepatocyte protective activity and the structure-activity relationships of quinic acid and caffeic acid derivatives from the flower buds of Lonicera bournei. Planta Med., 2001, 67, 4, p. 322-325. PMid:11458447
View Article PubMed/NCBIInbathamizh L., Padmini E. Quinic acid as a potent drug candidate for prostate cancer - a comparative pharmacokinetic approach. Asian J Pharm Clin Res. 2013, 6, 4, p. 106-112.
Aljović I., Gojak-Salimović S. Evaluation of the antioxidant activity of ferulic, homovanillic and vanillic acids using the Briggs-Rauscher oscillating reaction method. Glas. hem. tehnol. Bosne Herceg.. 2017, 49, p. 35-38.
Marcelis M., Suckling J., Hofman P., Woodruff P., Bullmore E., Van Os J., Evidence that brain tissue volumes are associated with HVA reactivity to metabolic stress in schizophrenia. Schizophr Res., 2006, 86, p. 45-53. PMid:16806836
View Article PubMed/NCBIStuart E.C., Scandlyn M.J., Rosengren R.J. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sciences, 2006, 79, p. 2329-2336 PMid:16945390
View Article PubMed/NCBIDu G.J., Zhang Z., Wen X.D., Yu C., Calway T., Yuan C.S., Wang C.Z. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients, 2012, 4 p. 1679-1691. PMid:23201840 PMCid:PMC3509513
View Article PubMed/NCBIArts I.C.W., Putte B.V., Hollman P.C.H. Catechin Contents of Foods Commonly Consumed in The Netherlands. 1. Fruits, Vegetables, Staple Foods, and Processed Foods. J Agric Food Chem. 2000, 48, p. 1746-1751. PMid:10820089
View Article PubMed/NCBIManochai B., Ingkasupart P., Lee S.H., Hong J.H., Evaluation of antioxidant activities, total phenolic content (TPC), and total catechin content (TCC) of 10 sugar apple (Annona squamosa L.) cultivar peels grown in Thailand. Food Sci. Technol. 2018, p. 1-7.
View ArticleMalta L.G., Ghiraldini F.G., Reis R., Oliveira M.V., Silva L.B., Pastore G.M., In Vivo analysis of antigenotoxic and antimutagenic properties of two brazilian cerrado fruits and the identification of phenolic phytochemicals. Food Res Int. 2012, 49, p. 604-611.
View ArticleWang- H.Q., Li D.L., Du Z.Y., Huang M.T., Cui X.X., Lu Y.J., Zhang K. Antioxidant and anti-inflammatory properties of chinese ilicifolius vegetable (Acanthopanax Trifoliatus (L) Merr) and its reference compounds. Food Sci Biotechnol. 2015, 24, p. 1131-1138.
View ArticleHao Y., Gao R., Liu D., He G., Tang Y., Guo Z. Selective extraction and determination of chlorogenic acid in fruit juices using hydrophilic magnetic imprinted nanoparticles. Food Chem. 2016, 200, p. 215-222. PMid:26830581
View Article PubMed/NCBIGordon A., Cruz A.P.G., Cabral L.M.C., Freitas S.C., Taxi C.D., Donangelo C.M., Mattietto R.A., Friedrich M., Matta V.M., Marx F. Chemical characterization and evaluation of antioxidant properties of Açaí frutis (Euterpe oleraceae Mart.) during ripening. Food Chem. 2012, 133, p. 256-263. PMid:25683393
View Article PubMed/NCBIFeng J-Y., Liu Z-Q. Phenolic and enolic hydroxyl groups in curcumin: Which plays the major role in scavenging radicals? J. Agric. Food. Chem. 2009, 57, 22, p. 11041-11046. PMid:19736944
View Article PubMed/NCBISroka Z., Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol. 2003, 41, 6, p. 753-758. 00329-0
View ArticleSalucci M., Stivala L.A. Maiani G., Bugianesi R., Vannini V. Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2). Br J Cancer, 2002, 86, p. 1645-1651. PMid:12085217
View Article PubMed/NCBISeeram N.P., Adams L.S., Henning S.M., Niu Y., Zhang Y., Nair M.G., et al. (2005). In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005, 16, 6, p. 360-367. PMid:15936648
View Article PubMed/NCBIBecker, M.M.; Ribeiro, D.B.; Silva, F.E.P.S.; Catanante, G.; Marty, J.L. Nunes, G.S. Determination of the antioxidant capacity of red fruits by miniaturized spectrophotometry assays. J Braz Chem Soc. 2019, 30, 5, p. 1108-1114.
View ArticleSingleton V.L., Rossi J.R. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 3, p. 144-158.
