Danilo Wilhelm Filho
Departamento de Ecologia e Zoologia, CCB, Universidade Federal de Santa Catarina, Trindade, Florianópolis, SC, 88040-900, Brazil; Tel: +55-48-23316917; FAX: +55-48-3315156; dawifi@ccb.ufsc.br.
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 2 ISSUE: 1
Page No: 90-104
Danilo Wilhelm Filho
Departamento de Ecologia e Zoologia, CCB, Universidade Federal de Santa Catarina, Trindade, Florianópolis, SC, 88040-900, Brazil; Tel: +55-48-23316917; FAX: +55-48-3315156; dawifi@ccb.ufsc.br.
Pereira, N.C.1; Cantoviski, K.1; Sell, F.1, Parisotto, E.B.1, Pedrosa, R.C.2; Zamoner, A.2 and Wilhelm Filho, D.1*
1Departamento de Ecologia e Zoologia and 2Departamento de Bioquímica, CCB, Universidade Federal de Santa Catarina, Florianópolis, SC, Brazil.
Ines Martins(ines.martins@mare-centre.pt)
Lani U Gleason(lani.gleason@csus.edu)
Aneta Spyra(aneta.spyra@us.edu.pl)
Pedro Mart%C3%ADnez-Paz(pjdepaz@ccia.uned.es)
Danilo Wilhelm Filho, Risk assessment of a coastal lacustrine environment using oxidative stress biomarkers present in the digestive gland of the Brazilian clam Anomalocardia brasiliana.(2018)SDRP Journal of Aquaculture, Fisheries & Fish Science 2(1)
Background: Lacustrine systems are environments of great ecological and economical relevance, however, in the last decades they have been progressively threatened by several contaminants.
Methods: Total and fecal coliform concentrations in the environment, several biomarkers of oxidative stress as well as trace metal concentrations were examined in the digestive gland of the clam Anomalocardia brasiliana sampled at four contaminated sites and compared to a reference site in a coastal lacustrine environment localized at the Santa Catarina Island, Southern Brazil. Comparisons of contaminated sites and the reference site were analyzed using one-way ANOVA with a minimal confidence interval of 5% (p<0.05).
Results: Compared to the reference site, lipoperoxidation (TBARS levels) in the digestive gland and coliform contents in the water were higher at all contaminated sites. Trace metal concentrations were high and cromium (2.65-0.55 mg g-1) and lead (0.63-0.77 mg g-1) concentrations were found to be above the limit values allowed by international legislation (FDA). Antioxidant enzymes such as superoxide dismutase, catalase, glutathione reductase, glutathione peroxidase and glutathione S-transferase showed generally increased activity, while levels of reduced glutathione (GSH) showed decreased contents in clams sampled at the contaminated sites.
Conclusions: The results indicate that A. brasiliana is facing a severe oxidative stress at the contaminated sites, which seems to be related to different contaminants but especially to fecal coliforms found in the water and to trace metals detected in the digestive gland. The clam Anomalocardia brasiliana is suitable as a useful bioindicator species for aquatic risk assessments.
Keywords: Mollusk; Anomalocardia brasiliana; Oxidative stress; Trace metals; Sewage.
The increased deterioration of the lacustrine system of the Lagoa da Conceição as well as other aquatic environments in the Santa Catarina Island is being demonstrated by several indicators of water quality in the last three decades [1-3], with some exceptions [4]. As a consequence, it shows increased nautic activity, diminished water quality, declining biodiversity and biomass production, and is contaminated, among others, by municipal sewage discharges and also trace metals mostly derived from intense nautic activity [4, 5]. Araújo [1] detected very high total and coliform contents (≥200 - ≥100,000/100ml) in different aquatic systems of the island especially associated with local mangroves. Beside the intense harvesting of the clam to supply the numerous restaurants of the island, the mean length and weight of this species is being rapidly decreasing in the last years in this lagoon and other parts of the island as well as the coast of the Santa Catarina state [6].
Brazilian and worldwide coastal lagoon systems are environments of great ecological and economical relevance because they sustain the development of early stages of many invertebrate and vertebrate species [7]. Nevertheless, in the last decades they have been progressively threatened by several contaminants, including among others, sewage discharges, which may include trace metals, thereby posing severe environmental concerns, as in the particular case of the present study, the lacustrine environment of Lagoa da Conceição in south Brazil [5, 8]. Lacustrine sediments are important sinks for trace metals, which are released either from different natural or anthropogenic sources [9].
The biological effects of reactive oxygen species (ROS) are generally similar in aerobic organisms and these ROS, beside their important benefits involving cell signaling and development, immune system, among other functions, are capable to damage biologically important molecules [10]. An imbalance in redox reactions involving ROS and antioxidants can lead to a condition called oxidative stress (OS)[11], which typifies the toxicity induced by different xenobiotics present in the aquatic environment, including trace metals [12-15].
Bivalve mollusks are good bioindicators for aquatic pollution associated with augmented ROS generation and changes in their antioxidant defenses [12, 14-22]. Also, many studies on bivalve mollusks revealed positive correlations between different OS biomarkers and the presence of trace metals in the digestive gland [9, 12-17, 19-21, 23-34]. However, the related literature is very limited regarding the exposure of aquatic bioindicators to sewage discharges reflected on OS biomarkers either in mollusks [35] or even in fish [36, 37].
Therefore, the present study was conducted in order to evaluate the environmental risk assessment of contaminants of different anthropogenic sources such as coliform concentrations in the water and trace metals on OS biomarkers present in the digestive gland of the Brazilian clam Anomalocardia brasiliana, sampled at four different contaminated sites, which were compared to a reference site in a coastal lacustrine environment localized at the Santa Catarina Island, Brazil.
Study area
The study area is a mesohaline lacustrine environment connected to the Atlantic Ocean through a permanent channel of approximately 2 km of extension and around 20 km2 of surface, called Lagoa da Conceição, a relatively large lacustrine system existent in the Santa Catarina Island, southern Brazil [4](Figure 1).
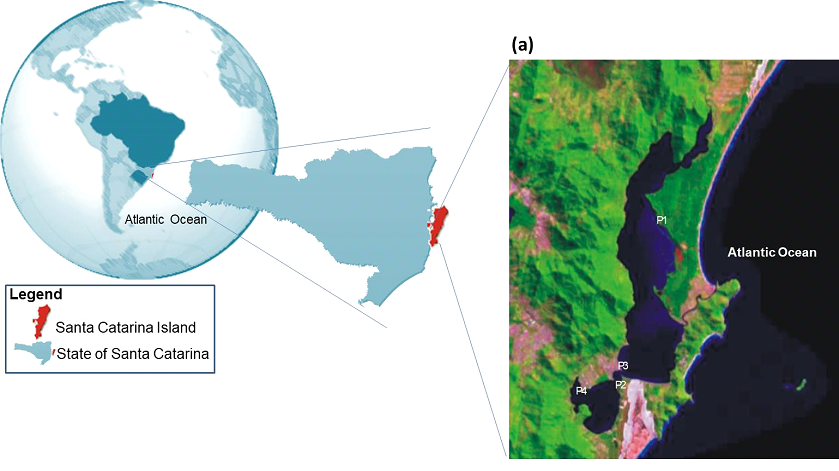
Figure 1. Geographical localization of the study at Santa Catarina Island and space photograph from part of the Santa Catarina Island showing the Lagoa da Conceição and the four contaminated sites. P1: Rio Vermelho; P2: Osni Ortiga; P3: Ponta das Almas; P4: LIC; Pc: Reference Site (Pontal Daniela) is not shown, which is localized in the north part of the island. The space photograph is available at www.embrapa.gov.br
In the present study five sites were examined: a reference site (“Praia da Daniela”, the reference site; 27°27’9.74”S - 48°32’17.73”W), here named Pc, localized near the Ratones river, inside the “Estação Ecológica de Carijós”, a local conservation unity criated in 1987, at the north part of the Santa Catarina island. This area is characterized by a very low anthropogenic influence mainly because of its status of a protected area (Pereira, 2003). Four contaminated sites were also evaluated at the Lagoa da Conceição, which is localized in the city of Florianópolis, Santa Catarina Island, southern Brazil (27○34'14”- 27○35'31”S; 48○30'07” - 48○31'33”W). The four contaminated sites are the following (Figure 1): site “Rio Vermelho” (27°33’5.65”S - 48°26’51.57”W) here named P1, which faces, beside sewage discharges, a strong influence of acid resins coming from a Pinus forest existent near the border of the lagoon. Site P2 or “Osni Ortiga” (27°36’31.74”S - 48°27’47.32”W), which is localized near a local urban area with intense car traffic. Site P3 or “Ponta das Almas” (27°36’17.13”S - 48°27’45.42”W), localized at the “Canto dos Araçás”, near an urban area and a recreational beach club. Finally, site P4 or “LIC” (27°36’44.60”S - 48°28’51.29”W), which is localized near a dense urban area and next to a relatively large recreational beach club that has an intense nautic (mainly sailing) activity (Figure 1).
Analysis of physicochemical parameters and coliform contents
Analyses of physicochemical parameters such as water temperature, pH, conductivity, salinity, dissolved oxygen, as well as the analysis of total and fecal coliforms of the water were measured according to standard methods for water and wastewater examination [38]. Water was sampled at 30 cm of depth at the sites where the clams were collected.
Study model
Fifteen adult individuals of A. brasiliana (Gmelin, 1791) with length shell rangeing from 20.5 cm to31.8 cm, irrespective of sex evaluation were collected at the five sampling sites during the low tide period, in the same day, during early Fall (beginning of April). The 75 specimens were immediately transported to the laboratory in containers with water and sediment, which was also collected at the corresponding site. Immediately after arrival and before dissection, the clams were weighed (weight range: 4.51 - 8.13 g) and total length (length range: 22.8 - 34.2 mm) was measured. The digestive glands were excised and the corresponding portions were weighed for preparation of the homogenates and acid extracts. Aliquots of the supernatants were frozen and stored in liquid nitrogen (-170°C) regarding the enzymatic analysis, while those for GSH and TBARS quantifications were analyzed in same day in fresh homogenates to avoid interferences related to frozen samples [39].
Preparation of tissue homogenates
The digestive glands were carefully excised, surface-dried with filter paper, weighed, washed in ice-cold saline solution and homogenized individually in a PBS solution (pH 7.4) containing 0.1% Triton X-100, 0.12 M NaCl, 30mM Na-PO4, (1:9, wet tissue weight: buffer volume), containing freshly prepared protease inhibitors (0.3 mM PMSF and 0.05 mM trypsin inhibitor). Homogenizations were carried out at in broken ice (5°C) using a tissue tearor for approximately 30 s, followed by centrifugation at 10,000 g for 5 min at 5°C. Aliquots of the supernatants were stored in liquid nitrogen and examined separately for each assay of enzymatic activity and TBARS quantification. All the biochemical measurements were carried out spectrophotometrically in a GBC UV/VIS 916 spectrophotometer (Sydney, NSW, Australia).
Trace metal analysis
The soft parts of the clams were carefully excised, dried at 60°C and then pulverized to a homogeneous particle size. Aliquots of 200 mg from a pool of 15 individuals from each site were submitted to a digestion process with 3 ml of 0.5M HNO3 at 120°C for 5 h, and 1 ml of 0.1M H2O2 solution at 60°C for 1 h [40]. After digestion, each sample was diluted in 100 ml of water (Millipore) and analyzed by graphite furnace atomic absorption spectrophotometry (Varian Spectra–640Z, with Zeeman background corrector) regarding the concentrations of Pb, Cr, Zn, Ni and Cd. Values were reported as μg.g-1.
Enzymatic activity
Superoxide dismutase activity was measured at 550 nm according to the method of cytochrome c reduction promoted by the superoxide anion [41]. In short, the activity was measured in aliquots of supernatants, which were added to a cuvette containing 2.0 ml of phosphate buffer 50 mM pH 7.8, EDTA 0.1 mM, cytochrome c 20 mM, xanthine 50 mM, 0.4 U of xanthine oxidase, obtaining a decrease of absorbance at A550 , which was monitored during 2 minutes. Values were expressed in USOD g-1 of tissue, considering that one arbitrary unit of SOD corresponds to the amount of sample able to inhibit 50% of the rate of cytochrome c reduction formation in the cuvette by superoxide anion. Catalase activity was evaluated by measuring the decrease in hydrogen peroxide concentration at 240nm [42] promoted by the enzyme present in the sample. Decays in A240 were registered during the first minute, in a cuvette containing 50 mM Na-phosphate, pH 7.0, and a freshly prepared 10 mM hydrogen peroxide solution. Hydrogen peroxide stock solution was previously titrated to ascertain the concentration. Values were expressed in mmol min-1 g-1 of tissue. Glutathione peroxidase (GPx) was measured at 340 nm through the glutathione/NADPH/glutathione reductase system, by the dismutation of terc-butylhydroperoxide [43], and expressed in μmol min-1g-1. The activity of glutathione reductase (GR) was measured at 340 nm through the oxidation rate of NADPH, which is proportional to the GSSG formation, in a reaction medium containing 0.1M NaPO4 buffer, pH 7.0, 0.1% DPTA and 1mM GSSG [44], and expressed in μmol min-1g-1. The enzyme glutathione S-transferase (GST) was measured at 340 nm according to Habig et al. [45], using CDNB (1-chloro-2,4-dinitrobenzene) as substrate. In a cuvette containing 10 ml of CDNB 0.1 M, 10 ml of GR 0.1 M (GSH) and 970 ml of phosphate buffer 0.15 M, pH 7.0, while in the reference cuvette a medium containing 980 ml of the same phosphate buffer was used. The activity values were also expressed in μmol min-1g-1.
Lipoperoxidation levels (TBARS)
Determination of thiobarbituric acid-reactive substances (TBARS) was used to assay endogenous lipid oxidation according to Ohkawa [46] and Bird and Draper [47], in fresh homogenates. Frozen samples were not used because even when reacted with BHT they showed further enhanced lipid autoxidation, and therefore, overestimation of TBARS levels [48]. Fresh homogenates were added to 0.2mM butylhydroxytoluen (BHT) to avoid further lipid oxidation. Tissue acid extracts were obtained by the addition of the homogenate to 12% trichloroacetic acid (1:4 v/v), followed by centrifugation. Supernatants were centrifuged at 5,000g for 5 min at 5°C, added to 0.67% (w/v) 2-thiobarbituric acid, maintained in boiling water for 60 min, cooled at 5°C for 30 min, and then measured spectrophotometrically at 535 nm. Absorbances were expressed as nmol TBARS g tissue-1 (Ƹ535 = 153 mM-1 cm-1).
Glutathione assay
Reduced glutathione (GSH) was measured according to Anderson [49] using the Elmann's reagent (DTNB). Tissue acid extracts were obtained by the addition of 12% trichloroacetic acid (1:4 v/v), followed by centrifugation at 5,000 g for 3 min at 5°C. Supernatants from the acid extracts were added to 0.25 mM DTNB in 0.1M Na-PO4, pH 8.0, and the formation of thiolate anion was determined at 412 nm during 3 minutes. Total glutathione (TG) was also measured at 412 nm according to the method of Tietze [50], and oxidized glutathione (GSSG) was calculated in equivalents of GSH (2 GSH = 1 GSSG). Values were expressed in mmol g-1 of tissue using e = 14,1 mM-1 cm-1.
Chemicals
All reagents were purchased from Sigma Chemical Co. (Ohio, USA), with the exception of the standard working solutions for trace metal analysis, which were prepared from Merk Titrisol solution (Germany).
Statistics
All experimental data are represented as mean ± S.E.M. (n=15 per site). For testing the normality the following tests were carried out: D'Agostino & Pearson omnibus normality test and Shapiro-Wilk normality test. Statistically significant differences from the reference site were determined by one-way ANOVA and post hoc tests with multiple comparisons (Tukey-Kramer) posttest using GraphPad InStat version 3.01 and GraphPad Prism version 5.0, GraphPad software Inc, (La Jolla, California, USA). Differences were considered to be significant when p<0.05.
Physicochemical parameters analyses
The physicochemical analyses revealed relatively small differences among the different sites examined (Table 1). Nevertheless, a relatively low value for dissolved oxygen was detected at site P4, which was approximately half (4.5 mg g-1) the values found in the other sites (6.9-10.7 mg g-1) (Table 1).
Table 1. Physicochemical parameters and total and fecal coliform concentrations in water collected at different sites of the Lagoa da Conceição. P1: Rio Vermelho; P2: Osni Ortiga; P3: Ponta das Almas; P4: LIC; Pc: Reference Site (Pontal Daniela).
|
Parameter |
P1 |
P2 |
P3 |
P4 |
PC |
Unit |
|
Conductivity |
36.9 |
31.1 |
39.8 |
29.2 |
49.2 |
mS/Cm |
|
Color |
20.0 |
15.0 |
15.0 |
10.0 |
30.0 |
u.C. |
|
BOD |
12.0 |
9.0 |
10.2 |
9.6 |
5.4 |
mg/L |
|
QOD |
515.0 |
360.0 |
750.0 |
570.0 |
420.0 |
mg/L |
|
Dissolved O2 |
10.7 |
9.3 |
7.2 |
4.5 |
6.9 |
mg/L |
|
pH |
8.5 |
8.3 |
8.27 |
7.87 |
8.30 |
Unity |
|
Salinity |
23.4 |
19.2 |
25.1 |
18.0 |
31.08 |
%o |
|
STD |
21.60 |
18.60 |
24.30 |
16.90 |
29.53 |
mg/L |
|
Temperature |
21.7 |
24.0 |
19.1 |
18.4 |
17.7 |
°C |
|
Turbidity |
6.0 |
2.4 |
6.0 |
1.2 |
14.6 |
NTU |
Sites P1: Rio Vermelho; P2: Osni Ortiga; P3: Ponta das Almas; P4: Praia do LIC; PC: Pontal da Daniela (Reference Site).
Coliforms, trace metals and Pinus resins
High total (≥1732.9/100ml) and fecal coliform contents (1732.9 – 111.9/100ml) were found in all contaminated sites examined compared to the reference site (81.6 and 18.3 total and fecal coliforms/100ml, respectively; Table 1). Site P2 also showed high TBARS contents together with high coliform contents and low trace metal contents in the digestive gland of A. brasiliana (Table 1; Figure 1). Similarly, site P4 showed the lowest coliform and TBARS contents together with a relatively low trace metal content (Tables 1,2).
Trace metal concentrations in the digestive gland of A. brasiliana showed values according to the following decreasing sequence: P3>P1>P2>PC>P4 (Table 2). Ni showed the highest and Cd the lowest concentrations in the digestive gland of A. brasiliana (Table 2). However, these concentrations were relatively high when calculated in a wet weight basis. Pb showed 0.02; 0.09; 0.63; 0.77; 0.16 mg g-1 and Cd showed 0.04; 0.09; 0.10; 0.12; 0.10 mg g-1 for sites Pc, P1, P2, P3, and P4 (reference site), respectively (Table 2).
Table 2 . Concentrations of trace elements (Pb, Cr, Zn, Ni and Cd) in the digestive gland of Anomalocardia brasiliana (n=15) sampled at different sites of the Lagoa da Conceição. Site P1: Rio Vermelho; Site P2: Osni Ortiga; P3: Site Ponta das Almas; Site P4: LIC; Site Pc: Reference site (Pontal Daniela).
|
|
Trace metal (mg.g-1) |
Coliforms (N 100 ml-1) |
||||
|
|
Pb |
Cr |
Zn |
Ni |
Cd |
|
|
P1 RIO VERMELHO |
0.02 |
1.28 |
0.20 |
0.77 |
0.04 |
≥2.419 |
|
P2 OSNI ORTIGA |
0.09 |
0.68 |
0.38 |
0.44 |
0.09 |
1.733 |
|
P3 PONTA ALMAS |
0.62 |
2.65 |
8.21 |
0.80 |
0.10 |
961 |
|
P4 PRAIA DO LIC |
0.77 |
0.55 |
9.36 |
0.41 |
0.10 |
112 |
|
PC PONTAL DANIELA |
0.16 |
0.67 |
10.09 |
0.50 |
0.10 |
18.3 |
Site P1: Rio Vermelho; Site P2: Osni Ortiga; P3: Site Ponta das Almas; Site P4: LIC; Site Pc: Reference site (Pontal Daniela).
Biomarkers of oxidative stress
Figure 2 shows the OS biomarkers in the digestive gland of A. brasiliana samples from different sites of the Lagoa da Conceição (content of TBARS, enzymatic activity of SOD, CAT, GPx, GR and GST; as well as TG, GSH and GSSG levels). The levels of TBARS found in the digestive organ of A. brasiliana collected in the reference site (Pc) and in the contaminated sites P1, P2, P3 and P4 were: 57.65±5.98; 870.6±39.1; 534.5±40.3; 160.8±12.7 and 72.16±7.31 nmol TBARS g-1, respectively. Excepting for sites P3 and P4, high TBARS contents were found in the digestive organ of A. brasiliana collected in the other contaminated sites P2 and P3 compared to the reference site Pc (p<0.0001 when compared to Pc; Figure 2). In addition, the digestive organ of A. brasiliana collected at P1 presented the higher levels of TBARS when compared to all other contaminated sites (p<0.001), while their levels at P2 were higher than the TBARS content from samples collected at P3 and P4 sites (p<0.001).
The mean GSH concentration found in the digestive gland of A. brasiliana from the reference site Pc (1.96±0.10 mmol g-1) was higher (p<0.0001) than those from the polluted sites (P1: 0.90±0.14; P2: 1.00±0.07; P3: 1.18±0.08; P4: 1.55±0.14 mmol g-1). Moreover, GSH concentration in samples collected at P3 was higher and at P4 was lower than those obtained in samples from P2 (p<0.0001). As expected, an inverse correlation between GSH and GSSG concentrations was evident in the digestive gland sampled in the contaminated sites of the Lagoa da Conceição (Pc: 0.58±0.05; P1: 2.26±0.31; P2: 0.46±0.02; P3: 1.01±0.26; P4: 1.96±0.13 mmol g-1)(Figure 2). The digestive glands of animals sampled at the reference site showed the lowest contents of oxidized glutathione compared to the contaminated sites P1 and P4 (p<0.0001), while sites P2 and P3 GSSG concentrations similar to those obtained at the reference site. Total glutathione (TG) contents found in clams collected at the reference site (2.44±0.11 mmol g-1) were similar to those found in site P1 (3.16±0.43 mmol g-1), site P2 (1.46±0.06 mmol g-1) and site P3 (2.19±0.32 mmol g-1), while higher contents were found in site P4 (3.51±0.22 mmol g-1; p<0.0001). On the other hand, GSSG content from digestive glands of animals sampled at P2 were lower than that observed at P1 (p<0.001). Significant difference in GSSG content was also observed after comparing data from P3 and P4 samples versus P2 (P<0.001)(Figure 2).
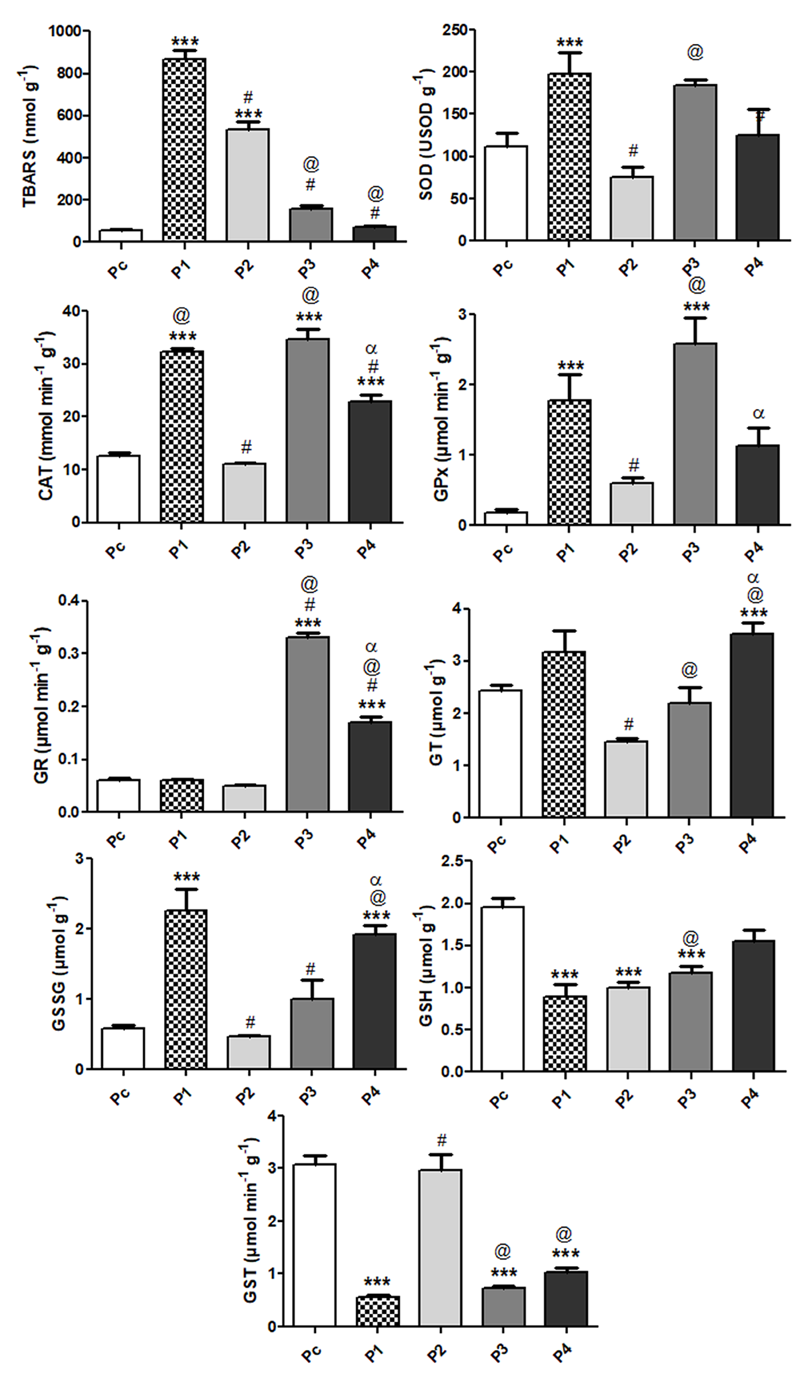
Figure 2. OS biomarkers in the digestive gland of Anomalocardia brasiliana (n=15) sampled at different sites of the Lagoa da Conceição: contents of TBARS; reduced glutathione (GSH); total glutathione (TG); oxidized glutathione (GSSG); activity of superoxide dismutase (SOD); catalase (CAT); glutathione peroxidase (GPx); glutathione reductase (GR) and glutathione S-transferase (GST). Data are reported as means ± SEM. Statistically significant differences, as determined by one-way ANOVA followed by Tukey-Kramer multiple comparison test are indicated: ***P<0.0001 compared with Pc (reference site); #P<0.001 compared with P1; αP<0.001 compared with P2; αP<0.001 compared with P3. Sites P1: Rio Vermelho; P2: Osni Ortiga; P3: Ponta das Almas; P4: Praia do LIC; PC: Pontal da Daniela (Reference Site).
SOD activity showed higher values at the contaminated site P1 (198.2±25.5 U SOD g-1) when compared to the reference site (111.6±6.2 U SOD g-1). The activity of the enzyme in the samples collects at site P2 (74.9±13.0 U SOD g-1) was similar to that observed in the digestive organ of A. brasiliana collected at Pc, but lower than the SOD activity from P1 samples (p<0.001). Moreover, significant difference in SOD activity was obtained between P2 and P3 samples (75.3±13.0 and 184.9±13.0 U SOD g-1, respectively; p<0.001)(Figure 2).
Catalase activity showed variable responses at the different contaminated sites when compared to the reference site (12.6±0.7 μmol min-1 g-1). Sites P1, P3 and P4 showed higher catalase activity than Pc (32.3±3.6; 64.8±1.8 and 22.9±1.2 μmol min-1 g-1, respectively; p<0.001), while site P2 showed the lowest activity (11.2±0.2 μmol min-1 g-1; p<0.001) among the contaminated sites, which was not significantly different from the reference site. Although the SOD activity in the digestive organ of A. brasiliana collected at P4 was higher than that obtained in samples collected at reference site (P<0.0001), it was lower than that observed at P1 and P3 (p<0.001)(Figure 2).
Glutathione peroxidase activity at the reference site was much lower (0.19±0.04 µmol min-1 g-1) than that found in the contaminated sites P1 and P3 (1.78±0.37; 2.59±0.37 µmol min-1 g-1, p<0.001). Site P3 showed the highest value (2.59±0.37 µmol min-1 g-1; p<0.0001), almost 14 times higher compared to the reference site, while sites P1 and P4 showed roughly ten and six fold higher GPx activity compared to the reference site (1.78±0.04 and 1.13±0.26 mmol min-1g-1, respectively) (Figure 2). Site P2 showed the lowest value (0.60±0.08 µmol min-1g-1) among the contaminated sites, even so the value was ca. 3 times higher compared to the reference site. Glutathione reductase activity found at sites P1 and P2 (0.06±0.01 µmol min-1g-1 and 0.04±0.01 µmol min-1g-1, respectively) were similar to that of the reference site (0.06±0.01 µmol min-1g.1; Figure 2). On the other hand, sites P3 and P4 showed higher GR activity (0.32±0.01 µmol min-1g-1 and 0.16±001 µmol min-1 g.1) compared to the reference site (p<0.0001) and to P1 and P2 sites (p<0.001)(Figure 2).
The enzymatic activity of GST found in the digestive organ of A. brasiliana collected in the reference site and in the contaminated sites P1, P2, P3 and P4 were respectively: 3.09±0.17; 0.56±0.04; 2.97±0.30; 0.73±0.050 and 1.03±0.08 µmol min-1 g-1. The GST activity observed in samples collected at reference site and at site P2 presented very similar values. The activity of this enzyme in the digestive organ of A. brasiliana collected at P1, P3 and P4 contaminated sites was lower than those showed at Pc or P2 sites (p<0.0001; p<0.001, respectively)(Figure 2).
Physicochemical parameters
Very high contents of total and fecal coliforms were detected in three contaminated sites, especially in P1 and P2, and also in P3, when compared to the reference site Pc. Sites P1 and P2 revealed values that were well above the limit allowed by the local environmental agency (FATMA) and Brazilian legislation [51]. These results clearly indicate that there is a strong anthropogenic environmental stress, considering that all the contaminated sites are under an accelerated and often illegal urban occupation, including the use of frequent clandestine (in natura) sewage discharges directly to this lacustrine system [2, 52, 53], as well as in other aquatic environments existent in the Santa Catarina island [1].
The clams were collected by purpose after the summer season (middle April of 2002, beginning of Fall) to take into account the seasonal anthropogenic impact on this lacustrine system. Corroborating the results obtained in present study, the time of sampling occurred just after the high tourist influx during high summer (January-February), when such transient population often promotes temporary increases of 3-5 fold of the normal population of the Santa Catarina Island. Accordingly, near the site P4 is very common to visualize algae bloom associated with a local and predominant bad smell during spring and summer [53]. Such conditions are probably consequence of augmented sewage discharges together with the particularly local low water circulation near site P4, with the consequent oxygen deficit, which coincided with the lowest dissolved oxygen concentrations found at this site in the present study, and also with the high local H2S production [53]. In this regard, the evaluation of trace metal contamination in surface sediments of the Guanabara Bay (Brazil) showed the importance of hydrogen sulfide, which are trapped in the bottom thereby maintaining these metals less oxygenated and less soluble for contamination of the benthic fauna [54].
In contrast, P2 showed the second highest coliform content in the water, thus indicating a contaminated site, but also showed the lowest value for the chemical oxygen demand, thus conversely suggesting a relatively low contaminated site. On the other hand, total and coliform values obtained for the reference site as well as considering the physicochemical parameters obtained, ensure its use as a control site in the present study. Accordingly, the measured biochemical oxygen demand (BOD) was approximately half at the reference site compared to the contaminated sites.
Coliforms, trace metals, Pinus resins and lipoperoxidation levels
High total and fecal coliform contents were detected in all contaminated sites compared to the reference site, indicating a strong anthropogenic influence on these areas, most of them probably related to urban sewage discharges. Site P2 showed high TBARS contents together with high coliform contents and low QOD in the water, together with low trace metal contents in the digestive organ of A. brasiliana. Coherently, site P4 showed the lowest coliform and TBARS contents combined with a relatively low trace metal contents. These results suggest that TBARS concentrations in the digestive gland of A. brasiliana could be more associated with the coliform contents in the surrounding water rather than with the trace metal contents per se. Accordingly, not all heavy metals induce OS in different organisms, as was found in the digestive gland of Mytillus galloprovincialis, showing that only copper was able to enhance lipoperoxidation while cadmium and zinc did not [26].
Likewise, site P1 was the solely site here examined that was heavily contaminated by several acid resin components such as primaric, adiabetic and dehydroabietic acids coming from a vicinal forest of Pinus elliotti, which drains into the lagoon and are cumulative and highly toxic to aquatic organisms [52]. On the other hand, elevated lipoperoxidation processes were directly associated with trace metal contents in other mussels sampled in polluted areas [26, 31]. Similarly, a high correlation between trace metal and TBARS concentrations was also detected by our research group in the digestive gland of the local mangrove mussel Mytella guyanensis[19]. Also similar to the present findings, enhanced OS biomarkers were detected after 1-3 weeks in the freshwater clam Unio tumidus transplanted to contaminated sites was well correlated with the worsening of water quality [55],
However, low TBARS contents were found in the mollusks Chamaelea gallina and Cassostrea gigas sampled in polluted areas of the Spanish coast [56], and the authors suggested that the lowering of such lipoperoxidation could be a compensation of the elevation of the antioxidant defenses. This interpretation coincides with ours, excepting for the relatively low GST activity detected in site P1, probably as a consequence of the inhibitory effect produced by the resin contamination derived from Pinus elliotti. Accordingly, Adler-Ivanbrook and Breslin [30] also found elevated levels of Cu, Cr and As in the blue mussel Mytilus edulis exposed to wood treated with these heavy metals.
Thus, the apparent association between the water contents of fecal coliforms and the different biomarkers of OS found in the digestive gland of the clam including the TBARS contents, seems not to be sufficient to explain the antioxidant responses here obtained. Such antioxidant responses may be attributable to the trace metals found in the digestive gland, probably reflecting the concentrations found in the sediment and water of this lacustrine system [5].
Very similar profiles were found between the trace metal concentrations of the digestive gland of A. brasiliana and those found in the sediments in the same lacustrine system [3, 5], probably due to the bioaccumulation process associated with bivalve filter-feeding [7]. In this regard, many bivalve species can accumulate trace metals in concentrations directly proportional to that of the environment, and the main site of trace metal metabolism and accumulation is the digestive gland [28, 57-59]. In the present study the digestive gland of A. brasiliana also showed a similar profile, exhibiting roughly 20 to 100 times lower trace metal concentrations than those found in the sediments [3, 5], although still higher when compared to the concentrations found in the surrounding water. As mentioned in the related literature, such heavy metal concentrations vary according to seasonal and other environmental changes such as the intensity of rainfalls [28]. In addition, antioxidants in mollusks also respond to seasonal and ontogenetic variations, irrespective to the presence of pollutants present in the environment [14, 39, 60-62]. Therefore, such natural contributions must be taken into consideration when focusing environmental contamination in monitoring programs.
When the concentrations of Pb, Ni and Cd found in the digestive gland of A. brasiliana were compared to those found in the digestive gland of the mangrove mussel Mytella guyanensis, they showed very similar values [4, 19]. Accordingly, other related study on the bivalves Chione sp., C. palmula and C. corteziensis from Mexico [63] revealed concentrations of Cd, Ni, Cr, Zn and Pb close to those found in the digestive gland of A. brasiliana (this study). Most of these concentrations were within the values recommended by FDA for marine animals (0.6 – 0.9 mg.g-1 for Cd and 0.4-0.6 mg.g-1 for Pb). However, for Cr concentrations the values found in A. brasiliana sampled at all sites examined were above (range 0.55 – 2.65 mg.g-1) those allowed by the FDA (0.3 – 0.4 mg.g-1), as well as the Pb concentrations found in the sites P3 and P4 (0.62 and 0.77 mg.g-1, respectively), which were also above the limit recommended by the FDA.
The concentrations of Cd and Pb found in the digestive gland of Mytilus galloprovincialis caged for one month in contaminated sites of Gulf of Patras (Greece) were even higher [64]. Not surprisingly, they found similar trace metal concentration ratios between sediments and digestive gland for Zn and Cu, higher values for Hg (1-3 times), Pb (2-4 times), Cr (15-40 times), while in the present study these ratios for Zn and Pb were much higher (~40 and ~60 times, respectively). Surprisingly, Cd concentrations in the digestive organ of M. galloprovincialis were even higher than in the contaminated sediment [64], indicating a high trace metal bioaccumulation in this mussel species. The highest trace metal accumulated by the digestive organ of A. brasiliana was also Cd, but the values were roughly five fold lower than those found in the surrounding sediment [3, 5]. All these results reinforce that bivalve mollusks have a bioaccumulation capability related to their filter-feeding nutrition [21], which are directly proportional to levels existent in the environment [14, 28, 57-59].
Biomarkers of oxidative stress
Excepting in site P2, the SOD activity showed higher values in clams sampled at the contaminated sites. Similar results were obtained in Mytilus sp [65], in the oyster Saccostrea cucullata [66], and in the clam Tapes philippinarum exposed to aquatic contaminants [67]. Conversely, Mytella guyanensis did not show differences in the SOD activity for contaminated sites compared to a reference site [19], and similar finding was also obtained for Mytilus edulis [68] and Perna viridis [31]. At site P2 SOD activity, as well as the other antioxidant enzymes examined (see below) was not different from the reference site. However, this site revealed the second highest coliform content in the water and the lowest value for the chemical oxygen demand (360 mg/L). While the former value corroborates the category of a contaminated site, the latter value suggests a relatively low contamination. Thus, considering the site P2 and excepting for the relatively high fecal coliform contents found in the water, all the antioxidant enzymes here evaluated were downregulated, in accordance to the apparent low levels of environmental contamination of this site compared to the other contaminated sites.
Similarly, catalase activity showed generally significant higher activity in clams sampled at all the contaminated sites compared to the reference site, again with the exception of site P2, which showed a similar value of that found at the reference site. High CAT activity was also found by our research group in the digestive gland of the mangrove mussel Mytella guyanensis, also comparing contaminated sites with a reference site [19]. Similar responses were also obtained in Saccostrea cucullata [66], in Mytilus sp [65] and in Crassostrea rhizophora and Perna perna exposed to pollutants [69], while Cheung and collaborators [31] in Perna viridis and Matozzo and collaborators [67] in Tapes philippinarum did not detect such correlation.
In parallel to SOD and CAT activity, glutathione peroxidase activity in clams sampled at the reference site were much lower than those found at the contaminated sites, sites P1 and P4 showing GPx activity roughly ten fold higher than that of the reference site. This similar enzymatic profile is not surprisingly, because both antioxidant enzymes possess a very close functional convergence in vertebrates and invertebrates [70]. Accordingly, enhanced GPx activity was also found in other mollusk species exposed to aquatic contaminants [19, 31, 68]. However, the present results are not in agreement with authors that do not considered GPx as a good OS biomarker in mussels [71], contrasting with others who also have recommended GPx as an excellent biomarker of environmental contamination in the pearl oyster Pinctada martensii [72].
As occurred in the other antioxidant enzymes, excepting for the clams collected at site P2, glutathione reductase activity showed higher activity compared to those collected at the reference site. The main role of GR is to maintain constitutive high cellular GSH levels, which are important to cell homeostasis and to avoid OS derived from xenobiotic exposure [73]. Therefore, elevated GR activity suggests a compensatory response to recover the depletion of GSH [10]. Mytella guyanensis sampled in contaminated sites also revealed elevated GR activity [19], while Cheung and collaborators [31] did not find differences in GR activity in Perna viridis collected in polluted sites from Hong Kong.
GST activity showed the highest value at the reference site, while the digestive gland from clams collected at the different contaminated sites showed a priori an unexpected lower activity, again with the exception of clams sampled at site P2, which showed a mean value similar to that of the reference site. Mussels acutely exposed to contaminated areas usually show enhanced GST activity and/or expression [19, 31, 74]. However, unchanged GST activity was found in Unio tumidus [75], and in Perna perna and Crassostrea gigas [69] exposed to different pollutants. The apparent inhibition of GST activity detected in all the contaminated sites combined with the upregulation of all other antioxidant enzymes probably indicates that this clam is facing a severe and chronic oxidative insult at all the contaminated sites, excepting at P2 and at the reference site. Similar results on GST inhibition were already described in fish [48, 61, 76].
An inverse correlation between GSH and GSSG concentrations was evident in the digestive gland of A. brasiliana sampled at the contaminated sites of the Lagoa da Conceição. The mean GSH concentrations found in the digestive gland of A. brasiliana sampled at all the four contaminated sites were lower compared to the reference site, indicating that GSH is being depleted by such contaminants. Low GSH contents were also found in other mussel species exposed to contaminated areas [31], and in a mussel exposed to metal contaminants, which was inversely correlated with the lipid peroxidation values [24]. Likewise, the digestive gland of clams sampled at the reference site showed the lowest contents of oxidized glutathione (GSSG) compared to the contaminated sites, again excepting site P2, which exhibited a value very similar to that of the reference site. High GSSG contents were also fund by our research group in the digestive organ of M. guyanensis sampled in two polluted mangroves compared to a reference site [19]. Despite the relatively high TBARS and coliform contents found respectively in the digestive gland and in the surrounding water, and according to the responses obtained in all antioxidant enzymes, site P2 coherently revealed lower GSSG contents, suggesting that these clams were less affected by local contaminants compared to the other sites.
Total glutathione contents found in the digestive gland of A. brasiliana collected at the reference site were similar to those found in site P3, while higher contents were found in sites P1 and P4, and again a different profile was obtained for site P2, exhibiting lower TG values. The relatively high total glutathione contents found in the two contaminated sites may reflect the necessity of A. brasiliana to further synthetize this important antioxidant tripeptide to compensate the OS associated with local contamination. In accordance with such inference, the lower apparent investment in glutathione in clams collected at site P2 was well in line with the results obtained for the other OS biomarkers, which also showed minor changes related to this site when compared to the other contaminated sites.
In summary, the trace metal concentrations found in the digestive gland of A. brasiliana collected in contaminated sites showed a very similar profile with the concentrations formerly found in the sediments [3, 5], probably reflecting the bioaccumulation process associated with bivalve filter-feeding foraging process. Some of these metal concentrations (Pb and Cr) were higher than those allowed by the related legislation of different countries (Brazil, USA and Spain). Accordingly, the concentrations of total and fecal coliforms were elevated in the water collected at all contaminated sites compared to the reference site, while one site (P1) was particularly contaminated by acid resins drained from a Pinus forest. Similarly, the biomarkers of oxidative damage such as TBARS and GSSG concentrations were generally higher in the digestive gland of clams collected in contaminated sites, results that are in parallel to the response obtained for most of the antioxidant enzymes, which showed generally higher activity in the contaminated sites compared to the reference site. Also, excepting in one contaminated site (P2), which seems to be less affected by the different contaminants here examined, GST activity was lower in clams collected in all contaminated sites. This inhibition of GST together with the concomitant depletion of GSH suggests that A. brasiliana is gradually losing a full compensatory antioxidant compensation in the contaminated sites. However, as mentioned by Regoli and Giuliani [77] caution must be taken when making such inferences with OS biomarkers, because “in monitoring studies (…) antioxidants could represent a snapshot of cell activity at a given time, not an effective endpoint of environmental pollutants”.
Araujo, N.B., Contribuição ao estudo da qualidade da agua da bacia hidrografica do Rio Tavares : poluição organica Florianopolis-Santa Catarina., in Centro de Ciências humanas. 1993, Universidade Federal de Santa Catarina: Florianópolis. p. 126.
Logullo, R.T., Influência das condições sanitárias sobre a qualidade das águas utilizadas para à maricultura no Ribeirão da Ilha-Florianópolis, SC., in Environmental Engineering. 2005, Federal University of Santa Catarina: Florianópolis, SC, Brazil.
Garcia, A.A., Diagnóstico Ambiental da Lagoa da Conceição e do Canal da Barra Através de Indicadores Físico-Químicos dos Sedimentos de Fundo e dos indicadores Sócio-Ambientais., in Engenharia Ambiental. 1999, Universidade Federal de Santa Catarina. p. 298.
Masutti, M.B., O manguezal do Itacorubi como barreira biogeoquímica: estudo de caso, in Engenharia Ambiental. 1999, Federal University of Santa Catarina: Florianópolis. p. 2013.
da Silva, M.R., et al., Metal Contamination in Surface Sediments of Mangroves, Lagoons and Southern Bay in Florianopolis Island. Environmental Technology, 1996. 17(10): p. 1035-1046.
View ArticlePezzuto, P.R. and D.S.e. Souza, A pesca e o manejo do berbigão (Anomalocardia brasiliana) (Bivalvia: Veneridae) na Reserva Extrativista Marinha do Pirajubaé, SC, Brasil. Desenvolvimento e Meio Ambiente; v. 34 (2015)DO - 10.5380/dma.v34i0.39758, 2015.
Goldberg, E.D., The mussel watch — A first step in global marine monitoring. Marine Pollution Bulletin, 1975. 6(7): p. 111. 90271-4
View ArticleTirelli, N.C., Diagnóstico da qualidade da água e da carne das ostras da espécie Crassostrea gigas na Baía Sul da Ilha de Santa Catarina, in Engenharia de Aqüicultura. 2003: Florianópolis. p. 70.
Yao, Z. and P. Gao, Heavy metal research in lacustrine sediment: a review. Chinese Journal of Oceanology and Limnology, 2007. 25(4): p. 444-454.
View ArticleHalliwell, B. and J.M.C. Gutteridge, Free Radicals in Biology and Medicine. 2007: Oxford University Press. 888.
Sies, H., 1 - Oxidative Stress: Introductory Remarks, in Oxidative Stress. 1985, Academic Press: London. p. 1-8.
View ArticleWinston, G.W., Oxidants and antioxidants in aquatic animals. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 1991. 100(1-2): p. 173-176. 90148-M
View ArticleWhitacre, D.M., Reviews of Environmental Contamination and Toxicology - 2012, D.M. Whitacre, Editor. 2012, Springer: Amsterdam. p. 147.
Canesi, L., Pro-oxidant and antioxidant processes in aquatic invertebrates. Ann N Y Acad Sci, 2015. 1340: p. 1-7. PMid:25428611
View Article PubMed/NCBIKumar, N., K.K. Krishnani, and N.P. Singh, Oxidative and cellular stress as bioindicators for metal contamination in freshwater mollusk Lamellidens marginalis. Environ Sci Pollut Res Int, 2017. 24(19): p. 16137-16147. PMid:28537033
View Article PubMed/NCBILa Touche, Y.D. and M.C. Mix, Seasonal variations of arsenic and other trace elements in bay mussels (Mytilus edulis). Bulletin of Environmental Contamination and Toxicology, 1982. 29(6): p. 665-670. PMid:7159776
View Article PubMed/NCBILivingstone, D.R., et al., Oxyradical Production as a Pollution-Mediated Mechanism of Toxicity in the Common Mussel, Mytilus edulis L., and Other Molluscs. Functional Ecology, 1990. 4(3): p. 415-424.
View ArticleLivingstone, D.R., The fate of organic xenobiotics in aquatic ecosystems: quantitative and qualitative differences in biotransformation by invertebrates and fish. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 1998. 120(1): p. 43-49. 10008-9
View ArticleTorres, M.A., et al., Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Marine pollution bulletin, 2002. 44(9): p. 923-932. 00142-X
View ArticleValavanidis, A., et al., Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf, 2006. 64(2): p. 178-89. PMid:16406578
View Article PubMed/NCBIWang, S.L., et al., Heavy metal pollution in coastal areas of South China: a review. Mar Pollut Bull, 2013. 76(1-2): p. 7-15. PMid:24084375
View Article PubMed/NCBIMeng, F., et al., The assessment of environmental pollution along the coast of Beibu Gulf, northern South China Sea: an integrated biomarker approach in the clam Meretrix meretrix. Marine environmental research, 2013. 85: p. 64-75. PMid:23422511
View Article PubMed/NCBIPhillips, D.J.H., The use of biological indicator organisms to monitor trace metal pollution in marine and estuarine environments—a review. Environmental Pollution (1970), 1977. 13(4): p. 281-317. 90047-7
View ArticleViarengo, A., et al., Effects of heavy metals on lipid peroxidation in mussel tissues. Marine Environmental Research, 1988. 24(1-4): p. 354. 90338-8
View ArticleViarengo, A., Heavy metals in marine invertebrates: mechanisms of regulation and toxicity at the cellular level. CRC Reviews in Aquatic Sciences. 1989, Boca Raton, Florida: CRC Press. 295-317.
Viarengo, A., et al., Heavy metal effects on lipid peroxidation in the tissues of mytilus gallopro vincialis lam. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 1990. 97(1): p. 37-42. 90168-9
View ArticleCoimbra, J. and S. Carraça, Accumulation of Fe, Zn, Cu, and Cd during the different stages of the reproductive cycle in Mytilus edulis. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 1990. 95(2): p. 265-270. 90115-P
View ArticleWalsh Andrew, R. and J. O'Halloran, Accumulation of chromium by a population of mussels (Mytilus edulis (L.)) exposed to leather tannery effluent. Environmental Toxicology and Chemistry, 2009. 17(7): p. 1429-1438.
Regoli, F. and G. Principato, Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquatic Toxicology, 1995. 31(2): p. 143-164. 00064-W
View ArticleAdler‐Ivanbrook, L. and V.T. Breslin, Accumulation of copper, chromium, and arsenic in blue mussels (Mytilus edulis) from laboratory and field exposures to wood treated with chromated copper arsenate type C. Environmental Toxicology and Chemistry, 1999. 18(2): p. 9.
View ArticleCheung, C.C., et al., Relationships between tissue concentrations of chlorinated hydrocarbons (polychlorinated biphenyls and chlorinated pesticides) and antioxidative responses of marine mussels, Perna viridis. Mar Pollut Bull, 2002. 45(1-12): p. 181-91.
Géret, F., et al., Influence of metal exposure on metallothionein synthesis and lipid peroxidation in two bivalve mollusks: the oyster (Crassostrea gigas) and the mussel (Mytilus edulis). Aquatic Living Resources, 2002. 15(1): p. 61-66. 01147-0
View ArticleLiang, L.N., et al., Evaluation of mollusks as biomonitors to investigate heavy metal contaminations along the Chinese Bohai Sea. Sci Total Environ, 2004. 324(1-3): p. 105-13. PMid:15081700
View Article PubMed/NCBIWanick, R.C., et al., Use of the digestive gland of the oyster Crassostrea rhizophorae (Guilding, 1828) as a bioindicator of Zn, Cd and Cu contamination in estuarine sediments (south-east Brazil). Chemistry and Ecology, 2012. 28(2): p. 103-111.
View ArticleAvery, E.L., R.H. Dunstan, and J.A. Nell, The use of lipid metabolic profiling to assess the biological impact of marine sewage pollution. Arch Environ Contam Toxicol, 1998. 35(2): p. 229-35. PMid:9680515
View Article PubMed/NCBIK. Isamah, G., S. Asagba, and H. Coker, Comparative Evaluation of the Levels of Some Antioxidant Enzymes and Lipid Peroxidation in Different Fish Species in Two Rivers in the Western Niger Delta. Vol. 65. 2000. 351-356.
Oakes, K.D., M.E. McMaster, and G.J. Van Der Kraak, Oxidative stress responses in longnose sucker (Catostomus catostomus) exposed to pulp and paper mill and municipal sewage effluents. Aquatic toxicology (Amsterdam, Netherlands), 2004. 67(3): p. 255-271. PMid:15063075
View Article PubMed/NCBIGreenberg, A.E., L.S. Clesceri, and A.D. Eaton, Standard methods for the examination of water and wastewater, A.P.H.A.A.W.W.A. Water and E. Federation., Editors. 1992, American Public Health Association.: Washington, DC.
Wilhelm-Filho, D., C.G. Fraga, and A. Boveris, Seasonal and ontogenetic changes modulate oxygen consumption and antioxidant defenses in the cutlassfish Trichiurus lepturus (Pisces, Trichiuridae). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2017. 199: p. 90-97. PMid:28347745
View Article PubMed/NCBIManly, R., et al., Trace metal concentrations in Mytilus edulis L. from the Laguna San Rafael, Southern Chile. Marine Pollution Bulletin, 1996. 32(5): p. 444-448. 00006-9
View ArticleFlohe, L. and F. Otting, Superoxide dismutase assays. Methods Enzymol, 1984. 105: p. 93-104. 05013-8
View ArticleAebi, H., Catalase in vitro. Methods Enzymol, 1984. 105: p. 121-6. 05016-3
View ArticleFlohe, L. and W.A. Gunzler, Assays of glutathione peroxidase. Methods Enzymol, 1984. 105: p. 114-21. 05015-1
View ArticleCarlberg, I. and B. Mannervik, Glutathione reductase. Methods Enzymol, 1985. 113: p. 484-90. 13062-4
View ArticleHabig, W.H., M.J. Pabst, and W.B. Jakoby, Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem, 1974. 249(22): p. 7130-9. PMid:4436300
PubMed/NCBIOhkawa, H., N. Ohishi, and K. Yagi, Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 1979. 95(2): p. 351-358. 90738-3
View ArticleBird, R.P. and H.H. Draper, Comparative studies on different methods of malonaldehyde determination. Methods Enzymol, 1984. 105: p. 299-305. 05038-2
View ArticleWilhelm Filho, D., et al., Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture, 2001. 203(1-2): p. 149-158. 00599-3
View ArticleAnderson, M.E., Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol, 1985. 113: p. 548-55. 13073-9
View ArticleTietze, F., Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Analytical Biochemistry, 1969. 27(3): p. 502-522. 90064-5
View ArticleCONAMA, RESOLUÇÃO CONAMA Nº 20, C.N.d.M.A. CONAMA, Editor. 1986: Brazil.
Soares, C.H.L., Resinas ácidas-biodegradação e toxicidade 2001, 4ª Reunión anual de SETAC Latino América/Society of Environmental Toxicology and Chemistry: Buenos Aires.
Pereira, N.C., Diagnóstico ambiental da Lagoa da Conceição utilizando o berbigão Anomalocardia brasiliana (Gmelin, 1791) como bioindicador de poluição aquática, in Engenharia Ambiental. 2003, Universidade Federal de Santa Catarina: Florianópolis. p. 91. PMid:12770520
PubMed/NCBIPerin, G., et al., A five-year study on the heavy-metal pollution of Guanabara Bay sediments (Rio de Janeiro, Brazil) and evaluation of the metal bioavailability by means of geochemical speciation. Water Research, 1997. 31(12): p. 3017-3028. 00171-1
View ArticleCharissou, A.M., C. Cossu-Leguille, and P. Vasseur, Relationship between two oxidative stress biomarkers, malondialdehyde and 8-oxo-7,8-dihydro-2'-deoxyguanosine, in the freshwater bivalve Unio tumidus. Sci Total Environ, 2004. 322(1-3): p. 109-22. PMid:15081742
View Article PubMed/NCBIRodríguez-Ariza, A., et al., Biochemical and genetic indices of marine pollution in Spanish littoral. Science of The Total Environment, 1993. 134: p. 109-116. 80009-9
View ArticlePhillips, D.J.H. and P.S. Rainbow, Strategies of trace metal sequestration in aquatic organisms. Marine Environmental Research, 1989. 28(1): p. 207-210. 90226-2
View ArticleRainbow, P.S., Heavy metals in marine invertebrates, in Rainbow Heavy metals in the Marine Environment, R.W. Furness and P.S. Rainbow, Editors. 1990: Boca Raton. p. 67-79.
Roesijadi, G. and W. Robinson, Metal regulation in aquatic animals: mechanisms of uptake, accumulation and release. Aquatic toxicology, 1994. 102: p. 125-133.
Sheehan, D. and A. Power, Effects of seasonality on xenobiotic and antioxidant defence mechanisms of bivalve molluscs. Comparative biochemistry and physiology. Part C, Pharmacology, toxicology & endocrinology, 1999. 123(3): p. 193-199. 00033-X
View ArticleWilhelm Filho, D., et al., Influence of season and pollution on the antioxidant defenses of the cichlid fish acará (Geophagus brasiliensis). Brazilian Journal of Medical and Biological Research, 2001. 34(6): p. 719-726.
View ArticleShaw, J.P., et al., Seasonal variation in cytochrome P450 immunopositive protein levels, lipid peroxidation and genetic toxicity in digestive gland of the mussel Mytilus edulis. Aquat Toxicol, 2004. 67(4): p. 325-36. PMid:15084409
View Article PubMed/NCBIMiladinovic, T., M.G. Nashed, and G. Singh, Overview of Glutamatergic Dysregulation in Central Pathologies. Biomolecules, 2015. 5(4): p. 3112-41. PMid:26569330 PMCid:PMC4693272
View Article PubMed/NCBIKalpaxis, D.L., et al., Biomonitoring of Gulf of Patras, N. Peloponnesus, Greece. Application of a biomarker suite including evaluation of translation efficiency in Mytilus galloprovincialis cells. Environ Res, 2004. 94(2): p. 211-20. 00048-3
View ArticlePorte, C., et al., Responses of mixed-function oxygenase and antioxidase enzyme system of Mytilus sp. to organic pollution. Comp Biochem Physiol C, 1991. 100(1-2): p. 183-6. 90150-R
View ArticleNiyogi, S., et al., Antioxidant enzymes in brackishwater oyster, Saccostrea cucullata as potential biomarkers of polyaromatic hydrocarbon pollution in Hooghly Estuary (India): seasonality and its consequences. Sci Total Environ, 2001. 281(1-3): p. 237-46. 00850-6
View ArticleMatozzo, V., L. Ballarin, and M.G. Marin, Exposure of the clam Tapes philippinarum to 4-nonylphenol: changes in anti-oxidant enzyme activities and re-burrowing capability. Mar Pollut Bull, 2004. 48(5-6): p. 563-71. PMid:14980472
View Article PubMed/NCBISolé, M., et al., Effects of the "Aegean Sea" oil spill on biotransformation enzymes, oxidative stress and DNA-adducts in digestive gland of the mussel (Mytilus edulus L.). Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 1996. 113(2): p. 257-265. 02095-0
View ArticleAlves de Almeida, E., et al., Oxidative stress in Perna perna and other bivalves as indicators of environmental stress in the Brazilian marine environment: antioxidants, lipid peroxidation and DNA damage. Comp Biochem Physiol A Mol Integr Physiol, 2007. 146(4): p. 588-600. PMid:16626983
View Article PubMed/NCBIWilhelm Filho, D., et al., Comparative antioxidant defenses in vertebrates—emphasis on fish and mammals. Trends Comp. Biochem. Physiol, 2000. 7: p. 33-45.
Marek, P., et al., Glutathione‐dependent detoxifying enzymes in rainbow trout liver: Search for specific biochemical markers of chemical stress. Environmental Toxicology and Chemistry, 1997. 16(7): p. 1417-1421.
View ArticleXiang, N., et al., Dynamic responses of antioxidant enzymes in pearl oyster Pinctada martensii exposed to di(2-ethylhexyl) phthalate (DEHP). Environ Toxicol Pharmacol, 2017. 54: p. 184-190. PMid:28763719
View Article PubMed/NCBIKretzschmar, M. and W. Klinger, The hepatic glutathione system--influences of xenobiotics. Experimental pathology, 1990. 38(3): p. 145-164. 80201-X
View ArticleFitzpatrick, P.J., et al., Assessment of a glutathione S-transferase and related proteins in the gill and digestive gland of Mytilus edulis (L.), as potential organic pollution biomarkers. Biomarkers, 1997. 2(1): p. 51-56. PMid:23899155
View Article PubMed/NCBIPetushok, N., et al., Comparative study of the xenobiotic metabolising system in the digestive gland of the bivalve molluscs in different aquatic ecosystems and in aquaria experiments. Aquat Toxicol, 2002. 61(1-2): p. 65-72. 00030-9
View ArticleGallagher, E.P., T.S. Gross, and K.M. Sheehy, Decreased glutathione S-transferase expression and activity and altered sex steroids in Lake Apopka brown bullheads (Ameiurus nebulosus). Aquat Toxicol, 2001. 55(3-4): p. 223-37. 00158-8
View ArticleRegoli, F. and M.E. Giuliani, Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res, 2014. 93: p. 106-17. PMid:23942183
View Article PubMed/NCBI