J. Wang
Email: jtwang@sdau.edu.cn
Tel: +86 538 8242593 ext 8107 Fax: +86 538 8241419.
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 1
Page No: 250-256
J. Wang
Email: jtwang@sdau.edu.cn
Tel: +86 538 8242593 ext 8107 Fax: +86 538 8241419.
Zhen Wang a, Haojun Han a, Jiting Wang *, Dongyan Guan, Huiwen Sun, Yang Li , Xiang Li , Tingting Fang
Shandong Provincial Key Lab. of Animal Biotechnology and Disease Control and Prevention; Lab of Aquatic Animal Nutrition & Environmental Health; Shandong Agricultural University, 61 Daizong Street, Taian City, Shandong Province, 271018, China.
a Both authors contribute equally to this work.
Zhen Wang, Haojun Han, Jiting Wang , Dongyan Guan, Huiwen Sun, Yang Li , Xiang Li , Tingting Fang , The effects of different carbon sources on growth, tyrosine kinase activity and gene expression of insulin receptor 1 in GIFT tilapia (2022) Journal of Aquaculture, Fisheries & Fish Science 4(1) : 257-266
A feeding trial was conducted to evaluate the effects of different sugar sources on growth performance, serum biochemistry, immunity, insulin receptor 1 and tyrosine kinase activity of GIFT tilapia. Four hundred and eighty fish with specifications of 31.70±0.68 g were selected and placed in 16 aquariums. The experiment was divided into four treatment with four replicates of thirty fish per tank. After 50 days of feeding, the results were as follows: From the indexes of final average weight, weight gain rate and specific growth rate, the growth performance was sucrose, glucose, starch and dextrin group in turn, and the sucrose group performed best (P<0.05). The activcities of acid phosphotase, alkaline phosphotase, superoxide dismutase and lysozyme in sucrose group were significantly higher than that of the other three groups (P<0.05). The serum glucose level of sucrose group and glucose group is significantly higher than that of dextrin group and starch group (P < 0.05). In terms of liver glycogen level, the content of sucrose group was significantly higher than that of dextrin group and starch group (P < 0.05). In terms of muscle glycogen level, the content of sucrose group was the highest and that of starch group was the lowest. The tyrosine kinase activity of glucose group was the highest and significantly different from dextrin group (P< 0.05). The tyrosine kinase activity in muscle was always higher than that in liver (P < 0.05). The IR-1 mRNA in starch group and dextrin group was significantly higher than that in sucrose group. Different sugar source diets had a significant effect on the expression of IR-1 mRNA in the muscle of tilapia. In conclusion, compared with dextrin and starch, sucrose and glucose can be better utilized by GIFT tilapia and show good growth performance.
Key words: GIFT tilapia; Carbohydrates; Growth; Immunity; Insulin Receptor 1; Tyrosine Kinase
Carbohydrate is widely distributed in nature and is a low-cost energy material. Fish and terrestrial animals have many different characteristics in the demand and utilization of carbohydrates in feed. Fish, which are considered to have a congenital "diabetes constitution" (Wilson, 1994), have a low ability to use sugar. If the sugar level in the feed exceeds a certain limit, it will cause low disease resistance, slow growth and high mortality, form fatty liver, and further lead to liver damage. Improving the utilization efficiency of feed sugar and reducing the energy role of feed protein, so as to reduce the feed cost, is an important topic in the field of fish nutrition research. The saving effect of sugar on protein refers to that containing an appropriate amount of sugar in feed can reduce the decomposition and energy supply of feed protein, so as to improve the utilization of protein and save feed protein (Helland and Grisdale, 1998;Lee and Lee,2004). Feed sugar can not only reduce the waste of feed protein and feed cost, but also reduce the pollution of protein metabolites to aquaculture water. However, excessive carbohydrates will reduce the specific growth rate and weight gain rate of fish (Hemre et al., 2002), cause the continuous increase of glucose content in plasma (Wilson, 1994), lead to a large amount of fat accumulation, and then lead to fatty liver disease (Lie et al., 1988; Zhang et al., 2004), and reduce the immune ability of fish (Maule et al., 1989). In order to improve the utilization ability of fish to sugar and improve the efficiency of sugar saving protein, the most important thing is to deeply study the mechanism of sugar metabolism, so as to put forward technical measures to solve the problem of efficient utilization in theory.
The low utilization ability of sugars in fish is not only related to the species of fish, but also related to the differences utilization of different kinds of sugars (Christiansen et al, 1987). Different kinds of carbohydrates will not only affect the utilization of feed protein and lipid, but also affect the growth and health of fish. At present, in the research of fish nutrition, the nutritional effect and mechanism of different sugar sources on fish have become a hot spot (Enes et al, 2008). To explore the optimal addition types of sugars to different fish, the previous research results can be divided into the following aspects. Different kinds of fish have different utilization levels of the same kind of sugars (Ren, 2012), different sugar sources have different effects on the growth and metabolism of the same kind of fish (Dong et al., 2016), the utilization capacity of fish for macromolecular sugars is lower than that of small molecules sugars (Mohanta et al., 2007), and the utilization capacity of fish for small molecules sugars is lower than that of macromolecular sugars (Shiau et al., 1997), the ability of carnivorous fish to utilize sugar is lower than that of omnivorous and herbivorous fish (Furuichi and Yone, 1980), etc. The related research of different sugar sources on the growth and metabolism of fish has made a breakthrough, but there are few related studies on the effects of monosaccharides, disaccharides and polysaccharides on the growth, glucose metabolism enzymes and immune function of tilapia, and the practical application is insufficient. Moreover, the research direction has been extended to the study of the activities of key enzymes involved in the process of glucose and lipid metabolism in fish and the differences of their mRNA levels, as well as the regulatory effects of different carbohydrates on the expression of these genes. GIFT tilapia (Oreochromis niloticus) is one of the most important commercial freshwater fish worldwide due to high growth, desirable taste and high market value. Therefore, the purpose of this study was to explore the effects of glucose, sucrose, dextrin and starch on the breeding effect, whole fish body composition and immune function of GIFT tilapia, and to study the gene expression of insulin receptor-1 and tyrosine kinase activity in liver and muscle, so as to understand the nutritional effect and mechanism of different sugar sources on fish. Optimize the preparation of feed sugar source and protein source of tilapia, reduce the feed processing cost, further reduce the pollution of aquaculture water, and then realize the sustainable and healthy development of aquaculture.
2.1. Experimental design and diets
Four isonitrogenous and isoenergetic diets with 28% different carbohydrates were designed. Before preparing the feed, all the raw materials were crushed and passed through a 60-mesh sieve. All crushed feed materials were mixed evenly according to the feed formula in Table 1, and then corn oil was added. The tiny oil particles were rubbed by hand, and finally distilled water was added to convert the powder feed form into a hard mass. The wet mash was extruded into a 2-mm diameter particle strip by using a small-sized flat die pelletiser, dried naturally, and stored at 4℃.
Table 1. Formulation and proximate chemical composition of test diets (%)
|
Ingredient |
Glucose |
Sucrose |
Dextrin |
Starch |
|
Fish meal (CP 65.5%) |
30.00 |
30.00 |
30.00 |
30.00 |
|
Casein (CP 88.7%) |
10.00 |
10.00 |
10.00 |
10.00 |
|
Glucose |
28.00 |
0.00 |
0.00 |
0.00 |
|
Sucrose |
0.00 |
28.00 |
0.00 |
0.00 |
|
Dextrin |
0.00 |
0.00 |
28.00 |
0.00 |
|
α-Starch |
0.00 |
0.00 |
0.00 |
28.00 |
|
CMC |
24.70 |
24.70 |
24.70 |
24.70 |
|
Corn oil |
6.50 |
6.50 |
6.50 |
6.50 |
|
Vitamin premix 1 |
0.20 |
0.20 |
0.20 |
0.20 |
|
Mineral premix 2 |
0.20 |
0.20 |
0.20 |
0.20 |
|
Choline chloride (50%) |
0.40 |
0.40 |
0.40 |
0.40 |
|
Total |
100.00 |
100.00 |
100.00 |
100.00 |
|
Proximate chemical composition |
||||
|
Crude protein (%) |
28.52 |
28.52 |
28.52 |
28.52 |
|
Crude lipid (%) |
8.26 |
8.26 |
8.26 |
8.26 |
|
Gross energy (MJ/kg) |
15.98 |
16.27 |
16.27 |
16.38 |
|
Total phosphorous (%) |
1.02 |
1.02 |
1.02 |
1.02 |
1 Vitamin premix (mg kg-1 diet): retinol acetate 30 mg; cholecalciferol 5 mg; alpha-tocopherol 60 mg; ascorbic acid 600 mg; vitamin K3 7 mg; thiamin 20 mg; riboflavin 20 mg; pyridoxine HCL 12 mg; vitamin B12 0.05 mg; inositol 100mg; pantothenic acid 50 mg; niacin acid 35 mg; folic acid 8 mg; biotin 0.06 mg.
2 Mineral premix (mg or g/kg diet): KI (1%) 60 mg; CoCl2·6H2O (1%) 7 mg; CuSO4·5H2O 20 mg; FeSO4·H2O 300 mg; ZnSO4·H2O 200 mg; MnSO4·H2O 60 mg; Na2SeO3·5H2O (1%) 60 mg; MgSO4·7H2O 2600 mg.
2.2. Fish and growth experiment
The experimental tilapias were purchased from a local fry farm. Before the formal trial, the fish were domesticated in the controlled water circulation system for two weeks and fed with basic diet. At the begining of feeding trial, four hundred and eighty fishes with good health condition were randomly allocated into sixteen water-circulated tanks, and thirty fish per tank. In this feeding experiment of sixty days, food was supplied according to fish requirements, and the uneaten feed was gathered after every meal by using plastic nets, dried and weighed. The fish were fed three times a day. Water quality parameters included temperature 24.5 ± 2.5 ℃, pH 7.3 ± 0.3, dissolved oxygen 5.8 ± 0.4 mg/L, ammonia-N less than 0.05 mg/L, and nitrite-N less than 0.03 mg/l. After the feeding experiment, the fish were fasted for 24 hours, the total weight of each aquarium was weighed, the feed intake of each tank was recorded, and the growth performance index of each group of fish was calculated.
2.3 Sample collection and chemical analysis
At the end of the feeding experiment, five fish were randomly selected from each aquarium and frozen (-20°C) for whole fish body composition analysis. Another five fish from each aquarium were selected for serum biochemical index analysis. Blood was collected from the caudal vein of fish, and then centrifuged (4000 g at 4°C for 10 mins). The separated serum was frozen at -80°C for further analysis. Another five fish each tank were anesthetised and then dissected to obtain the muscle tissue and liver. Each five fish sample was collected in a bag, indicating the sample number and date. These samples were immediately stored at -80°C for further use. The serum biochemical indexes were analyzed using the colorimetric enzymatic method.
The contents of blood glucose and liver/muscle glycogen were measured through peroxidase and colorimetry respectively. The insulin concentrations were determined following the double antibody sandwich method. All indices were tested using the assay kit of Nanjing Jiancheng Bioengineering Co., Ltd (China) according to the instructions. According to AOAC (2000) method, the content of dry matter, crude protein, crude lipid, and crude ash in whole fish body was analysed.
2.4 Immune index analysis
The lysozyme (LZM) activity was measured using turbidimetry. The freeze-dried lyophilized Micrococcus lysodeikticus (0.3 mg/ml) was used as the LZM substrate in 0.05 M sodium phosphate buffer (pH 6.2). The test serum (diluent 1:2, 10 μ L) was then added to 200 μl bacterial suspension, and the absorbance recorded at 450 nm was tested after 0.5 and 4.5 min, respectively. The unit of LZM activity is the number of enzymes that causing a 0.001 reduction in absorbance per minute. The acid phosphatase (ACP) and alkaline phosphatase (AKP) activities were detected using the test kits. The unit definition of ACP and AKP activities corresponds to 1 mg phenol (mol) production when a 100 mL supernatant sample interacts with the matrix at 37 ° C for 30 min. Superoxide dismutase (SOD) activity was measured by its ability to inhibit superoxide anion generated by the xanthine and xanthine oxidase reaction system. One unit of SOD activity was defined as the amount of enzyme that produced a 50% inhibition in color formation measured at 550 nm.
2.5 Determination of insulin receptor tyrosine kinase activity
Insulin receptor was partially purified from the livers of 50 male Sprague Dawley rats (ap-proximately 150 g) by WGA chromatography, as described previously [Arnold and Newton,1991]. Insulin receptor was stored at -20℃in buffer containing 0.2 M N-acetylglucosamine, 40%glycerol, 0.1% Triton X-100,0.5 mM EDTA,0.5 mM EGTA, 25 mM HEPES, pH 7.5 (storage buffer). For the experiments in Figures 3B and 4, insulin receptor was partially purified from National Institutes of Health (NIH)3T3 HIR 3.5 cells, which express on the order of 10 6 copies of the human insulin receptor per cell [Whittaker etal.,1987]. Confluent cells were digested with enzyme-free solution. The cells were pelleted by centrifugation at 1,300g for 1 min at room temperature, lysed in 50 mM HEPES,pH 7.5 containing 1 mM EDTA,and 1 mM PMSF followed by homogenization. Membranes were pelleted at 543,000g for 10 min at 4℃ and resuspended in 50 mM HEPES, pH 7.5, containing 0.1% Triton X-100. The resuspended membranes were further homogenized and then centrifuged at 627,000g at 4℃for 10 min. Insulin receptor kinase activity in the supernatant (containing solubilized membrane proteins) was assayed directly or after reconstitution of proteins with exogenous lipids.
2.6. Gene expression analysis of IR-1 in the liver and dorsal muscle
2.6.1. RNA extraction and cDNA synthesis
A small piece of the dorsal muscle or liver (80 mg) of the test fish was ground into a fine powder with a mortar and pestle and placed in liquid nitrogen. The frozen powder was transferred to a 1.5 mL Eppendorf tube, and 1mL of Trizol reagent was added. Total RNA was isolated with Trizol according to the manufacturer's instructions. The content of RNA in the samples was determined by spectrophotometry; the integrity (quality) of the samples was detected by denaturing gel electrophoresis (1 % agarose gel). The 260/280 nm absorbance ratio of all samples was between 1.8 and 2.0 indicating the purity of the RNA samples.
The first-strand cDNA was synthesized from 1 μg of RNA using the PrimeScript RT Reagent Kit with the gDNA Eraser (Perfect Real Time; TaKaRa Biotechnology, Dalian, China) following the manufacturer’s protocol. The synthesized cDNA was diluted 10-fold for the following qRT-PCR analysis.
2.6.2. Expression Analysis by quantitative PCR
The gene expression levels were measured by quantitative real-time PCR (qPCR) on the prepared cDNA using the ABI7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) with the SYBR Premix Ex Taq (Takara Bio Inc. China) detection reagents. Gene-specific primer sequences are shown in Table 2. All PCR reactions were run in duplicate and normalized using (Livak & Schmittgen 2001) the β-actin gene as a reference.
Table 2. Nucleotide sequences of the primers for real-time qRT-PCR amplification
|
Primer pairs |
Sequence (5’→3’) |
GenBank No. (Gene symbol) |
|
β-actin -F |
GGTGGGTATGGGTCAGAAAG |
AB037865 |
|
β-actin -R |
CTCCTCAGGGGCAACTCTC |
AB037865 |
|
IR-1-F |
TCAAGACGGTCAACGAAT |
JN967750 |
|
IR-1-R |
TCATCAGCTCCATCACTAC |
JN967750 |
F: upstream primer R: downstream primer
2.7. Statistical analysis
The SPSS 22.0 software was used to process and analyze the experimental data. The data were expressed as mean ± SD. Duncan’s multiple range test was used for multiple comparisons based on one-way ANOVA. The relative expression of the target genes was calculated by the 2-ΔΔCt method, P<0.05 indicated significant differences in the results.
3.1. Growth performance
The results in table 3 showed that diets with different sugar sources had significant effects on the growth indexes of tilapia. From the indexes of final average weight, weight gain rate and specific growth rate, the growth performance was sucrose, glucose, starch and dextrin group in turn, and the sucrose group performed best (P < 0.05).
Table 3. The effects of different carbohydrate sources on growth performance of tilapia
|
Items |
Glucose |
Sucrose |
Dextrin |
Starch |
|||
|
IAW (g)1 |
31.61±0.04 |
31.82± 0.12 |
31.74±0.04 |
31.59±1.21 |
|||
|
FAW (g)2 |
94.92±2.67ab |
98.75±2.38b |
90.43±3.05 a |
91.83±2.49a |
|||
|
FI (g) 3 |
102.55±2.32 |
104.71±1.59 |
99.14±2.18 |
99.66±3.03 |
|||
|
FCR4 |
1.62±0.07ab |
1.56±0.06a |
1.68±0.08b |
1.65±0.05ab |
|||
|
WGR (%)5 |
200.28±8.95ab |
210.34±8.22b |
184.90±5.38a |
190.69±9.91ab |
|||
|
SGR (%/d)6 |
1.83±0.12ab |
1.89±0.05b |
1.74±0.04a |
1.77±0.09ab |
|||
*Data represent means ± SD (n = 4). Values with different letters are significantly different (P < 0.05). The absence of letters indicates no significant difference between treatments.
1 IAW: Initial average weight (g).
2 FAW: Final average weight (g).
3FI: Feed intake (g).
4 FCR: Feed conversion ratio = feed intake / (final body weight - initial body weight)
5 WGR: Percent weight gain rate (%) = (final body weight–initial body weight) ×100/initial body weight
6 SGR: Specific growth rate (% day-1) = 100 × [(Ln (final body weight) - Ln (initial body weight)) / duration (60 days)]
3.2. Immune index
The results in table 4 show that the activcities of acid phosphotase, alkaline phosphotase, superoxide dismutase and lysozyme in sucrose group were significantly higher than that of the other three groups (P < 0.05), and there was no significant difference among the other three groups (P > 0.05).
Table 4. The effects of different carbohydrate sources on serum immune indices of tilapia
|
Items |
Glucose |
Sucrose |
Dextrin |
Starch |
|
ACP (U/L) |
233.25±16.45a |
304.88±17.93b |
239.86±14.75a |
227.73±17.19a |
|
AKP (U/L) |
375.59±19.85a |
472.63±23.06b |
382.49±24.13a |
374.75±13.95a |
|
SOD (U/mL) |
166.98±1.69 |
169.68±1.64 |
167.37±1.54 |
158.90±1.25 |
|
LSZ (U/mL) |
30.58±1.70ab |
38.15±1.24b |
27.78±1.49a |
26.64±1.34a |
*Data represent means ± SD (n = 4). Values with different letters are significantly different (P < 0.05). The absence of letters indicates no significant difference between treatments. ACP: acid phosphatase; AKP: alkaline phosphatase; SOD: superoxide dismutase; LSZ: lysozyme.
3.3. The contents of insulin and glycogen of liver and muscle
The table 5 shows that the serum glucose level of sucrose group and glucose group is significantly higher than that of dextrin group and starch group (P < 0.05). There was no significant difference in insulin concentration among the four groups (P > 0.05). In terms of liver glycogen level, the content of sucrose group was significantly higher than that of dextrin group and starch group (P < 0.05), but there was no significant difference with glucose group (P > 0.05). In terms of muscle glycogen level, the content of sucrose group was the highest and that of starch group was the lowest, there was no significant difference between glucose group and dextrin group (P < 0.05).
Table 5. Serum insulin level and glycogen of liver and muscle in tilapia
|
Items |
Glucose |
Sucrose |
Dextrin |
Starch |
|
Blood glucose (mmol/l) |
9.20±1.27b |
9.43±1.40b |
8.07±2.12a |
8.14±2.41a |
|
Blood Insulin (ng/l) |
3.35±0.03 |
3.36±0.04 |
3.32±0.07 |
3.19±0.12 |
|
Hepatic glycogen (mg/g) |
31.63±2.37b |
34.17±2.20b |
27.14±3.70ab |
21.15±4.73a |
|
Muscle glycogen (mg/g) |
3.26±0.23ab |
4.40±0.17b |
3.16±0.57ab |
2.21±0.36a |
*Data represent means ± SD (n = 4). Values with different letters are significantly different (P < 0.05). The absence of letters indicates no significant difference between treatments.
3.4 Tyrosine kinase activites and relative mRNA level of IR-1 in liver and muscle
The effects of different sugar sources on tyrosine kinase activity of GIFT tilapia are shown in figure 1. There was no significant difference in tyrosine kinase concentration (TKA) between sucrose group and glucose group in liver, but it was significantly different from that of dextrin group and starch group (P < 0.05). The tyrosine kinase activity of glucose group was the highest and significantly different from dextrin group (P < 0.05). The tyrosine kinase activity in muscle was always higher than that in liver (P < 0.05).
The effects of diets with different sugar sources on the IR-1 mRNA in liver and muscle of tilapia are shown in figure 2. The results showed that there was no significant difference in the IR-1 mRNA in sucrose group, glucose group and dextrin group, which was slightly higher than that in starch group (P < 0.05). The IR-1 mRNA in starch group and dextrin group was significantly higher than that in sucrose group. Different sugar source diets had a significant effect on the expression of IR-1 mRNA in the muscle of tilapia.
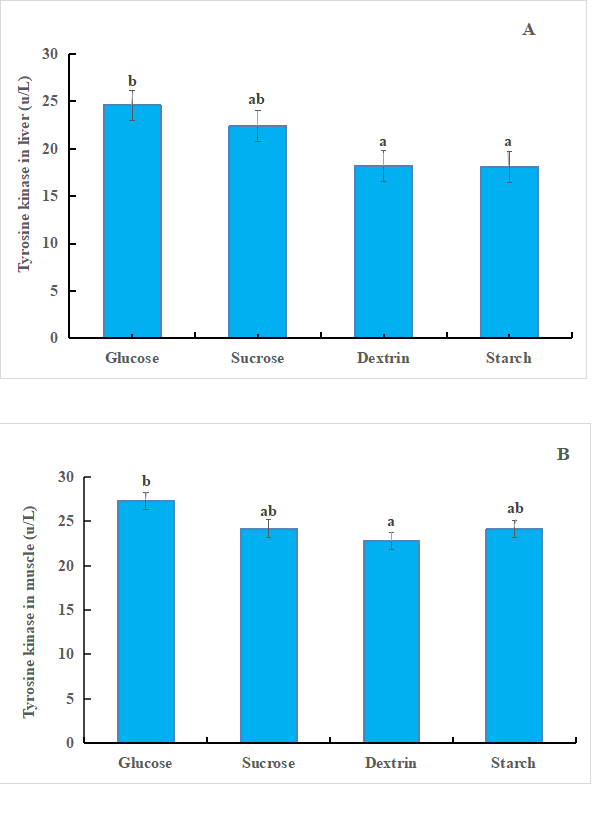
Figure 1. A. Tyrosine kinase in liver of tilapia. Bars with different letters are significantly different (P < 0.05). B. Tyrosine kinase in muscle of tilapia. Bars with different letters are significantly different (P < 0.05).
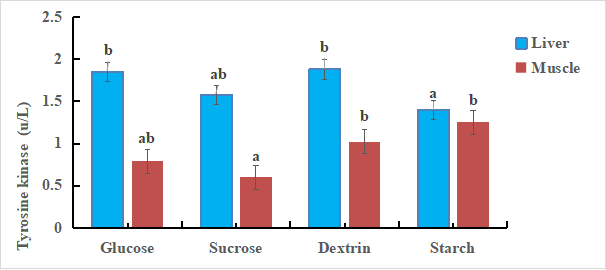
Figure 2. Relative mRNA level of IR-1 in liver and muscle. Bars with different letters are significantly different (P < 0.05).
The utilization efficiency of the same kind of fish to different sugar sources is different, and the utilization efficiency of different kinds of fish to the same sugar source is also different. Some studies believe that the utilization capacity of small molecular sugars is lower than that of large molecular sugars, such as turbot (Miao, 2013), black carp (Zhao, 2009), crucian carp (Cai et al., 2006) and cobia (Ren, 2012); Some studies believe that the utilization effect of macromolecular sugars is lower than that of small molecules, such as salmon (Buhler, 1961), grass carp (Tian et al., 2000) and grouper (Shiau et al,2001); Some studies have found that the size of sugar molecules has little effect on the ability of fish to use sugar diet, such as tilapia (Wu et al., 2013). The reason that the utilization level of small molecule sugar in fish is lower than that of macromolecule sugar is that the digestive system of fish can be directly converted and utilized after ingesting small molecule sugar like glucose. When glucose reaches the blood, it will cause the increase of blood sugar due to the rapid absorption rate, which will have negative physiological effects. On the contrary, the reaction mechanism of macromolecules is reversed, which requires a series of decomposition and transformation such as hydrolysis to become glucose which can be absorbed and utilized by fish body. Therefore, the poor utilization of sugar in some fishes results in different fish growth. The effects of different diets with sugar sources on growth performance of hybrid sturgeon larvae were studied (Song et al., 2016). The final average body weight gain, weight gain rate and specific growth rate of glucose group were the lowest and the dextrin group was the highest.
In this study, the growth rate and specific growth rate of sucrose group were the highest, followed by glucose group, which was significantly higher than that of dextrin group and starch group. However, studies on grouper (Shiau and Lin, 2001), golden bream (Enes et al., 2008) and grass carp (Tian and Liu, 2000) have found that their utilization of small molecular sugar is higher than that of large molecular sugar, which may be different among different fishes. The low utilization rate of small molecule sugar in some fishes may be due to the fact that the fish ingest the diet of glucose so quickly that they can not use the glucose effectively in a short time, which causes stress due to the high blood sugar content in the fish body, thus affecting the growth and development of fish. Macromolecular sugars need to undergo enzymatic decomposition, transformation and hydrolysis before they can be better absorbed and utilized by the fish body, which is more conducive to the fish growth. However, when herbivorous fish eat a diet containing glucose, they can rapidly oxidize and decompose glucose into CO2 and release ATP, which can more effectively improve protein synthesis and improve growth and metabolism of fish (Tian et al., 2001).
Fish mainly rely on non-specific immunity to improve their resistance to disease. AKP and ACP are marker enzymes of macrophage lysosomes within the animal immune system. These enzymes can hydrolyze invading pathogens, promote phagocytosis, degrade phagocytes, and play a significant role in immune function (Huang et al., 2005). The higher its content, the stronger the immune capability of the animal body. Lysozyme is a nonspecific humoral immune factor widely present among fishes. The lysozyme activity reflects the strength of nonspecific immunity (Li and Wu, 2001). The results of this study showed that the activcities of ACP, AKP and LSZ in sucrose group were significantly higher than that of the other three groups (P < 0.05). In this test, sucrose group has better immunity, which may be due to the fact that tilapia has better sucrose absorption ability than other sugars, which can promote digestion and absorption ability of digestive system and thus enhance immunity of the body, eventually manifested as improvement of growth performance.
Insulin is an important endocrine hormone in animals. It regulates growth, development and metabolism of animals and has a wide range of biological functions. Insulin is the only hormone that lowers blood sugar in animals. The main reason for the low tolerance to sugar is considered to be insufficient insulin secretion in fish. The results showed that there was no significant difference in serum insulin level of tilapia after feeding diets with different sugar sources.The energy of fish is mainly stored in the glycogen of fish body, which mainly exists in muscle and liver. The results of this study showed that the content of liver glycogen in glucose group was significantly higher than that in dextrin group and starch group.The results of this experiment are similar to the research results of turbot fish. After Miao et al.(2013) fed turbot with glucose, sucrose and dextrin as carbohydrate sources, the results showed that the liver glycogen content of turbot was glucose group > sucrose group > dextrin group > control group.The reason for this result may be that tilapia can absorb glucose quickly after eating the feed containing glucose, resulting in the rapid rise of blood glucose content in the fish. However, the fish can not secrete insulin quickly to digest glucose, which leads to the fish can not make good use of it after absorbing glucose. Therefore, Excess glucose in fish is stored in the liver in the form of glycogen (Ma et al., 2017). Another study found that the muscle glycogen content of the dextrin group of hybrid sturgeon was significantly higher than that of other groups, and the liver glycogen content of the dextrin group was the lowest, which was significantly lower than that of the glucose group and wheat starch group (Song et al., 2016). Chu et al. (2017) found that the liver glycogen of starch group in juvenile Dabry’s sturgeon was significantly higher than that of glucose, sucrose, dextrin, corn starch and wheat starch group, the muscle glycogen in starch and glucose group was significantly higher than that in other groups.The results of this study showed that the content of muscle glycogen in sucrose group was significantly higher than that in other groups. There was no significant difference between glucose group and dextrin group, but it was significantly higher than that in starch group. However, in the study of liver muscle glycogen content of young turbot (Li et al., 2011), it is found that there is no significant change in muscle glycogen in its body. The reason may be that there are differences among fish varieties.
Insulin is considered to be the most powerful important hormone to promote anabolism, which can improve the transformation and metabolism of sugars, proteins and fats and inhibit their degradation (Saltiel et al., 2001). Insulin receptors play a key role in fish and are differentially expressed in different tissues and cells, indicating that the role of insulin in vivo is tissue-specific (Caruso and Sheridan, 2011). After releasing the signal, insulin binds to insulin receptor (IR), and then activates protein tyrosine kinase (PTK) containing ß subunit; PTK phosphorylates insulin receptor substrate (IRS) and activates it. Secondly, PTK phosphorylates insulin receptor and protein; IRS combines with signal molecules containing sarcoma homologous domain to activate many downstream signal pathways, resulting in a cascade reaction between protein kinase and phosphatase, so as to play a wide range of biological functions. At present, there are many reports on the insulin signal transduction system in mammals, but compared with mammals, there are still few studies on the insulin signal transduction system in fish.
The results of this study found that there were significant differences in the mRNA level of IR-1 in liver and muscle. Similar to this result was the study of Nile tilapia (Liu, 2012). Liu (2012) found that after injecting glucose into the abdomen of Nile tilapia, the mRNA level of IR-1 in tilapia was highly expressed in muscle, heart and liver, which was relatively low in liver and the highest in muscle. This study more clearly confirmed that the response of insulin in fish has a certain regularity. There was no significant difference in the mRNA level of IR-1 in the liver of tilapia fed with four sugar source diets, but it had a significant effect on the mRNA level of IR-1in muscle tissue, especially in the starch group, the mRNA level of IR-1in muscle was significantly higher than that in other groups. The results showed that different kinds of sugars in the diet affected the expression of insulin receptor in tilapia.
In terms of specific growth rate and weight gain rate, compared with dextrin and starch, sucrose and glucose can be better utilized by GIFT tilapia and show good growth performance. The results show that GIFT tilapia has different performance on different sugar source diets. In the breeding and production of GIFT tilapia, appropriate sugar can be added to reduce the dietary protein level, and show better growth performance and immunity.
This study was supported by the National Natural Science Foundation of China (Grant No. 31472288) to Jiting Wang; Shandong Science and Technology Development Plan Program to Jiting Wang (Grant No. 2014GGH210010); The Key Technology R&D Program of Shandong Province to Jiting Wang (Grant No. 2019GNC106078); and Funds of Shandong “Double Tops” Program.
Conflict interest
The authors declare that they have no conflict of interest.
Orcid
Jiting Wang https://orcid.org/0000-0003-1483-333X
Zhang H, Li G, Sun C, et al., 2004. Effects of nutrients on fatty liver disease in fish. Marine bulletin, 23(1):82-89.
ZhaoY.Advanced in the study of utilization of carbohydrate by black carp (Mylopharyngodln piceus Richardson) [D].Suzhou: Suzhou University, 2009
Wu B, Peng Q, Chen B, 2013. Effect and mechanism of different sugar sources in diet on juvenile tilapia. Oceanologia Et Limnologia Sinica,44(4):1050-1055.
Wilson R P, 1994. Utilization of dietary carbohydrate by fish. Aquaculture, 124(1-4):67-80. 90363-8
View ArticleTian L, Liu Y, Liu D, 2001.Study on the utilization of glucose and starch as energy in grass carp. Journal of Sun Yat sen University: Natural Science Edition,40 (2):104-106.
Tian L X, Liu Y J, Liu D H, et al. 2000. Effects of glucose and corn starch on growth and the fat deposition in the mesentery of grass carp. Journal of Fisheries of China, 24(5): 438-441.
Song J, Jiang H, Jiang Z, 2016. Effects of different sugar sources in feed on growth performance, serum biochemical indexes and Muscle Nutrients of juvenile hybrid sturgeon. Journal of Dalian Ocean University, 31(1):58-64.
Shiau,S.Y., Lin,Y.H., 2001.Carbohydrate utilization and its protein-sparing effect in diets for grouper, Epinephelus malabaricus. Animal Sci.73,299-304.
View ArticleSaltiel A R, Kahn C R. 2001. Insulin signallig and the regulation of glucose and lipid metabolism. Nature, 414: 799-806. PMid:11742412
View Article PubMed/NCBIShiau S Y. 1997. Utilization of carbohydrates in warmwater fish-with particular reference to tilapia, Oreochromis niloticus X 0.aureus . Aquaculture, 151(1):79-96. 01491-3
View ArticleRen.Study on carbohydrate nutritional physiology of cobia and rainbow trout [D].Qingdao: China Ocean University,2012.
Mohanta K N, Mohanty S N, Jena J K, et al. 2006. Apparent protein,lipid energy digestibility coeffcients of some commonly used feed ingredients in formulated pelleted diets for silver barb, Puntius gonionolus. Aquaculture Nutrition, 12(3):211-218.
View ArticleMaule A G,Tripp R A, Kaatttari S L, et al., 1989. Stress alters immune function and disease resistance in chinook salmon (Oncorhtnchus tshawytscha). J Endocrinol,120:135-142. PMid:2918264
View Article PubMed/NCBIMiao S, Miao H, 2013. Effects of different kinds of carbohydrates in feeds on growth performance and metabolic response of turbot. Chinese Journal of Fisheries,37 (6):910-919.
View ArticleMa H, Wang M, Lu Y, Yuan Y, Sun P, 2017. Effects of carbohydrate types and levels on growth performance, serum biochemical indexes, liver glucose metabolism related enzyme activities and liver glycogen content of Pseudosciaena crocea. Chinese Journal of animal nutrition, 29 (3) :824-835.
Liu H. Effects of glucose injection on glucose metabolism, insulin receptor and GLUT4 gene expression in tilapia [D]. Shandong Agricultural University, 2012.
Livak, K.J., Schmittgen, T.D. 2001. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25, 402-408. PMid:11846609
View Article PubMed/NCBILie O, Lied E, Lambertsen G,1988. Feed optimization in atlantic cod (Gadus morhua ): Fat versus protein content in the feed. Aquaculture, 69(3):333-341. 90340-7
View ArticleLi, L., Wu, Z. 2001. Research progress of fish humoral immunity. Marine science, 11, 20-22.
Li X. Effects of feed sugar level on growth, physiological parameters and body composition of turbot and Paralichthys olivaceus [D] Ocean University of China, 2011.
Lee S M, Lee J H, 2004. Effect of dietary glucose, dextrin and starch on growth and body composition of juvenile starry flounder Platichthys stellatus. Fisheries Science, 70(1):53-58.
View ArticleHuang, Z., Chen, Y., Zhao, Y., Zuo, Z., Chen, M., Wang, C. 2005. Effects of tributyltin on the activities of acid phosphatase, alkaline phosphatase, and Na+, K+ - ATPase in the gills of Meretrix meretrix. Marine Environmental Science, 3, 56-59.
Hemre G I, Mommsen T P, Krogdahl A, 2002. Carbohydrates in fish nutrition: effects on growth,glucose metabolism and hepatic enzymes. Aquacult Nutr, 8:175-194.
View ArticleHelland S J, Grisdale-Helland B, 1998. Growth, feed utilization and body composition of juvenile Atlantic halibut (Hippoglossus hippoglossus) fed diets differing in the ratio between the macronutrients. Aquaculture, 166(1-2):49-56. 00273-7
View ArticleFuruichi M, Yone Y. 1980. Effects of dietary dextrin levels on growth and feed efficiency, the chemical composition of liver and dorsal muscle and the absorption of dietary protein and dextrin in fishes. Bull Jpn Soc Sci Fish, 46: 225-229.
View ArticleEnes P, Panserat S, Kaushik S, et al. 2008. Growth performance and metabolic utilization of diets with native and waxy maize starch by gilthead sea bream (Sparus aurata) juveniles. Aquaculture, 274(1): 101-108.
View ArticleDrew M D, Schafer T C, Zijlstra R T, 2012. Glycemic index of starch affects nitrogen retention in grower pigs. Journal of Animal Science, 90(4):1233. PMid:21984722
View Article PubMed/NCBIDong L. Effects of different sugar sources and sugar levels on growth and glucose metabolism of pompano ovata [D]. Guangxi University, 2016.
Chu Z, Wei Q, Du H, Liu W, Zhang L, Xie J, Di J, 2017. Effects of different carbohydrate sources on growth performance, body composition, and physiological and biochemical parameters of juvenile Dabry's sturgeon (Acipenser dabryanus). Journal of Fishery Sciences of China, 24(2): 284-294.
View ArticleChristiansen D C, Khngsoyr L, 1987. Metabolic, utilization of nutrients and the effects of insulin in fish. Comp Biochem Physiol, 88: 701-711 90232-X
View ArticleCaruso M A, Sheridan M A. 2011. New insights into the signaling system and function of insulin in fish. General and Comparative Endocrinology, 173(2): 227-247. PMid:21726560
View Article PubMed/NCBICai C, Wang Y, 2006. Effects of feed sugar types and levels on the growth and body composition of herring and crucian carp. Chinese fisheries science,13 (3):452-459.
Buhler D R,Halver J E, 1961. Nutrition of salm on oid fish: Ⅸ.Carbohy-drate requirements of Chinook salmon. The Journal of Nutrition,74 (3) : 307-318.
View ArticleAOAC 2000. Association of official analytical chemists-official methods of analysis (17th ed.) Arlington, Virginia.
