Rajesh Kumar Singh
E-mail: rajeshsingh999@gmail.com
Tel./Fax: +86-15676711050
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 6 ISSUE: 1
Page No: 313-324
Rajesh Kumar Singh
E-mail: rajeshsingh999@gmail.com
Tel./Fax: +86-15676711050
Pratiksha Singh1-3. Rajesh Kumar Singh2,3*. Mohini Prabha Singh4. Pooja Nikhanj4. Param Pal Sahota4. Wenxia Fang1. Yang Rui Li2,3
1State Key Laboratory of Non-Food Biomass and Enzyme Technology, Guangxi Academy of Sciences, Nanning- 530007, Guangxi, China.
2Key Laboratory of Sugarcane Biotechnology and Genetic Improvement (Guangxi), Ministry of Agriculture, Sugarcane Research Center, Chinese Academy of Agricultural Sciences, Guangxi Key Laboratory of Sugarcane Genetic Improvement, Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning- 530007, Guangxi, China.
3Guangxi Key Laboratory of Crop Genetic Improvement and Biotechnology, Nanning- 530007, Guangxi, China.
4Punjab Agricultural University, Ludhiana-141004, Punjab, India.
Pratiksha Singh, Rajesh Kumar Singh, Mohini Prabha Singh, Pooja Nikhanj, Param Pal Sahota, Wenxia Fang, Yang Rui Li. Yeast α-L-Rhamnosidase: Sources, Properties, and Industrial Applications(2021) Journal of Food Science & Technology 6(1) p:313-324
Yeasts have been used for the heterologous production of a range of enzymes. However, α-L-rhamnosidase production in yeasts as well as its vast potential for biotechnological processes is less reported. α-L-Rhamnosidase is one of the important biotechnologically attractive enzymes in several industrial and biotechnological processes. In food and agriculture industries, the enzyme catalyzes the hydrolysis of hesperidin to release L-rhamnose and hesperidin glucoside, industrial removal of bitterness from citrus juices caused by naringin, and enhancing aroma in grape juices and derived beverages. In pharmaceutical and chemical industries, this enzyme is used in the structural determination of polysaccharides, glycosides and glycolipids, metabolism of gellan, conversion of chloropolysporin B to chloropolysporin C, and production of prunin. Rhamnosidases are extensively distributed in fungi and bacteria while their production from yeast sources is less reported. Yeast rhamnosidase is very important as it is produced in short-duration fermentation, with enhanced shelf life, high thermal stability, capable of retaining juice flavor, and is non-toxic for human consumption. In this review, an attempt has been made to fill up this gap by focusing on production, purification, characterization, structural and molecular biological studies of yeast rhamnosidase and its potential biotechnological applications.
Keywords: Industrial applications, Naringin, Rhamnosidase, Yeast
Yeasts are groups of unicellular fungi that belong to the phylum Dikaryomycota. Two main groups of yeasts classified are Ascomycetes or Basidiomycetes (Barnett et al. 2000). The yeasts are characterized by single cells that reproduce by budding from a narrow or broad base (e.g. Saccharomyces) or fission from a broad base (e.g. Schizosaccharomyces). Additionally, pseudohyphae or true hyphae or both may be present (Kurtzman and Fell 1998). The application of yeasts is not only restricted to the traditional processes of making bread, wine, and beer, though yeasts are also a rich source of a variety of industrially essential enzymes such as rhamnosidase, amylase, protease, invertase, etc. In this chapter, we targeted the enzyme α-L-rhamnosidases produced by yeast. In addition, recent research has generally focused on yeasts as biocontrol on plant disease management for managing postharvest diseases, mostly of fruit in agriculture sectors (Pimenta et al. 2009).
α-L-Rhamnosidase (3.2.1.40) are exo-type enzymes that remove terminal α-L-rhamnosyl groups at the ends of polysaccharides and glycosides containing L-rhamnose. The enzyme converts the bitter glycoside naringin to the less-bitter prunin by cleavage of an α-(1→2) bond between L-rhamnose and glucose (Magario et al. 2009) (Fig. 1). Many other natural glycosides, which include rutin, quercitrin, hesperidin, diosmin, and terphenyl glycosides, containing terminal α-rhamnose can act as substrates of α-L-Rhamnosidase (Ribeiro 2011). Rhamnosidases are important industrial enzymes of great significance in the current biotechnological area with applications in food (Spagna et al. 2000), agriculture, and pharmaceutical (Monti et al. 2004) industrial processes for the bioconversion of natural or synthetic rhamnosides e.g. the elimination of hesperidin crystals in orange juice, for aroma enhancement of wine, in steroid transformation, in the structural study of bacteria polysaccharide, and in the production of L-rhamnose from glycosides (Gallego et al. 2001; Soria and Ellenrieder 2002; Manzanares et al. 2001; Mutter et al. 1994; Puri and Banerjee 2000).
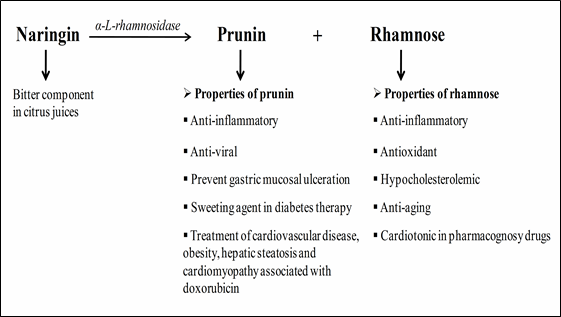
Fig. 1 Hydrolysis of naringin into prunin and rhamnose by α-L-rhamnosidase.
Several microorganisms use α-L-rhamnosidase to release L-rhamnose as a source of carbon and energy (Twerdochlib et al. 1994). The enzyme has been reported from animal tissues, plants, microbes like yeasts, fungi, and bacteria (Yadav et al. 2010). Despite α-L-rhamnosidases being biotechnologically important enzymes, only a few crude rhamnosidase preparations hesperidinase, and naringinase are commercially available so far. All of these preparations are presently obtained from the genera Aspergillus and Penicillium. In the case of yeast strains, there is a lack of information on the production, purification, characterization, preparation, and evaluation of α-L-rhamnosidase. Only a few yeast strains Saccharomyces, Hansenula, Debaryomyces, Candida, Aureobasidium pullulans (Miklosy and Polos 1995; Rosi et al. 1995; Mcmahon et al. 1999; Yadav et al. 2010), Pichia angusta (Yanai and Sato 2000) and Clavispora lusitaniae (Fig. 2) (Singh et al. 2015 and 2018) have been reported. Yeast α-L-rhamnosidase possesses characteristics of high specificity, increased retention of juice flavor, nutrients, and economic viability with a strong ability to remove the bitter taste from citrus fruits.
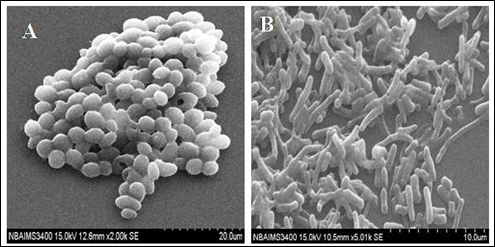
Fig. 2 Scanning electron micrographs of yeast strain Clavispora lusitaniae producing rhamnosidase enzymes. (A) Absence of naringin and (B) Presence of 0.2% naringin (Singh et al. 2015).
There is only one review available on α-L-rhamnosidases by Yadav et al. 2010. Therefore, our responsibilities to research and focus on this area and provide more information on rhamnosidases. In this article, an attempt has been made to review the recent literature and summary of yeast rhamnosidases and their potential applications.
Sources
Naringinase (α-L-rhamnosidase and ß-D-glucosidase) has been reported in the literature since the earliest of 1938, from celery seeds (Hall1938). Other plant sources of α-L-rhamnosidase are grapefruit leaves (Thomas et al.1958), Rhamnus daurica (Suzuki 1962), and Fagopyrum esculentum (Bourbouze et al. 1976). The α-L-rhamnosidases from only two animal sources, viz. Turbo cornutus liver and pig liver have been reported (Kurosawa et al. 1973; Qian et al. 2005). Microorganisms are the main sources of α-L-rhamnosidase, mainly filamentous fungi such as Aspergillus, Circinella, Eurotium, Fusarium, Penicillium, Rhizopus, and Trichoderma (Scaroni et al. 2002). The induction of α-L-rhamnosidases production in several fungal strains such as Acremonium persicinum, Circinella muscae, Emericella nidulans, Fusarium oxysporum, Mortierella alpine, Penicillium oxalicum, Rhizopus arrhizus, Talaromyces flavus, and Trichoderma harzianum, using L-rhamnose, naringin, rutin, hesperidin as inducers (Monti et al. 2004). Aspergillus niger and Penicillium decumbens are the most commonly used species for their production with a potential value in oenology, and their enzymatic activities have been well characterized (Gallego et al. 1996; Manzanares et al. 2003; Orejas et al. 1999; Singh et al. 2015).
The first bacterial α-L-rhamnosidase was purified from the genus Bacteroides (Jang and Kim 1996). Other bacterial strains which produce α-L-rhamnosidases are thermophilic bacterium (Birgisson et al. 2004), Fusabacterium (Park et al. 2005), Pseudoalteromonas species, Ralstonia pickettii (Orrillo et al. 2007), Lactobacillus acidophilus (Beekwilder et al. 2009), Pediococcus acidilactici (Michlmayr et al. 2011), Clostridium stercorarium (Zverlov et al. 2000), Sphingomonas paucimobilis (Hashimoto and Murata 1998), Bacillus sp. (Hashimoto et al. 1999) and Corticium rolfsii (Kaji and Ichimi 1973). In a few reports, low levels of activity have been found in yeast belonging to genus Saccharomyces, Hansenula, Debaryomyces, Candida, and Aureobasidium (McMahon et al. 1999; Miklosy and Polos 1995; Rosi et al. 1995; Yadav et al. 2010), P. angusta (Yanai and Sato 2000), Clavispora lusitaniae (KF633446), Clavispora lusitaniae (KF633447), Candida sp. YS12A (KF680225) and Candida hyderabadensis (KF680226) (Singh et al. 2015). All yeast strains producing α-L-rhamnosidase are summarized in Table 1.
Table 1. List of yeast strains producing α-L-rhamnosidase enzyme
|
S. No. |
Yeast |
Enzyme activity |
pH optima |
Temperature optima (oC) |
Molecular Mass (kDa) |
pI |
Reference |
|
1. |
Clavispora lusitaniae KF633446 |
0.058 UmL-1 |
4 |
50 |
- |
- |
Singh et al. 2018, Singh et al. 2015 |
|
2. |
Clavispora lusitaniae KF633447 |
0.046 UmL-1 |
- |
- |
- |
- |
Singh et al. 2015 |
|
3. |
Candida sp. YS12A KF680225 |
0.033 UmL-1 |
- |
- |
- |
- |
Singh et al. 2015 |
|
4. |
Candida hyderabadensis KF680226 |
0.029 UmL-1 |
- |
- |
- |
- |
Singh et al. 2015 |
|
5. |
Saccharonryces cerevisiae |
- |
- |
- |
- |
- |
Miklosy et al. 1995 |
|
6. |
Hansenula anomala |
- |
- |
- |
- |
- |
Miklosy et al. 1995 |
|
7. |
Debaryomyces ploymorphus |
- |
- |
- |
- |
- |
Rosi et al. 1995 |
|
8. |
Aureobasidium pullulans |
- |
- |
- |
- |
- |
Mcmahon et al. 1999 |
|
9. |
Candida guillermondii |
- |
- |
- |
- |
- |
Mcmahon et al. 1999 |
|
10. |
Saccharomyces cerevisiae IAM 4S61 |
13.7 mUL-1 |
- |
- |
- |
- |
Yanai and Sato 2000 |
|
11. |
Crptococcus terreus IFO 0727 |
6.5 mUL-1 |
- |
- |
- |
- |
Yanai and Sato 2000 |
|
12. |
Pichia angusta X349 |
34 mUL-1 |
6 |
40 |
90 |
4.9 |
Yanai and Sato 2000 |
|
13. |
Pichia capsulate X91 |
28.8 mUL-1 |
- |
- |
- |
- |
Yanai and Sato 2000 |
|
14. |
Pichia guilliermondii NPCC1053 |
33 Ug-1 |
6 |
- |
- |
- |
Rodriguez et al. 2010 |
|
15. |
Pichia pastoris MutS |
- |
- |
- |
- |
- |
Markošová et al. 2015 |
Assay methods
There are various methods available for measuring α-L-rhamnosidase activity, some are mentioned below:
1. Romero et al.'s (1985) method using p-nitrophenyl α-L-rhamnopyranoside as a substrate is a convenient and most common method for assaying α-L-rhamnosidase activity. The absorbance of the mixture is determined spectrophotometrically at 400 nm (A400). The amount of enzyme that released 1 µmol of p-nitrophenol in 1 min is defined as one unit of α-L-rhamnosidase activity (E400nm p-nitrophenolate= 21.44 mM- 1cm- 1) (Fig. 3). The use of a synthetic substrate did not affect the pH, temperature, or ionic strength optima of the enzyme (Puri 2012).
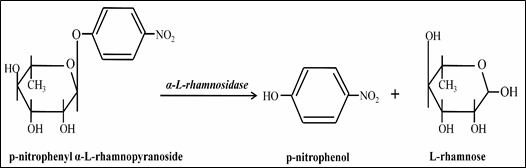
Fig. 3 Hydrolysis of p-nitrophenyl α-L-rhamnopyranoside into p-nitrophenol and rhamnose by α-L-rhamnosidase.
2. Spectrophotometric determination of flavanones according to the alkaline diethylene glycol method of Davis (1947) is used to evaluate naringinase (α-L-rhamnosidase + ß-D-glucosidase) activity. In this method, naringin reacts with diethylene glycol in an alkaline solution to produce a yellow color which is measured at a wavelength of 420 nm (A420) (Davis 1947). 2,4-Dinitrosalicylic acid (DNS) method through rhamnose and glucose determination was also applied for naringinase activity (Miller 1959).
3. Ribeiro and Ribeiro (2008) developed an effective HPLC-PAD method for the simultaneous determination of naringin, prunin, and naringenin. The method was linear, precise, and selective for naringin and naringenin identification and quantification (Puri 2012).
4. The enzyme activity toward naringin and hesperidin was assayed for determining rhamnose release by using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) on a CarboPac PA1 anion-exchange column. One unit of rhamnosidase activity was defined as the amount of enzyme required to release rhamnose at 40 °C and pH of 4.0 at the rate of 1 μmol min− 1 (Koseki et al. 2008).
5. Assays with nonchromogenic substrates (naringin, hesperidin, rutin, and rutinose) were conducted by Michlmayr et al. (2011). Rhamnose, glucose, and rutinose were quantified by HPLC analysis with a CarboPac PA 1 column and pulsed amperometric gold electrode detection. One unit of rhamnosidase activity is expressed as released rhamnose (μmol) per min at 37°C and pH 5.5.
6. In few reports, L-rhamnose was separated using TLC and quantified to measure α-L-rhamnosidase activity by Victor et al. 1987 and Feng et al. 2005.
Yeast rhamnosidase
The fungal and bacterial sources of α-L-rhamnosidases have been thoroughly explored; although yeast rhamnosidase has rarely and is less studied and needs to explore more extensive research studies. At present, there are only one α-L-rhamnosidases of yeast origin Pichia angusta X349 that has been purified and characterized (Yanai and Sato 2000). Here we documented some reports on α-L-rhamnosidase activity from yeast that have been published (Table 1).
Production, purification, and characterization
Optimization of rhamnosidase production from Clavispara lusitaniae KF633446 by using multivariate response surface methodology was studied by Singh et al. (2018). The observed optimized parameter for highest rhamnosidase production in the minimal medium was (w/v) 0.6% rhamnose, 0.4% yeast extract, 35±5 °C temperature, and 4 pH.
Yanai and Sato (2000) examined 386 yeast strains for α-L-rhamnosidase activity using L-rhamnose as the inducer. The screening results showed that α-L-rhamnosidase activity was generally restricted to species S. cerevisiae, C. terreus, P. angusta, and P. capsulata. One strain, P. angusta X349, was selected as the best producer of the enzyme. Selected strain P. angusta X349 has been purified to homogeneity using ammonium sulphate precipitation and column chromatography on concanavalin A-Sepharose, DEAE Bio-Gel A agarose, Rhamnose-Sepharose 6B, and hydroxyapatite. The P. angusta X349 rhamnosidase was a monomeric protein with a molecular mass of 90 kDa and the isoelectric point at 4.9. The enzyme was optimally active at pH 6.0 and around 40 oC. The Ki for L-rhamnose inhibition was 25 mM. The enzyme was inhibited by Cu2+, Hg2+, and p-chloromercuribenzoate. The α-L-rhamnosidase was highly specific for α-L-rhamnopyranoside and liberated rhamnose from naringin, rutin, hesperidin, and 3-quercitrin.
The α-L-rhamnosidase activity of Pichia guilliermondii NPCC1053 indigenous wine strain from the North-Patagonian region was evaluated on various culture media (Rodriguez et al. 2010). The αRh activity of P. guilliermondii was associated with its cellular growth and the maximum value of total enzymatic activity (33 Ug-1 dry weight) under optimal conditions (YNB- rhamnose supplemented with ammonium sulphate) was at the end of the exponential growth phase (sixth day). This study contributes to the knowledge of α-L-rhamnosidases from yeast origin and the enzyme that could be used in the production of more aromatic young wines.
A total of thirty yeast strains were isolated from a whey beverage and screened for α-L-rhamnosidase enzyme production (Singh et al. 2015). Of these, only four isolates Clavispora lusitaniae (KF633446), Clavispora lusitaniae (KF633447), Candida sp. YS12A (KF680225) and Candida hyderabadensis (KF680226) were capable of producing the α-L-rhamnosidase enzyme by hydrolyzing naringin. The production of α-L-rhamnosidase varied in the range of 0.029 ± 0.00 to 0.058 ± 0.02 U mL–1. Clavispora lusitaniae KF633446 was the best producer of an enzyme. The enzyme was most active at pH 4 and temperature 50 °C using p-nitrophenyl-α-L-rhamnopyranoside as the substrate (Singh et al. 2015). Maximum activity at pH 4 is close to that of fruit juice, making it suitable for use in fruit juices and wine.
Pichia pastoris is presently one of the most favored microorganisms for recombinant enzyme production owing to its proficient expression system. The upscaling of recombinant α-L-rhamnosidase production cloned in the MutS strain of P. pastoris KM71H was optimized by simplified methanol feeding protocol. Results showed that the specific activity of α-L-rhamnosidase improved from 35 Umg-1 up to 82 Umg-1 in the upscaled fermentation, which is the maximum specific activity of other recombinant and also wild producers described so far (Markosova et al. 2015).
Molecular and structural biology
Only some reports on the molecular and structural properties of α-L-rhamnosidase are available. The ram A is the first cloned α-L-rhamnosidase gene obtained from the anaerobe thermophilic bacterium, which belongs to the new type of glycoside hydrolase family (Zverlov et al. 2000). Several microorganisms including yeast use α-L-rhamnosidase to release L-rhamnose as a source of carbon and energy. In the oxidative pathway for the catabolism of L-rhamnose, L-rhamnose is first oxidized to L-rhamnono-γ-lactone by a NAD-utilising L-rhamnose-1-dehydrogenase. This inducible enzyme activity has been reported in various yeast species including Scheffersomyces stipitis, Aureobasidium (Pullularia) pullulans, and Debaryomyces polymorphus (Rigo et al. 1976; Vieira et al. 1979; Twerdochlib et al. 1994). Koivistoinen et al. (2012) studied the characterization of the gene cluster for L-rhamnose catabolism in the yeast Scheffersomyces (Pichia) stipitis. The genes for L-rhamnose catabolism RHA1, LRA2, LRA3, and LRA4 but not LADH are clustered in Scheffersomyces (Pichia) stipitis and related fungal species, located next to the cluster is a transcription factor, TRC1, which is conserved among related species. Transcriptome analysis shows that all the catabolic genes and all genes in the cluster are up-regulated on L-rhamnose. The LRA4 gene is not part of the cluster and it has several paralogues in L-rhamnose utilizing yeasts, so the function of one of the paralogues, LRA41 by heterologous expression and biochemical characterization was also studied by them.
To date, four structures have been resoluted from the GH78 rhamnosidase family. The first crystal structure of α-L-rhamnosidase RhaB from the Bacillus sp. GL1 is accessible at 1.9Ǻ resolution. This protein is homodimeric, consisting of four ß-sandwich domains, a core catalytic (α/α)6 barrel, contains 956 amino acid residues, and 106 kDa molecular mass (Cui et al. 2007). The second structure of Streptomyces avermitilis (SaRha78A; PDB code 3W5N) α-L-rhamnosidase was determined in complex with L-rhamnose, this protein is large, monomeric, and consisting of six domains (Fujimoto et al. 2013). The third structure, a putative α-L-rhamnosidase from Bacteroides thetaiotaomicron VP1-5482 (BT1001; PDB code 3CIH), determined in a structural genomics project is unpublished, also a homodimer. More recently, the crystal structure of KoRha, a putative α-L-rhamnosidase from Klebsiella oxytoca has been determined at 2.7 Ǻ resolution with rhamnose bound in the active site of the catalytic domain. The structure reveals an elongated homodimer which is significantly smaller than those of the other earlier determined GH78 structures. Asp 222, the putative catalytic acid, is preceded by an unusual non-proline cis-peptide bond which helps to project the carboxyl group into the active center (Neill et al. 2015).
No information on the three-dimensional structure of an α-L-rhamnosidase from yeast is available. So, there is a scientific demand to isolate the gene coding for α-L-rhamnosidase and crystallize α-L-rhamnosidases from yeast and other sources. Structure analysis of family GH78 enzymes will help to clarify their mechanisms of catalysis and substrate specificity and to improve their potential application in a wide variety of industries.
Industrial applications of α-L-rhamnosidase
α-L-Rhamnosidases have several technical applications in the food, chemical, and pharmaceutical industries for the bioconversion of natural or synthetic rhamnosides (Table 2).
Table 2. Biotechnological applications of α-L-rhamnosidase enzyme
|
Industries |
Applications |
|
|
Food and Agriculture
|
Beverages Debittering of fruit juices Removal of hesperidin crystals Aroma enhancement in wine Additives Gellan depolymerization Tomato pulp digestion |
|
|
Chemical |
Naringin extraction Rhamnose preparation Glycolipid’s production |
|
|
Pharmaceutical |
Lectin-directed enzyme activated prodrug therapy (LEAPT) Steroid’s biotransformation Ginsenosides production Antibiotics preparation Prunin preparation Flavonoids deglycosylation |
|
FOOD AND AGRICULTURE INDUSTRY
Beverages
Debittering of fruit juices
Citrus juice turns bitter after extraction due to the chemical naringin (flavanoid) and limonin (limonoid). Naringin is the major component in citrus fruit with a very bitter taste. The enzyme naringinase is composed of α-L-rhamnosidase and β-D-glucosidase. Naringin (4,-5,7’-trihydroxyflavonone-7-rhamnoglucoside) is first hydrolyzed by an α-L-rhamnosidase activity of naringinase to rhamnose and prunin (one-third of the bitterness of naringin) which can be further hydrolyzed into glucose and naringenin by the β-D-glucosidase component of naringinase (Singh et al. 2015). Rhamnose and prunin molecules have great potential, especially in the food and pharmaceutical industries (Fig. 1). Singh et al. (2015) produced debittered kinnow beverage using α-L-rhamnosidase producing Clavispora lusitaniae KF633446 yeast. The decrease of naringin with storage was 443.00 ± 10.00 to 143.70 ± 4.00 ppm due to the α-L-rhamnosidase activity.
Removal of hesperidin crystals
The crystallization of soluble hesperidin in the canned mandarin orange juice causes turbidity to the juice (Baker and Tatum 1986). The hesperidinase enzyme-containing α-L-rhamnosidase activity produced by several fungi, especially Aspergillus niger is used to prevent the presence of hesperidin crystals in citrus products (Soares and Hotchkiss1998). Hesperidin and hesperidin glycosides treated with α-L-rhamnosidase are highly soluble in water and even stored for a long period without crystal precipitation (Miyake and Yumoto 1999).
Aroma enhancement in wine
Rhamnosidase (with β-glucosidase and arabinosidase) is used for aroma enhancement in winemaking. Monoterpene alcohols such as geraniol, linalool, and α-terpineol are the most important flavoring substances in alcoholic beverages such as wine (Marais 1983; Mateo and Jiménez 2000) and sweet potato shochu (Ohta et al. 1990). A large number of mono-terpenes are present in odorless diglycoside conjugates, the hydrolysis of these diglycosides occur in two enzymatic steps (Günata et al. 1988; Zverlov et al. 2000). In a first reaction, depending on the conjugate, the glycosidic linkage is cleaved by either β-D-apiosidase, α-L arabinofuranosidase, or an α-L-rhamnosidase releasing the β-D glucoside. In a second reaction, the β-D-glucoside is hydrolyzed by the action of a β-D-glucosidase causing the release of glucose and a volatile compound aromatically active (Gunata et al. 1988; Gunata 2002; Rodrı´guez et al. 2010). α-L-Rhamnosidase from P. guilliermondii NPCC1053 is an indigenous yeast strain that was able to release monoterpenols and alcohols from grape glycosidic extracts (Rodriguez et al. 2010). P. angusta α-L-rhamnosidase may be useful in winemaking due to the high tolerance to glucose and ethanol, their specificity for the aglycone moieties of grape glycosides, and their hydrolytic activities to natural flavonoid rhamnoglycosides (Yanai and Sato 2000).
Additives
Gellan depolymerization
Gellan, a bacterial exopolysaccharide, has broad application in the food industry owing to its high viscosity in the presence of divalent cations (Cui et al. 2006). Used in the preparation of food additives from biopolymers as well as in the preparation of sweeteners (Giavasis et al. 2000). Bacillus sp. GL1 was isolated to enzymatically modify the viscous properties of gellan gum (Hashimoto et al. 1999) and has a set of enzymes including an α-L-rhamnosidase responsible for the complete depolymerization of gellan gum (Cui et al. 2006).
Tomato pulp digestion
Bacterial α-L-rhamnosidase activity was first attributed to the gut bacteria, which together with β-glucosidase convert ingested flavonoid glycosides into their aglycone forms (Griffiths and Barrow 1972, Macdonald et al. 1983). The rhamnosidases from L. plantarum have been shown to convert flavonoid rutin from tomato into well-absorbed glucosides (Puri 2012, Beekwilder et al. 2009).
Chemical industry
Naringin extraction
In industrial citrus processing, solid waste products are obtained, they are essentially constituted of peel and pulp. The citrus solid waste products are rich in fermentable materials as sugars and pectins, high concentrations of flavanone glycosides, hesperidin, and naringin, and lower amounts of many other flavonoids. The purified α-L-rhamnosidase established hydrolysis of naringin extracted from kinnow peel thus endorses its industrial applicability for producing rhamnose (Puri et al. 2011).
Rhamnose preparation
α-L-rhamnosidase had potential in the manufacture of L-rhamnose by hydrolysis of natural glycosides containing terminal L-rhamnose (Cheetham and Quail 1991) (Fig. 1). Recombinant α-L-rhamnosidase has industrial applicability for the production of rhamnose and prunin from citrus peel waste (Kaur et al. 2010).
Glycolipids production
Glucolipid production from Candida bombicola sophorolipids by P. decumbens naringinase (α-L-rhamnosidase + β-D-glucosidase) showed that the enzyme might be useful for the production of special fatty acids (Saerens et al. 2009).
Pharmaceutical Industry
Lectin-directed enzyme activated prodrug therapy
The lectin-directed enzyme-activated prodrug therapy (LEAPT) bipartite drug delivery system utilizes glycosylated enzyme, localized according to its sugar pattern, and capped prodrugs released by that enzyme. Prodrugs of doxorubicin and 5-fluorouracil capped by the nonmammalian L-rhamnosyl sugar unit have been efficiently synthesized and evaluated for use in the LEAPT system, released by synthetically rhamnosidase enzyme (Robinson et al. 2004; Garnier et al. 2010).
Steroids biotransformation
The hydrolysis of diosgene (a saponin) by α-L-rhamnosidase produces α-L-rhamnose and diosgenin which is used in the synthesis of clinically useful steroid drugs such as progesterone (Elujoba and Hardman 1987). Curvularia lunata rhamnosidase can remove L-rhamnose from a number of steroidal saponins (Feng et al. 2007).
Production of ginsenosides
Glycosyl groups of ginsenosides can be cleaved by α-L-rhamnosidase to produce ginsenosides with improved activities. The ginsenoside-Rh1 obtained from ginsenosides-Rg2 using α-L-rhamnosidase exhibits anticancer activity (Ribeiro 2011).
Antibiotics preparation
The glycopeptides antibiotic chloropolysporin C is prepared from the related compound chloropolysporin B by enzymatic hydrolysis using Rhase (Sankyo 1988). It exhibits antibacterial activity, useful in the treatment and prophylaxis of infections, and is a growth-promoting agent for animals.
Prunin preparation
The flavonoid prunin is produced from naringin using α-L-rhamnosidase activity. It possesses anti-inflammatory and antiviral activity against DNA/RNA viruses (Kaul 1985) (Fig. 1).
Flavonoids deglycosylation
Deglycosylation of flavonoids in Cleome arabica leaf extracts (CALE) with naringinase may be an important therapeutic factor in the treatment of chronic diseases (Bouriche and Arnhold 2010).
This review highlights the information of α-L-rhamnosidases from yeast origin. In our knowledge concerning rhamnosidases, there is a wide gap between yeast and other microorganisms. At present about yeast strains, only a few reports have addressed the enzymatic activity, fermentative production on a large scale, characterization, and its subsequent application. Also, its structural, functional, and molecular biology aspects have not been reported. Although several rhamnosidase coding genes are identified from various bacteria, none have been used for the commercial production of the enzyme. The studies in the above directions will be required for this purpose. Accordingly, there is a need for a process of producing and purifying α-L-rhamnosidase from more efficient new yeast strains which exhibit improved enzyme activity, with enhanced shelf life, high thermal stability, more efficient in food, chemical, and pharmaceutical industries, and non-toxic for human consumption.
Baker RA, Tatum JH (1986) Hesperidin precipitation from orange pulp wash. Proc Fla State Hort Soc 99:90-92.
Barnett JA, Payne RW, Yarrow D (2000) Yeasts: characteristics and identification. Cambridge University Press, Cambridge.
Beekwilder J, Marcozzi D, Vecchi S (2009) Characterization of rhamnosidases from Lactobacillus plantarum and Lactobacillus acidophils. Appl Environ Microbiol 75:3447-3454. PMid:19346347
View Article PubMed/NCBIBirgisson H, Hreggvidsson GO, Fridjonsson OH et al (2004) Two new thermostable α-L-rhamnosidases from a novel thermophilic bacterium. Enzyme Microb Technol 34:561-571.
View ArticleBourbouze R, Percheron F, Courtois JE (1976) α-L-rhamnosidase from Fagopyrum esculentum: purification and some properties. Eur J Biochem 63:331-337. PMid:1261553
View Article PubMed/NCBIBouriche H, Arnhold J (2010) Effect of Cleome arabica leaf extract treated by naringinase on human neutrophil chemotaxis. Nat Prod Commun 5:415-418. PMid:20420319
View Article PubMed/NCBICheetham PSJ, Quail MA (1991) Process for preparing L-rhamnose. US Patent 5,077,206.
Cui Z, Maruyama Y, Mikami B et al (2006) Crystallization and preliminary crystallographic analysis of the family GH 78 α-L-rhamnosidase RhaB from Bacillus sp. GL1. Acta Cryst 62:646-648. PMid:16820683
View Article PubMed/NCBICui Z, Maruyama Y, Mikami B et al (2007) Crystal structure of glycoside hydrolase family 78 α-L-rhamnosidase from Bacillus sp. GL1. J Mol Biol 374:384-398. PMid:17936784
View Article PubMed/NCBIDavis DW (1947) Determination of flavonones in citrus juice. Anal Chem 19:46-48.
View ArticleElujoba AA, Hardman R (1987) Diosgenin production by acid and enzymatic hydrolysis of fenugreek. Fitoterapia 58:299-303.
Feng B, Kang L, Ma B et al (2007) The substrate specificity of a glucoamylase with steroidal saponin-rhamnosidase activity from Curvularia lunata. Tetrahedron 63:6796-6812.
View ArticleFeng B, Ma B, Kang L et al (2005) The microbiological transformation of steroidal saponins by Curvularia lunata. Tetrahedron 61:11758-11763.
View ArticleFujimoto Z, Jackson A, Michikawa M et al (2013) The structure of a Streptomyces avermitilis alpha-L-rhamnosidase reveals a novel carbohydrate-binding module cbm67 within the six-domain arrangement. J Biol Chem 288:12376-12385. PMid:23486481
View Article PubMed/NCBIGallego MV, Pinaaga F, Ramoan D et al (1996) Production and characterization of an Aspergillus terreus α-L-rhamnosidase of oenological interest. Z Lebensm Unters Forsch 203:522-527.
View ArticleGallego MV, Pinaga F, Ramon D et al (2001) Production and characterization of an Aspergillus terreus α-L-rhamnosidase of oenological interest. J Food Sci 66:204-209.
View ArticleGarnier P, Wang XT, Robinson MA et al (2010) Lectin directed enzyme activated prodrug therapy: synthesis and evaluation of rhamnose capped prodrugs. J DrugTargeting 18:794-802. PMid:21047273
View Article PubMed/NCBIGiavasis I, Harvey LM, McNeil B (2000) Gellan gums. Crit Rev Biotech 20:177-211. PMid:11039329
View Article PubMed/NCBIGriffiths LA, Barrow A (1972) Metabolism of flavonoid compounds in germ-free rats. Biochem J 130:1161-1162. PMid:4656801
View Article PubMed/NCBIGunata YZ (2002) Flavour enhancement in fruit juices and derived beverages by exogenous glycosidases and consequences of the use of enzyme preparation. In: Whitaker JR (ed) Handbook of Food Enzymology, New York, Marcel Dekker, pp 303-330.
View ArticleGünata Z, Bitteur S, Brillouet JM et al (1988) Sequential enzymic hydrolysis of potentially aromatic glycosides from grape. Carbohydr Res 184:139-149. 80012-0
View ArticleHall D (1938) A new enzyme of the glycosidase type. Chem Ind 57:473.
View ArticleHashimoto W, Murata K (1998) α-L-Rhamnosidase of Sphingomonas sp. R1 producing an unusual Exopolysaccharide of Sphingan. Biosci Biotechnol Biochem 62:1068-1074. PMid:9692187
View Article PubMed/NCBIHashimoto W, Nankai H, Sato N (1999) Characterization of α-L-rhamnosidase of Bacillussp.GL1 responsible for the complete depolymerization of gellan. Arch Biochem Biophys 368:56-60. PMid:10415111
View Article PubMed/NCBIJang IS, Kim DH (1996) Purification and characterization of α-L-rhamnosidase from Bacteroidesn JY-6, a human intestinal bacterium. Biol Pharm Bull 19:1546-1549. PMid:8996636
View Article PubMed/NCBIKaji A, Ichimi T (1973) alpha-L-Rhamnosidase activity in culture filtrate of Corticium rolfsii. Enzymatic activity at low pH. Agric Biol Chem 37:431-432.
View ArticleKaul TN, Middleton E, Ogra PL (1985) Antiviral effect of flavonoids on human viruses. J Med Viro l15:71-79. PMid:2981979
View Article PubMed/NCBIKaur A, Singh S, Singh RS et al (2010) Hydrolysis of citrus peel naringin by recombinant α-L-rhamnosidase from Clostridium stercorarium. J Chem Technol Biotechnol 85:1419-1422.
View ArticleKoivistoinen OM, Arvas M, Headman JR et al (2012) Characterisation of the gene cluster for L-rhamnose catabolism in the yeast Scheffersomyces (Pichia) stipitis. Gene 492:177-185. PMid:22037608
View Article PubMed/NCBIKoseki T, Mese Y, Nishibori N et al (2008) Characterization of an α-L-rhamnosidase from Aspergillus kawachii and its gene. Appl Microbiol Biotechnol 80:1007-1013. PMid:18633609
View Article PubMed/NCBIKurosawa Y, Ikeda K, Egami F (1973) alpha-L-Rhamnosidases of the liver of Turbo cornutus and Aspergillus niger. J Biochem73:31-37.
Kurtzman CP and Fell JW (1998) Definition, classification and nomenclature of the Yeasts. In: Kurtzman CP and Fell JW (ed) The yeasts, a taxonomic study, Elsevier, Amsterdam, pp 3.
View ArticleKurtzman CP, Fell JW (1998) Definition, classification and nomenclature of the Yeasts. In: Kurtzman C P, Fell JW (ed) The yeasts, a taxonomic study, Elsevier, Amsterdam, pp 3.
View ArticleMacdonald IA, Mader JA, Bussard RG (1983) The role of rutin and quercitrin in stimulating flavonol glycosidase activity by cultured cell-free microbial preparations of human feces and saliva. Mutat Res 122: 95-102. 90044-1
View ArticleMagario I, Neumann A, Oliveros E et al (2009) Deactivation kinetics and response surface analysis of the stability of α-L-rhamnosidase from Penicillium decumbens. Appl Biochem Biotechnol 152:29-41. PMid:18754082
View Article PubMed/NCBIManzanares P, Broeck HCV, Graaff LHD et al (2001) Purification and characterization of two different α-L-rhamnosidases, RhaA and RhaB from Aspergillus aculeatus. Appl Environ Microbiol 67:2230-2234. PMid:11319105
View Article PubMed/NCBIManzanares P, Orejas M, Gil JV et al (2003) Construction of a genetically modified wine yeast strain expressing the Aspergillus aculeatus rhaA gene, encoding an α-L-rhamnosidase of enological interest. Appl Environ Microbiol 69:7558-7562. PMid:14660415
View Article PubMed/NCBIMarais J (1983) Terpenes in the aroma of grapes and wines: a review. S Afr J Enol Vitic 4:49-58.
View ArticleMarkosova K, Lenka W, Michal R et al (2015) Upscale of recombinant α-L-rhamnosidase production by Pichia pastoris MutS strain. Front Microbiol 6:1140. PMid:26539173
View Article PubMed/NCBIMateo JJ, Jimenez M (2000) Monoterpenes in grape juice and wines. J Chromatogr A 881:557-567 01342-4
View ArticleMcMahon H, Zoecklein BW, Fugelsang K et al (1999) Quantification of glycosidase activities in selected yeasts and lactic acid bacteria. J Ind Microbiol Biotechnol 23:198-203.
View ArticleMichlmayr H, Schümann C, Kulbe KD et al (2011) Heterologously expressed family 51 alpha-L-arabinofuranosidases from Oenococcus oeni and Lactobacillus brevis. Appl Environ Microbiol 77:1528-1531. PMid:21169445
View Article PubMed/NCBIMiklosy E, Polos V (1995) Yeasts with β-D-glucosidase activity: properties and possible application in winemaking processes. Acta Aliment 24:167-180.
Miller GL (1959) Use of Dinitrosalicylic Acid Reagent for determination of reducing sugar. Anal Chem 31:426-428.
View ArticleMiyake T, Yumoto T (1999) Enzyme treated hesperidin, process for producing the same and method for using enzyme. US Patent 5,885,969.
Monti D, Pisvejcova A, Kren V et al (2004) Generation of an α-rhamnosidase library and its application for the selective derhamnosylation of natural products. Biotechnol Bioeng 87:763-771. PMid:15329934
View Article PubMed/NCBIMutter M, Beldman G, Schols HA et al (1994) Rhamnogalacturonan α-L-rhamnopyronohydrolase. A novel enzyme specific for the terminal non reducing rhamnosyl unit in rhamnogalacturonan regions of pectin. Plant Physiol 106:241-250. PMid:7972516
View Article PubMed/NCBINeill ECO, Stevenson CEM, Paterson MJ et al (2015) Crystal structure of a novel two domain GH78 family α-rhamnosidase from Klebsiella oxytoca with rhamnose bound. Proteins 83:1742-1749. PMid:25846411
View Article PubMed/NCBIOhta T, Ikuta R, Nakashima M et al (1990) Characteristic flavor of Kansho-shochu (sweet potato spirit). Agric Biol Chem 54:1353-1357.
View ArticleOrejas M, Ibanez E, Ramon D (1999) The filamentous fungus Aspergillus nidulans produce an α-L-rhamnosidase of potential oenological interest. Lett Applied Microb 28:383-388.
View ArticleOrrillo AG, Ledesma P, Delgado OD et al (2007) Cold-active α-L-rhamnosidase from psychrotolerant bacteria isolated from a sub-Antarctic ecosystem. Enzyme Microb Technol 40:236-241.
View ArticlePark S, Kim J, Kim D (2005) Purification and characterization of quercitrin-hydrolyzing alpha-L-rhamnosidase from Fusobacterium K-60, a human intestinal bacterium. J Microbiol Biotechnol 15:519-524.
Pimenta RS, Morais PB, Rosa CA et al. (2009) Utilization of Yeasts in Biological Control Programs, in Yeast Biotechnology: Diversity and Applications. Satyanarayana T and Gotthard K (Editors) Springer, pp 170-199.
View ArticlePuri M (2012) Updates on naringinase: structural and biotechnological aspects. Appl Microbiol Biotechnol 93:49-60. PMid:22080346
View Article PubMed/NCBIPuri M, Banerjee U C (2000) Biotechnology Advances. 18:207-217. 00034-3
View ArticlePuri M, Kaur A, Schwarz WH et al (2011) Molecular characterization and enzymatic hydrolysis of naringin extracted from kinnow peel waste. Int J Biol Macromol 48:58-62 PMid:20920523
View Article PubMed/NCBIQian S, Yu H, Zhang C et al (2005) Purification and characterization of dioscin α-L-rhamnosidase from pig liver. Chem Pharm Bull 53:911-914. PMid:16079518
View Article PubMed/NCBIRibeiro IAC, Ribeiro MHL (2008) Kinetic modelling of naringin hydrolysis using a bitter sweet alfa-rhamnopyranosidase immobilized in k-carrageenan. J Mol Catal B: Enzym 51:10-18.
View ArticleRibeiro MH (2011) Naringinases: occurrences, characteristics and applications. Appl Microbiol Biotechnol 90:1883-1995. PMid:21544655
View Article PubMed/NCBIRigo LU, Nakano M, Veiga LA et al (1976) L-Rhamnose dehydrogenase of Pullularia pullulans Biochim Biophys Acta 445:286-293. 90083-8
View ArticleRobinson MA, Charlton ST, Garnier P et al (2004) LEAPT: lectin directed enzyme activated prodrug therapy. Proc Nat Acad Sci 40:14527-14532. PMid:15448212
View Article PubMed/NCBIRodriguez ME, Lopes CA, Valles S et al (2010) Characterization of α-rhamnosidase activity from a Patagonian Pichia guilliermondii wine strain. J Appl Microbiol 109:2206-2213. PMid:20860771
View Article PubMed/NCBIRomero C, Manjon A, Bastida J et al (1985) A method for assaying the rhamnosidase activity of naringinase. Anal Biochem 149:566-571. 90614-1
View ArticleRosi I, Domizio P, Vinella M et al (1995) Hydrolysis of grape glycosides by enological β-glucosidases. In: Charalambous G (ed) Food flavors: Generation, analysis and process influence. Eliesvier Science, Amsterdam, pp 1623-1635. 80254-2
View ArticleSaerens K, Bogaert IV, Soetaert W et al (2009) Production of glucolipids and specialty fatty acids from sophorolipids by Penicillium decumbens naringinase: optimization and kinetics. Biotechnol J 4:517-524 PMid:19194976
View Article PubMed/NCBISankyo (1988) Preparation of antibiotic chloropolysporin-C. Jap Patent 63146797.
Scaroni E, Cuevas L, Ellenrieder G (2002) Hydrolytic properties of crude alpha-L-rhamnosidases produced by several wild strains of mesophilic fungi Lett Appl Microbiol 34:461-465. PMid:12028430
View Article PubMed/NCBISingh P, Sahota PP, Bhadra F et al (2015) Optimization, production and scale up of debittered kinnow beverage by α-L-rhamnosidase producing yeast E J F A 27:548-555.
View ArticleSingh P, Sahota PP, Singh RK (2015) Evaluation and characterization of new α-L-rhamnosidase producing yeast strains. J Gen Appl Microbiol 61:149-156. PMid:26582283
View Article PubMed/NCBISingh P, Sahota PP, Singh RK (2018) Optimization of media components for production of α-L-rhamnosidase from Clavispora lusitaniae KF633446. Int J Curr Microbiol App Sci 7:2947-2959.
View ArticleSoares NFF, Hotchkiss JH (1998) Naringinase immobilization in packaging films for reducing naringin concentration in grapefruit juice. J Food Sci 63:61-65
View ArticleSoria F, Ellenrieder G (2002) Biosci Biotechnol Bioch 66:1442-1449. PMid:12224626
View Article PubMed/NCBISpagna G, Barbagallo RN, Martino A et al (2000) A simple method for purifying glycosidases: α-L-rhamnopyranosidase from Aspergillus niger to increase the aroma of Moscato wine. Enzyme Microb Tech 27:522-530 00236-2
View ArticleSuzuki H (1962) Hydrolysis of flavonoid glycosides by enzymes from Rhamnus and other sources. Arch Biochem Biophys 99:476-483. 90296-5
View ArticleThomas DW, Smythe CV, Labbee MD (1958) Enzymatic hydrolysis of naringin, the bitter principle of grapefruit. Food Res 23:591-598.
View ArticleTwerdochlib AL, Pedrosa FO, Funayama S et al (1994) L-Rhamnose metabolism in Pichia stipitis and Debaryomyces polymorphus. Can J Microbiol 40: 896-902.
View ArticleVictor DB, Cedric HLS, Jeanette W (1987) Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem J 248:953-956. PMid:3435494
View Article PubMed/NCBIVieira MM, Rigo LU, Marechal LR et al (1979) Induction and catabolite repression of L-rhamnose dehydrogenase in Pullularia pullulans. J Bacteriol 138:55-59. PMid:438135
View Article PubMed/NCBIYadav V, Yadav PK, Yadav S et al. (2010) α-L-rhamnosidase: A review. Process Biochem 45:1226-1235.
View ArticleYanai T and Sato M (2000) Purification and characterization of α-L-rhamnosidase from Pichia angusta X349. Biosci Biotechnol Bioch 64:2179-2185. PMid:11129592
View Article PubMed/NCBIZverlov VVC, Hertel K, Bronnenmeier A et al (2000) The thermostable α-L-rhamnosidase RamA of Clostridium stercorarium: biochemical characterization and primary structure of a bacterial α-L-rhamnosidase hydrolase, a new type of inverting glycoside hydrolase. Mol Microbiol 35:173-179. PMid:10632887
View Article PubMed/NCBI