Dr. HUSSAINI ALHASSAN MOHAMMED
Email Address: halhassanmohd@gmail.com, hussaini.alhasan@udusok.edu.ng
GSM NO: +2348039705336
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 1
Dr. HUSSAINI ALHASSAN MOHAMMED
Email Address: halhassanmohd@gmail.com, hussaini.alhasan@udusok.edu.ng
GSM NO: +2348039705336
ALHASSAN Hussaini Mohammed1,2, Abbas Mirshafiey1, Homayoun Vahedi3, Mir Saeed yekaninajad4, Ghodratollah Panahi5, Hadi Hassannia1, Fatemeh Mansoori1 and Ali Akbar Saboor-Yaraghi1
Dr. ALHASSAN Hussain Mohammed, The Regulations of Gene Expressions by 1α,25(OH)2D3 in Patients with Inflammatory Bowel Diseases(2019)Journal of Advances In Allergy & Immunologic Diseases 3(1)
Introduction: Changes in vitamin D serum levels have been associated with inflammatory diseases, such as in IBD. Genome and transcriptome wide studies indicate that vitamin D signaling modulates many inflammatory responses on several levels. Inflammatory bowel diseases (IBD) are chronic relapsing immune-mediated disorders that result from an aberrant immunological response. IBD comprises of Crohn's disease and ulcerative colitis. The increasing number of hospitalisation coupled with the high economic burden experienced by IBD patients, calls for more concerted research efforts, to design a potent and credible treatment option for these strata of patients.
Aims/Objectives: This research was designed to assess the efficacy of 1α,25(OH)2D3 on the expression of IL-17, RORC, IL-4, and GATA3 genes in PBMC of Inflammatory bowel diseases (IBD) patients.
Materials and Methods: Ten(10) ml of blood was aseptically collected from 24 IBD patients and 24 healthy controls. PBMC was isolated and stimulated with 1µg/ml of LPS and incubated for 4 hrs. The cells were later treated with 10-10 and 10-8 M of 1α,25(OH)2D3 and incubated at 370C under 5% CO2 and 100% humidity. The RNA extractions, cDNA synthesis, and QRT-PCR was later performed.
Results: The result shows a significant down-regulation of RORC gene and IL-17 expression, while the IL-4 and GATA3 gene expression were significantly up-regulated.
Conclusion: This result is an indication that 1α,25(OH)2D3 possesses not just immunomodulatory potentials, but also has immunosuppressive effects on cytokines that are pivotal in the pathogenesis of IBD , which is to say that, it can be used in management and treatment of both UC and CD patients.
Keywords: 1α,25(OH)2D3, IBD, Ulcerative colitis, Crohn's disease, Immunosuppression
Inflammatory bowel diseases (IBD) can be described as a group of idiopathic, chronic, and relapsing inflammatory disorders whose etiologic origins are yet to be understood, but many investigations have attributed the emergence of this disease to genetic susceptibility, immune dysregulations, environmental factors or bacterial infections [1,2]. IBD is categorised into two major classes. Ulcerative colitis (UC) and Crohn's disease (CD), each of with its distinguishing characteristic. Ulcerative colitis (UC) is a Th2-like inflammatory disorder where the morphological changes are restricted to the colon. In about 95% of patients diagnosed with ulcerative colitis, the rectum is involved[3]. Crohn's disease is a Th-1-mediated inflammatory disorder, which may extend from the oesophagus to the anus, but the ileocecal region and terminal ileum are the most commonly affected areas[4] . Inflammation in CD is usually transmural with a resultant fistula formation. In most cases, the affected parts are bridged by intervening normal bowel[4,5]. The 1α,25(OH)2D3 has multiple immunomodulatory, anti-inflammatory and immunosuppressant properties. Supplementation of 1α,25(OH)2D3 was shown to be therapeutically effective in various animal models such as type 1 diabetes mellitus[6], IBD[7] and systemic lupus erythematosis (SLE)[8]. 1,25(OH)2D3 seems to interact with the immune system through its actions on the regulation and differentiation of cells such as lymphocytes, macrophages and natural killer cells (NK). Some notable immunomodulatory and immunosuppressive effects of 1α,25(OH) 2D3, are down-regulation of IL-2, IL-12, IL-6, IFN-γ, TNF-α; and Upregulation in the production of IL-4, IL-5 and IL-10 [9] .
The proliferation and ultimate differentiation of Naïve T-cells play a pivotal role in the development of IBD[10-11]. IL4 is a major Th-2 cytokine subset; its expression is controlled by the master regulator, the GATA3. The therapeutic intervention that up-regulates the GATA3 gene expression will inadvertently affect the expression of IL-4 [4]. RORγt or RORC in human is the key transcription factor that orchestrates the differentiation of Th-17 effector cell lineage. RORC induces transcription of the genes encoding for IL-17 and the related cytokine IL-17F in naıve CD4+ T helper cells and is required for their expression in response to IL-6 and TGF-β, the cytokines known to induce IL-17 [12]. Mice with RORγt deficient T cells have attenuated autoimmune disease and lack tissue-infiltrating Th17 cells. Altogether, these findings suggest that RORC are the key regulator of immune homoeostasis and highlight their potential as a therapeutic target in IBD[12].
It is worthy to note that most of the immune modifiers, and anti-inflammatory drugs used in the management of IBD, are without side effects, such as over-dependence and damage to some vital organs of the body, such as liver, Kidney, lungs, and GIT, leading to the development of colorectal cancers and other debilitating diseases [13-15]. It is paramount, therefore to develop safe, effective, and affordable treatment options that will replace the ones presently available. This research was designed to assess the regulations and therapeutic efficacy of 1α,25(OH)2D3 on the expression of IL-4, GATA3, RORC, and IL-17 in the PBMC of IBD patients.
Study Population.
The participants were recruited from the Digestive Disease Research Institute (DDRI), Shariati Hospital and Gastroenterology Department, Firosgar Hospital, Iran University of Medical Sciences, Tehran, between Feb.,2016 to May, 2017. None of the selected participants were on immunosuppressive and anti-inflammatory agents, such as anti-TNF drugs, cortisone and other drugs that could interfere with the research findings. Pregnant and lactating mothers, Smokers, HIV positive patients and drug users were all excluded from the study. The research participants cut across all age groups and have fulfilled all the above mentioned inclusive criteria. The mean age ranges were 31.0417± 5.39306 for the patients and 29.8333±5.70024 for the normal controls.Samples were collected from IBD patients (n = 24) aged 19-64 (mean age= 30 ) and healthy adults (n = 24)
Ethical Approval and Informed Consent.
The study was approved by the Ethical committee of Tehran University of Medical Sciences (TUMS) Tehran, and the research participants were recruited after informed consent was obtained from them. The research was conducted in line with the international best practice in conformity with the international standard ethical protocol.
Blood collections and PBMC Isolations.
Ten ml of blood samples was obtained from 24 patients and 24 normal controls into EDTA container. Peripheral blood mononuclear cells (PBMC) were isolated using Ficol-Paque centrifugation method (Amersham Pharmacia Biotech, Uppsala-Sweden). The Isolated PBMC was suspended in RPMI 1640 (GIBCO) with 10% FBS (GIBCO) and 1% penstrip. The cells were counted using improved Neubauer hemacytometer counting chamber.
Experimental Groups.
There are four groups for each patients, these are matched by age to age and sex to sex with appropriate normal control. The groups are Normal controls(NC), Positive control(PC), Vitamin D low dose (VLD), and Vitamin D high dose (VHD). Both the normal control (NC) and the positive control (PC) were not treated with 1α,25(OH)2D3. Group VLD were treated with 10-10M low dose 1α,25(OH)2D3 While group VHD were treated with 10-8M of 1α,25(OH)2D3.
Stimulation and Treatment of PBMC In cell Culture
The stimulations and treatments of all individual groups were done in Step IV safety carbinet. To a 24 well culture plate, 1.5 x106 Cells were added to each well. The cells were stimulated with 10µg/ml of LPS (Sigma) and incubated for 4 hours at 370C in 5% CO2 and in 100% humidified air. After 4 hours of incubation, the PBMC was treated with 10-10 and 10-8 M of low and high doses of 1α,25(OH)2D3 respectively. The cells were again incubated for an additional 24 hours at 370C in 5% CO2 and in 100% humidified air.
RNA Extractions
The cells were harvested from the cell culture plates into 2ml Eppendorf tube and centrifuge at 12,000 RCF for 10 minutes to separate the cells from the supernatants. The supernatants were stored at -700C until ready for cytokines assay. Total RNA was extracted from 2.5×106 cells, using GeneAll® Hybrid-RTM kits, Cat.No.305-101 (Songpa-Gu, Seoul, Korea 138-859, based on the manufacturer's instructions. The final concentration and purity of the total RNA were measured by NanoDrop 1000 UV–Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and stored at -700C prior to cDNA synthesis.
cDNA Synthesis.
Cyclic Deoxyribonucleic Acid (cDNA) synthesis was carried out using cDNA primescriptTM reagent Kit, Takara BIO. INC (Perfect Real Time), Cat NO: RR037A, lot NO: AK5601 (Nojihigashi 7-4-38, Kusatsu, Shiga 525-0058 Japan), based on manufacturer's instructions. The cDNA synthesised was stored at -200, until required for qRT-PCR.
Real-Time PCR
The quantitative real-time PCR was done using SYBR® Premix Ex Taq™ II (Takara Co., Ltd.) with all the specific primers from Sigma-Aldrich as shown in Table:1. The cytokine analysis and gene transcriptions of IL-4, GATA3, IL-17, RORC and GAPDH were carried out using StepOnePlus™ Real-Time PCR Systems (Applied Biosystems, Foster City, CA, USA). The relative expressions of target gene mRNA compared to the endogenous control, GAPDH mRNA, were measured using a ΔCT method with reference to each amplification plot (fluorescence signal vs cycle number). The mean difference (ΔCT) between the values in the replicate samples of target genes and that of the endogenous control, GAPDH mRNA was calculated. The changes in the expressions of the target genes and the normal group were calculated using, ΔCTPatients – ΔCTControls = ΔΔCT. This is further expressed as a relative fold change or expression of the gene in the patients compared to the normal healthy control (2^-ΔΔCT).
Table 1: PRIMER SEQUENCES FOR qRT- PCR
|
Primers |
Forward |
Reverse |
|
IL-17 |
5’-CTGTCCCCATCCAGCAAGAG-3’
|
5’-AGGCCACATGGTGGACAATC-3’
|
|
RORC |
5’-GTGGGGACAAGTCGTCTGG-3’
|
5’-AGTGCTGGCATCGGTTTCG-3’
|
|
IL-4 |
5’-CCAACTGCTTCCCCCTCTG-3’
|
5’-TCTGTTACGGTCAACTCGGTG-3’
|
|
GATA3 |
5’-GAGGGCTGGTTTCCTTGACTG-3’
|
5’-AAAAAGGGGCGACGACTCTG-3’
|
|
GAPDH |
5’-ACAACTTTGGTATCGTGGAAGG-3’
|
5’-GCCATCACGCCACAGTTTC-3’
|
PCR= Polymerase chain reaction, IL-17= Interleukin-17, RORC= RAR-related orphan receptor C, IL-4= Interleukin-4, GATA3= Gata binding protein-3, GAPDH=Glyceraldehyde 3-phosphate dehydrogenase
Statistical Analysis
All statistical analysis was carried out by SPSS software (22.0; IBM Corporation, Chicago, IL, USA).. The analysis of variance (ANOVA) test was used to compare the quantitative variables between the groups and the Post hoc turkey was used to determine significant differences in the gene expression level between the controls and treated groups. P≤0.05 was statistically considered as a significant expression. Graphs were designed using, GraphPad Prism software version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
The results of this study as presented in Fig.1-4 has evidently shown the enormous immunosuppressive and immunomodulatory potentials of 1α,25(OH)2D3 in the regulation of cytokines that are critical in the pathogenesis of IBD.
The level of IL-17 gene expression in untreated patients’ PBMC was 4.12 fold higher than that in the normal controls. After 24 hours of incubation and treatment with low and high doses of 1α,25(OH)2D3, the relative expression of 2.26 and 1.17 fold decrease in low and high doses of 1α,25(OH)2D3 was observed, respectively. This is a significant down–regulation in IL-17 expression between the treated and untreated groups (Fig.1).
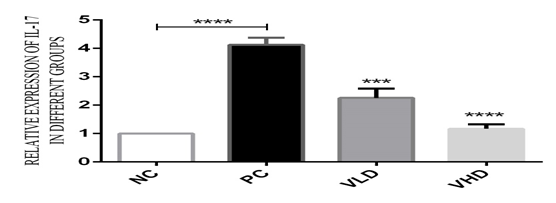
Fig. 1: The relative folds expression of IL-17 in untreated and treated groups: In the untreated groups the PBMC was stimulated with 10µg/ml of LPS and incubated for 24hours. The relative expression of IL-17 was 4.13 fold increase in the patients’ PBMCs compared to the normal control. In the treated group, after the stimulation of the PBMC with 10µg/ml of LPS, and incubated for 4 hours, the PBMC was treated with 10-8 and 10-10M of 1α,25(OH)2D3 and incubated for 24hours. The relative expression of IL-17 has indicated a 2.26 and 1.17 fold decrease in the treated groups compared to untreated groups. Statistically P<0.05 is considered significant. (***P<0.001, ****P< 0.0001 ). All data are representative of three independent qPCR experiments.
The level of RORC gene expressions in untreated patients’ PBMCs was 5.1 fold higher than in the normal control . After 24 hours of incubation and treatment of patients’ PBMC with 1α,25(OH)2D3, there was a statistically significant down-regulation in the relative fold expressions of RORC between the treated and untreated groups. The levels of RORC expressions in the PBMC of patients treated with low and high doses of 1α,25(OH)2D3 was 3.5 and 1.3 fold respectively, compared to the untreated patients’ PBMC (Fig.2).
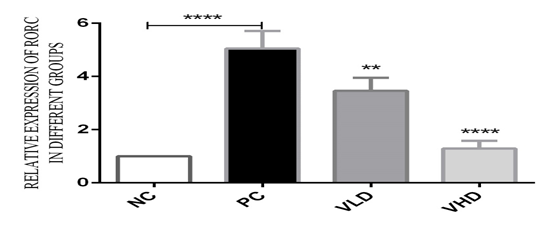
Fig.2: The relative folds expression of RORC in untreated and treated groups: In the untreated groups the PBMC was stimulated with 10µg/ml of LPS and incubated for 24hours. The relative folds expression of RORC was 5.1 fold increases in the patients PBMCs compared to the normal control. In the treated group, after the stimulation of the PBMC with 10µg/ml of LPS, and incubated for 4 hours, the PBMC was treated with 10-8 and 10-10M of 1α,25(OH)2D3 and incubated for 24hours. The relative expression of RORC has indicated a 3.5 and 1.3 fold decreases in the treated groups compared to untreated groups. Statistically P<0.05 is considered significant. ( **P< 0.01, ****P< 0.0001 ). All data are representative of three independent qPCR experiments.
There was a 0.23 fold decrease in the level of IL-4 expression in the untreated patients compared to the normal control. This is an indication that the level of circulating IL-4 cytokine is very low in IBD patients compared to the healthy subjects, but after 24 hours of treatment with 1α,25(OH)2D3, there was a sharp up-regulation in relative fold expression in the treated groups. The levels of IL- 4 gene expression in the PBMC of patients treated with low and high doses of 1α,25(OH)2D3 were 1.23 and 2.16 fold respectively, compared to the untreated patients PBMC (Fig.3).
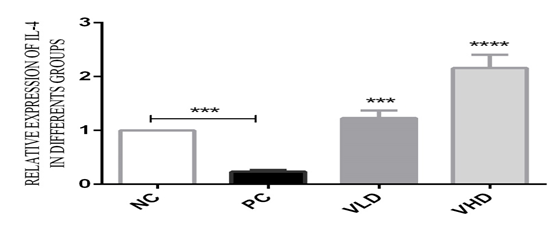
Fig. 3: The relative folds expression of IL-4 in untreated and treated groups: In the untreated groups the PBMC was stimulated with 10µg/ml of LPS and incubated for 24hours. The relative folds expression of IL-4 was 0.23 fold decreases in the patients PBMCs compared to the normal control. In the treated group, after the stimulation of the PBMC with 10µg/ml of LPS, and incubated for 4 hours, the PBMC was treated with 10-8 and 10-10M of 1α,25(OH)2D3 and incubated for 24hours. The relative expression of IL-4 has indicated a 1.23 and 2.16 fold increase in the treated groups compared to untreated groups. Statistically P<0.05 is considered significant. (***P<0.001, ****P< 0.0001). All data are representative of three independent qPCR experiments.
GATA3 gene expression in untreated patients PBMC was low when compared to the normal controls. The relative gene expression of GATA3 in untreated patients was 0.15 fold compared to the normal healthy controls, but after 24 hours of incubation and treatment with 1α,25(OH)2D3, there was a significant up-regulation of the GATA3 fold expression compared to untreated patients. The levels of GATA3 gene expression in the PBMC of patients treated with low and high doses of 1α,25(OH)2D3 has been up-regulated to 1.22 and 1.56 fold respectively, compared to the untreated patients PBMC (Fig.4). P<0.05 is statistically considered significant in the treatment groups.
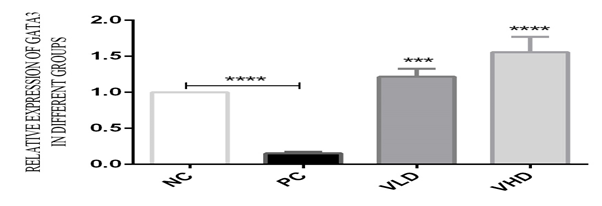
Fig.4: The relative folds expression of GATA3 in untreated and treated groups: In the untreated groups the PBMC was stimulated with 10µg/ml of LPS and incubated for 24hours. The relative folds expression of GATA3 was 0.15 fold decreases in the patients PBMCs compared to the normal control. In the treated group, after the stimulation of the PBMC with 10µg/ml of LPS, and incubated for 4 hours, the PBMC was treated with 10-8 and 10-10M of 1α,25(OH)2D3 and incubated for 24hours. The relative expression of GATA3 has indicated a 1.22 and 1.56 fold increases in the treated groups compared to untreated groups. Statistically P<0.05 is considered significant. (***P<0.001, ****P< 0.0001 ). All data are representative of three independent qPCR experiments
The 1α,25(OH)2D has diverse immunomodulatory functions. The vitamin D receptor (VDR) is widely expressed on most immune cellular subsets and VDR ligation by vitamin D results in activation of key innate immune cells such as monocytes, macrophages, and neutrophils leading to enhanced immunological activities [16–18]. The effects of 1,25(OH)2D3 as inhibitors of T cells have been well described. Since 1983 it has been described that 1α,25(OH)2D3 inhibites T cell proliferation and the secretion of selective cytokines after mitogen stimulation [19-20]. IL-17 is produced mainly by Th17 cells, although CD8+ T cells are also able to produce this cytokine during chronic inflammation [21]. IL-17 acts as a key mediator in delayed-type immune reactions by increasing chemokine production and recruiting monocytes and neutrophils to the inflammatory site[22]. In our study, there were significant up-regulation in the level of IL-17 gene expression in the PBMC of IBD patients compared with the control. This result was in conformity with other reported research findings in humans, where elevated levels of IL-17 in active CD and UC was reported[23]. Most recently, it has been shown that in CD patients increased numbers of circulating IL-17 and IFN-γ-producing CD161+ memory cells are present, and these cells constitute a high percentage of colonic mucosal cells[24]. In addition, CD patients have numbers of circulating IL-23R expressing T cells, which respond to IL-23 with increased production of IL-17, IL-22 and IFN-γ, which is further increased by the presence of IL-1β [24]. 1α,25(OH)2D3 has also been shown to inhibit IL-17 secretion by Th17 cells [25,26]. This is in agreement with our results, findings where after 24 hours of treatment and incubation of 1α,25(OH)2D3 with the PBMC of the patients, there was significant down-regulation of IL-17 relative gene expression when compared to the untreated patients’ PBMCs. Research has shown that Th1 and Th17 cells cause experimental autoimmune encephalomyelitis (EAE, murine model of multiple sclerosis), IBD and type-1 diabetes. In vivo, 1,25(OH)2D3 treatments suppressed the development and progression of these Th1/Th17 mediated diseases [27–29]. In addition, vitamin D and VDR deficiency exacerbated experimental type-1 diabetes and IBD in mice [27,30]. Moreso, in vitro treatment of T-cells with active Vitamin D suppresses Th17 development and inhibits production of IL-17 [31].
RORC in human is the master regulator for the Th17 differentiation. Individual deficient in RORC has limited Th17 differentiation, while overexpression RORC induced IL-17 expression in the absence Th17 polarising cytokines [13]. In our study, there was a 5.1 fold increase in the level of RORC gene expression in untreated patients than in the normal control. The relative overexpression of RORC observed in this study may be responsible for the increase in the expression of IL-17 observed in this study because of the synergy that existed between RORC and IL-17 gene expression [32]. This findings is in agreement with the research findings which states that in IBD, IL-23 promote differentiation of naïve CD4+ T cells into Th17 cells and this is characterized by increased expression of IL-17A and RORC in IBD patients [33], but in vitro treatment with 1α,25(OH)2D3, has shown a significant down-regulation in RORC and IL-17 [31] . This report is in line with our research findings, in which a significant down-regulation of RORC gene expression was observed in both low and high doses of 1α, 25(OH)2D3 after 24 hours of treatment and incubation.
IL4 is a major Th-2 cytokine subset; its expression is controlled by the master regulator, the GATA3, It is widely reported that IBD is characterised by a significant decrease in IL-4 gene expression[34]. This was confirmed in our study, where the level of IL-4 was significantly down-regulated compared to the healthy control, but after 24 hours of treatment and incubation with 1α,25(OH)2D3, a fold increase of 1.23 and 2.16 was observed in IL-4 gene expression in the treated groups compared to untreated groups. In vitro, 1,25(OH)2D3 treatment of T cells has been shown to increase IL-4 secretion by human and mouse Th cells [35,36,37]. In human PBMC 1,25(OH)2D3 induced the expression of IL-4 when added in vitro. The differentiation of Th2 requires GATA3 Master regulator, deficiency of GATA3 expression lead to loss of Th2 cells expression, resulting in low expression of IL-4 and other anti-inflammatory cytokines [37]. In untreated group, the relative folds expression of GATA3 was 0.15 fold decrease in the patients PBMCs compared to the normal control. In the treated group, the relative expression of GATA3 has indicated a 1.22 and 1.56 fold increases in the treated groups compared to the untreated group. In general, the 1,25(OH)2D3 regulation of IL-17, RORC, IL-4 and GATA3 is brought about by GATA3 dependant and GATA3 independent mechanism [38]. The 1,25(OH)2D3-mediated regulation of Th17 polarization occurs through GATA3-dependent mechanisms, including direct effects on RORC expression and IL-4-mediated inhibition of Th17 polarization. Moreover, GATA3-independent mechanisms are involved in the modulation of NFAT-C2. These mechanisms may play a role in the suppressive effect of 1,25(OH)2D3 in autoimmune disease activity [38].
The result of this research has indicated that the unmet needs for the development of novel therapies for IBD, could be fulfilled with the utilization of 1,25(OH)2D3. The potency and efficacy of this drug was elucidated by this research finding and others. The drug was able to significantly reduce, the relative gene expression of IL-17 and RORC to the barest minimum, while up-regulating the relative gene expressions of IL-4 and its transcription factor GATA3 in the PBMC of IBD. This research is an ex vivo study, there is a need for large scale in vivo clinical trials to determine the effects of this drugs in human subjects.
We wish to extend our sincere gratitude to the Research office International Campus, Tehran University of Medical Sciences, for their support towards the conduct of this research. We also wish to say thank you to the management of the Digestive Diseases Research Institute (DDRI), Shariati Hospital, and Gastroenterology and Endoscopy Department, Firouzgar Hospital for their roles in the recruitment of the research.
H. ALHASSAN Mohammed*, A Mirshafiey, H Vahedi, G Hemmasi, A. Moussavi Nasl Khameneh, K. Parastouei & A.A. Saboor-Yaraghi. Immunoregulation of inflammatory and inhibitory cytokines by Vitamin D3 in patients with inflammatory bowel diseases(IBD). Scandinavian Journal of Immunology 2017, 85(6):386-394, ISSN: 0300-9475. (DOI: 10.1111/sji.12547) PMid:28332200
View Article PubMed/NCBIPodolsky, D. K. The current future understanding of inflammatory bowel disease. Best practice & research Clinical gastroenterology 2002, 16, 933-943.
View ArticleMichener, W. M.; Whelan, G.; Greenstreet, R. L.; Farmer, R. G. Comparison of the clinical features of Crohn's disease and ulcerative colitis with onset in childhood or adolescence. Cleveland Clinic Quarterly 1982, 49, 13. PMid:7094330
View Article PubMed/NCBIFuss, I. J.; Neurath, M.; Boirivant, M.; Klein, J. S.; De La Motte, C.; Strong, S. A.; Fiocchi, C.; Strober, W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. The Journal of Immunology 1996, 157, 1261-1270.
View ArticleBamias, G.; Nyce, M. R.; Sarah, A.; Cominelli, F. New concepts in the pathophysiology of inflammatory bowel disease. Annals of internal medicine 2005, 143, 895-904. PMid:16365470
View Article PubMed/NCBIMathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Prevention of autoimmune diabetes in NOD mice by 1, 25 dihydroxyvitamin D 3. Diabetologia 1994, 37, 552-558. PMid:7926338
View Article PubMed/NCBICantorna, M. T.; Munsick, C.; Bemiss, C.; Mahon, B. D. 1, 25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. The Journal of nutrition 2000, 130, 2648-2652. PMid:11053501
View Article PubMed/NCBILemire, J. M.; Ince, A.; Takashima, M. 1, 25-Dihydroxyvitamin D3 attenuates of expression of experimental murine lupus of MRL/1 Mice. Autoimmunity 1992, 12, 143-148. PMid:1617111
View Article PubMed/NCBIMunger, K. L.; Levin, L. I.; Hollis, B. W.; Howard, N. S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama 2006, 296, 2832-2838. PMid:17179460
View Article PubMed/NCBISteinman, R. M.; Hawiger, D.; Nussenzweig, M. C. Tolerogenic dendritic cells. Annual review of immunology 2003, 21, 685-711. PMid:12615891
View Article PubMed/NCBIKohu, K.; Ohmori, H.; Wong, W. F.; Onda, D.; Wakoh, T.; Kon, S.; Yamashita, M.; Nakayama, T.; Kubo, M.; Satake, M. The Runx3 transcription factor augments Th1 and down-modulates Th2 phenotypes by interacting with and attenuating GATA3. The Journal of Immunology 2009, 183, 7817-7824. PMid:19933870
View Article PubMed/NCBIIvanov, I. I.; McKenzie, B. S.; Zhou, L.; Tadokoro, C. E.; Lepelley, A.; Lafaille, J. J.; Cua, D. J.; Littman, D. R. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121-1133. PMid:16990136
View Article PubMed/NCBISingh, J. A.; Furst, D. E.; Bharat, A.; Curtis, J. R.; Kavanaugh, A. F.; Kremer, J. M.; Moreland, L. W.; O'Dell, J.; Winthrop, K. L.; Beukelman, T. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis care & research 2012, 64, 625-639. PMid:22473917
View Article PubMed/NCBIGotzsche, P.; Johansen, H. Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev 2005, 1, CD000189.
View ArticleLaine, L.; Bombardier, C.; Hawkey, C. J.; Davis, B.; Shapiro, D.; Brett, C.; Reicin, A. Stratifying the risk of NSAID-related upper gastrointestinal clinical events: results of a double-blind outcomes study in patients with rheumatoid arthritis. Gastroenterology 2002, 123, 1006-1012. PMid:12360461
View Article PubMed/NCBIKongsbak, M.; Levring, T. B.; Geisler, C.; von Essen, M. R. The vitamin d receptor and T cell function. Frontiers in immunology 2013, 4, 148. PMid:23785369
View Article PubMed/NCBIBeard, J. A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. Journal of Clinical Virology 2011, 50, 194-200. PMid:21242105
View Article PubMed/NCBIWhite, A. N.; Ng, V.; Spain, C. V.; Johnson, C. C.; Kinlin, L. M.; Fisman, D. N. Let the sun shine in: effects of ultraviolet radiation on invasive pneumococcal disease risk in Philadelphia, Pennsylvania. BMC infectious diseases 2009, 9, 196. PMid:19961583
View Article PubMed/NCBIRigby, W.; Stacy, T.; Fanger, M. W. Inhibition of T lymphocyte mitogenesis by 1, 25-dihydroxyvitamin D3 (calcitriol). Journal of Clinical Investigation 1984, 74, 1451. PMid:6332829
View Article PubMed/NCBIProvvedini, D. M.; Tsoukas, C. D.; Deftos, L. J.; Manolagas, S. C. 1, 25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181-1183. PMid:6310748
View Article PubMed/NCBIShen, W.; Durum, S. K. Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochemical research 2010, 35, 940-946. PMid:19915978
View Article PubMed/NCBIMangan, P. R.; Harrington, L. E.; O'quinn, D. B.; Helms, W. S.; Bullard, D. C.; Elson, C. O.; Hatton, R. D.; Wahl, S. M.; Schoeb, T. R.; Weaver, C. T. Transforming growth factor-β induces development of the TH17 lineage. Nature 2006, 441, 231-234. PMid:16648837
View Article PubMed/NCBISakuraba, A.; Sato, T.; Kamada, N.; Kitazume, M.; Sugita, A.; Hibi, T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology 2009, 137, 1736-1745. PMid:19632232
View Article PubMed/NCBIKleinschek, M. A.; Boniface, K.; Sadekova, S.; Grein, J.; Murphy, E. E.; Turner, S. P.; Raskin, L.; Desai, B.; Faubion, W. A.; de Waal Malefyt, R. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. Journal of Experimental Medicine 2009, 206, 525-534. PMid:19273624
View Article PubMed/NCBIPalmer, M. T.; Lee, Y. K.; Maynard, C. L.; Oliver, J. R.; Bikle, D. D.; Jetten, A. M.; Weaver, C. T. Lineage-specific effects of 1, 25-dihydroxyvitamin D3 on the development of effector CD4 T cells. Journal of Biological Chemistry 2011, 286, 997-1004. PMid:21047796
View Article PubMed/NCBIBoonstra, A.; Barrat, F. J.; Crain, C.; Heath, V. L.; Savelkoul, H. F.; O'Garra, A. 1α, 25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. The Journal of Immunology 2001, 167, 4974-4980. PMid:11673504
View Article PubMed/NCBIZella, J. B.; McCary, L. C.; DeLuca, H. F. Oral administration of 1, 25-dihydroxyvitamin D 3 completely protects NOD mice from insulin-dependent diabetes mellitus. Archives of biochemistry and biophysics 2003, 417, 77-80. 00338-2
View ArticleGregori, S.; Giarratana, N.; Smiroldo, S.; Uskokovic, M.; Adorini, L. A 1α, 25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002, 51, 1367-1374. PMid:11978632
View Article PubMed/NCBIFroicu, M.; Zhu, Y.; Cantorna, M. T. Vitamin D receptor is required to control gastrointestinal immunity in IL‐10 knockout mice. Immunology 2006, 117, 310-318. PMid:16476050
View Article PubMed/NCBIBettelli, E.; Korn, T.; Kuchroo, V. K. Th17: the third member of the effector T cell trilogy. Current opinion in immunology 2007, 19, 652-657. PMid:17766098
View Article PubMed/NCBIBurgler, S.; Mantel, P.-Y.; Bassin, C.; Ouaked, N.; Akdis, C. A.; Schmidt-Weber, C. B. RORC2 is involved in T cell polarization through interaction with the FOXP3 promoter. The Journal of Immunology 2010, 184, 6161-6169. PMid:20427770
View Article PubMed/NCBILiu, Z.; Yadav, P. K.; Xu, X.; Su, J.; Chen, C.; Tang, M.; Lin, H.; Yu, J.; Qian, J.; Yang, P.-C. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. Journal of Leukocyte Biology 2011, 89, 597-606. PMid:21227898
View Article PubMed/NCBIKarttunnen, R.; Breese, E.; Walker-Smith, J.; MacDonald, T. Decreased mucosal interleukin-4 (IL-4) production in gut inflammation. Journal of clinical pathology 1994, 47, 1015-1018. PMid:7829675
View Article PubMed/NCBIMahon, B. D.; Wittke, A.; Weaver, V.; Cantorna, M. T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. Journal of cellular biochemistry 2003, 89, 922-932. PMid:12874827
View Article PubMed/NCBIColin, E.; Asmawidjaja, P.; van Hamburg, J. P.; Mus, A.; van Driel, M.; Hazes, J.; Van Leeuwen, J.; Lubberts, E. 1, 25‐dihydroxyvitamin D3 modulates Th17 polarization and interleukin‐22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis & Rheumatology 2010, 62, 132-142. PMid:20039421
View Article PubMed/NCBICantorna, M. T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1, 25 (OH) 2D regulation of T cells. Nutrients 2015, 7, 3011-3021. PMid:25912039
View Article PubMed/NCBIPai, J. K.; Pischon, T.; Ma, J.; Manson, J. E.; Hankinson, S. E.; Joshipura, K.; Curhan, G. C.; Rifai, N.; Cannuscio, C. C.; Stampfer, M. J. Inflammatory markers and the risk of coronary heart disease in men and women. New England Journal of Medicine 2004, 351, 2599-2610. PMid:15602020
View Article PubMed/NCBIDankers, W.; van Hamburg, J. P.; Mus, A.-M. M.; Asmawidjaja, P. S.; van Leeuwen, J. P.; Hendriks, R. W.; Boon, L.; Colin, E. M.; Lubberts, E. 59: 1, 25 (OH) 2D3 Inhibits Th17 cytokine production and RORγt expression through GATA3/IL-4-dependent and-independent mechanisms. Cytokine 2013, 63, 257.
View Article