Christopher Onyemaechi Ezike
Email: Christopher.ezike@esut.edu.ng
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 1
Christopher Onyemaechi Ezike
Email: Christopher.ezike@esut.edu.ng
Christopher Onyemaechi Ezike 1 ⃰ Awere, Sylvernus, U2. and Nwosu Callistus I1.
1Department of Animal/Fisheries Science and Management, Enugu State University of Science and Technology
Department of Agronomy and Ecological Management, Enugu State University of Science and Technology
Wensheng Liu(liuwsairr@163.com)
Christopher Ezike. Awere, Sylvernus, U Nwosu Callistus I, Benzo [a] Pyrene- carcinogenic and mutagenic equivalents of fingerlings of Clarias gariepinus (Burchell, 1822) exposed to water soluble fractions of Nigerian Bonny Light crude oil BLCO(2019)Journal of Aquaculture, Fisheries & Fish Science 3(1) p:186-191
The toxicity and PAH of C.gariepinus (0.9±0.01g) to 1, 3, 6, 9, 12 and 0.0 ml/L of BLCO oil was determined. There was no mortality in the control but 10, 20, 50 and 60% occurred in crude oil after 96 h exposure. The logarithmic probit line and R2: y = 1.372x + 3.724 , R² = 0.997 and 96-h LC50: 9.6 ml/L represented toxicity value for crude oil. Total PAH in exposed fish gave a value of 99.68±4.81 ng/µL The lowest value of 0.061(0%) Nap to the highest value of 384.68 (84%) in DahA for crude oil was determined but mean ∑▒ 16PAH gave 6% compared to the 11% that of its mean∑▒ 8PAH. The least observed difference LOD of PAH among 8PAHs was 0.02ng/µL and mean TEQ and MEQ gave 254.67 and 44.84 both corresponding to 11%. BaP-TEQ in BLCO ranged from 0.05(0%) in Chry to 1923(84%) in DahA. while BaP-MEQ ranged 0.09 (0%) Chry -119.25 (30%) DahA. The respective Benzo [a] Pyrene carcinogenic and mutagenic equivalents of Clarias gariepinus to BLCO was 2 and 9% respectively. In conclusion, the 96h median lethal concentration of BLCO to C.gariepinus fingerlings was 9.6 ml/L in which its mean ∑▒ 16PAH gave 6% compared to 11% that of mean 8PAH. Mean TEQ and MEQ in 8PAH both corresponded to 11% however BaP-TEQ and BaP-MEQ in BLCO ranged respectively from 0% in Chry to 84 and 30% in DahA. The respective Benzo [a] Pyrene carcinogenic and mutagenic equivalents of Clarias gariepinus to BLCO was 2 and 9% .
Key words: Toxic equivalent factor, carcinogenicity, mutagen, PAH, bonny light crude oil
Fishes are good indicators of pollution due to the lipophilic nature and high chemical stability of PAHs which accumulate in the fatty tissues of fish after an uptake from both inland and coastal waters [11, 10, 6]. Two broad groups exist based on their physical and biological properties including, high molecular weight (HMW) and low molecular weight (LMW) PAHs. The HMW PAHs consists of 4-6 aromatic rings and are less readily bio-degraded by indigenous microorganisms, hence can persist in the aqueous environment by bio-accumulating in aquatic organisms like fish and mussels and are more carcinogenic. The LMW PAHs consists of 2-3 aromatic rings and although less carcinogenic, also pose toxic effect to many aquatic organisms [2]. The concentrations of petroleum products toxic to aquatic organisms depend on the type and hydrocarbon constituents, as well as the species involved [14, 13]. Estimated concentrations of petroleum toxic to fish eggs and fingerlings to be 0.5-10 mg/L Benzo (a) pyrene binds to DNA to cause cancer and is frequently used as a marker for carcinogenic disorders and may provide the basis for predicting the impact of exposures of PAH to C.gariepinus fingerlings [20]. BaP-TEQ (carcinogenic equivalents and BaP- MEQ (mutagenic equivalents are measure for sum of total 8 number of particulate PAHs (∑8PAH), having molecular weight greater than 228 grams. ∑8PAH includes benzo (a) pyrene (BaP), benz (a) anthracene (BaA), chrysene/iso-chrysene (CHR), benz (b) fluoranthene (BbF), benzo (k) fluoranthene (BkF), indo (123-cd) pyrene (IP), Dibenz (a,h) anthracene (DahA) and benzo(ghi) pyrelene (BghiP) [17, 4, 5]. The hardy nature and possession of accessory air-breathing organs enable C. gariepinus to tolerate adverse aquatic conditions [23]. Nonetheless, Clarias gariepinus fingerlings are very delicate and sensitive to aquatic pollutants including crude oil and other petroleum products. The aim of the study was undertaken to determine the toxicity, PAH and carcinogen and mutagen equivalent levels of Nigerian- petroleum crude oil on C. gariepinus fingerlings.
2.1. Experimental fish and petroleum
A total of seven hundred and twenty (180) fingerlings of African catfish (mean weight 0.96 ± 0.1g) were obtained from local outskirts in Enugu Nigeria and transported to Fisheries Laboratory of the Department of Animal/Fisheries Science and Management, Enugu State University of Science and Technology ESUT, Enugu Nigeria. They were held in four fiber reinforced plastic (FRP) tanks, containing 320 L of de-chlorinated tap water. Aeration was provided to all tanks round the clock in order to maintain dissolved oxygen contents. Before the commencement of the study, the fish were acclimatized for two weeks and were fed with commercial fish diet composed of 40% crude protein. The faecal matter and other waste materials were siphoned off daily to reduce ammonia content in water. Petroleum (crude oil) was obtained from Nigerian National Petroleum Cooperation Enugu. The water soluble fraction WSF was prepared following the method of [28], which involved 20-h mixing of 10:1 clean water to petroleum with a rotator magnetic stirring rod, separated layers after resting for 12-hrs with separating flask before storing as stock solution in corked 50L plastic gallons. Ethical clearance from Enugu State University of Science and Technology Committee on Experimental Animal Care was obtained and followed.
2.2. Acute toxicity test
Toxicity of crude oil to C. gariepinus was carried out according to the OECD guideline for testing of chemicals No. 203 in a semi-static renewal system by using 200 L capacity glass aquaria. Thirty (30) fish per treatment were randomly exposed to 6 experimental treatments (1, 3, 6, 9, 12 and 0 ml/L of water soluble fractions WSF which served as the control in triplicate to determine 96h LC50 using the probit analysis proposed by [7, 26] and polycyclic aromatic hydrocarbons (PAH) in exposed fish [14] . The exposure was renewed each day and was analyzed using LC–MS/MS to ensure the agreement between nominal and actual concentrations of the petroleum in the aquaria. The experiment was conducted under the natural photoperiod of 12:12 light-dark cycle. The physico-chemical parameters of the test water were analyzed daily, using standard methods[1] and were recorded (dissolved oxygen 7.50 ± 0.45 mg L−1 , temperature 27.75 ± 0.5 0C, pH 7.8 ± 0.13 and free carbon dioxide 4.28 ± 0.6 mg L−1 ). The test fish of 9 and 12 ml/L were sampled to determine ∑16PAH, ∑8PAH, TEQ and MEQ. A portion of the sample using the GC-MC was taken for extraction and analysis of PAH [29, 19].
2.3 PAH extraction
The method described by [26,12,15] with slight modification for extraction and dosing of PAHs was employed. The toxic equivalent factors (BaPTEF) and mutagenic equivalent factors (BaPMEF) relating the carcinogenic mutagenic potency of individual PAH to BaP have been used [19, 25, 5]. The BaP carcinogenic equivalent (BaPTEF) and BaP mutagenic equivalent (BaPMEQ) for the individual PAHs was calculated: BAPTEQ = C1 X BAPTEF; BAPMEQ = C1 X BAPMEF, where Ci = concentration of PAHs.
2.4. Statistical Analysis
Data obtained were expressed as standard mean ± standard error of mean and analyzed using the statistical package SPSS 20.0 computer program (SPSS Inc. Chicago Illinois, USA). Figure of toxicity was done with logarithmic probit line graph while PAH and equivalent values of Bap were done on bar and pie charts.
3.1 Mortality and Logarithmic probit line for 96-h LC50 of petroleum products to C. gariepinus
Figure1 gave mortality values of fish to various concentrations (0, 1, 3, 6, 9 and 12 ml/L. There was no mortality in the control but 10, 20, 50 and 60% for crude oil occurred respectively in 1, 3, 6, 9 and 12 ml/L after 96-hour exposure. Figure 1 gave the logarithmic probit lines and R2: y = 1.372x + 3.724 , R² = 0.997 and 96-h LC50: 9.6 ml/L for crude oil to C. gariepinus.
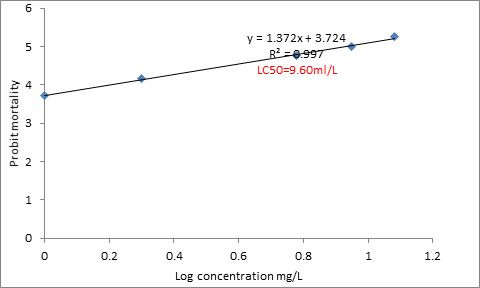
Figure1. Logarithimic probit line for determination of 96-h LC50 crude oil to C. gariepinus
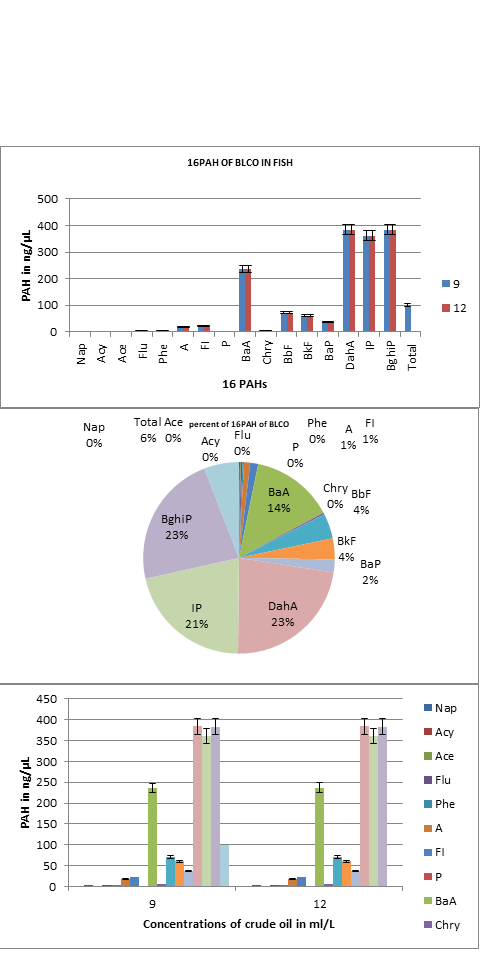
Figure 2. 16PAHs in fish exposed to 9 and 12ml/L of BLCO
Different superscripts in a row indicate significant difference between means (ANOVA, P< 0.05)
KEY:Nap = Naphthalene, Acy= Acenaphthylene, Ace= Acenaphthene Flu= Fluorene, Phe= Phenathrene , A =Anthracene, FI =Fluoranthene P = Pyrene BaA=Benz [a] anthracene, Chry = Chrysene, BbF= Benzo [b] fluoranthene, BkF=Benzo [k] fluoranthene, BaP = Benzo [a ]pyreneDahA = Dibenz [ ah] anthracene IP = Indeno [123-cd] pyrene,, B[ghi]P = Benzo [ghi] pyrene, CO=Crude oil, P= Petrol, K= kerosene, D= Diesel, pp=petroleum products, c= concentration in ml/L, nd= not detected.
3.2 PAHs in exposed fish to petroleum
Total PAHs values in ng/µL (figure 2) of petroleum products in exposed fish ranged from highest value of 99.68±4.81 ng/µL crude oil The lowest value of 0.061 was shown in Nap while highest value was 384.68 in DahA >383.47 B[ghi]P > 361.38 IP >236.41BaA >71.59 BbF>60.17 BkF >37.17 BaP> 22.82FI> 17.72A >5.33Chry >4.56 Flu> 4.35 Phe>2.79 Acy>2.40Ace >0.06Nap for crude oil. The mean ∑▒ 16PAH gave 6% compared to the 11% that of its mean∑▒ 8PAH.
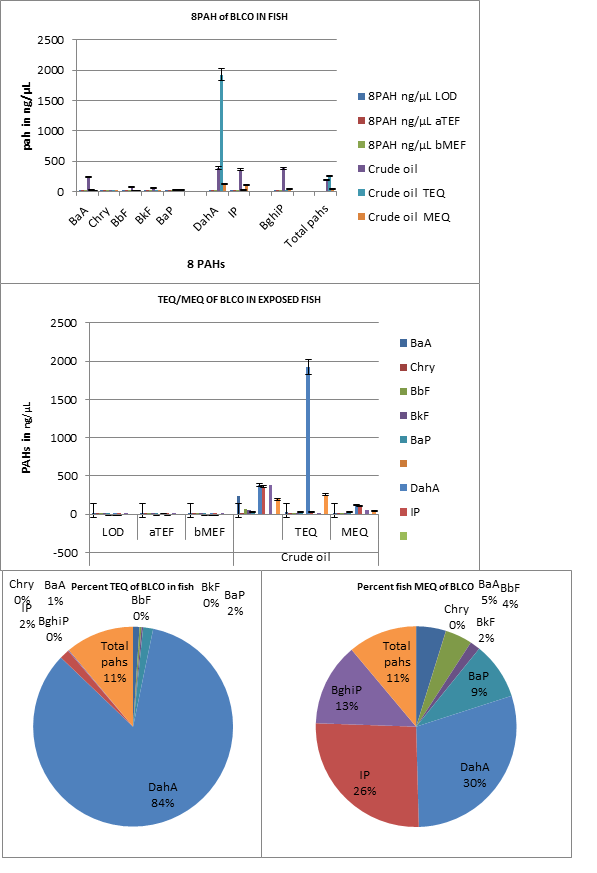
Figure 3. Bap-TEQ and Bap- MEQ of crude oil
TEF*: toxic equivalency factors for cancer potency relative to BaP (Nisbet and LaGoy et al. 1992)
MEF*: mutagenic potency factor relative to BaP (Durant et al. 1996 and 1999)
3.3 Carcinogenicity and mutagenicity equivalents
The least observed difference LOD of PAH among 8PAHs was 0.02ng/µL and mean TEQ and MEQ gave 254.67 and 44.84 both corresponding to 11%. BaP-TEQ in BLCO ranged from 0.05(0%) in Chry to 1923(84%) in DahA. Similarly, BaP-MEQ ranged 0.09 (0%) Chry -119.25 (30%) DahA. The respective Benzo [a] Pyrene carcinogenic and mutagenic equivalents of Clarias gariepinus to BLCO was 2 and 9% respectively.
The observed value of 9.6ml/L as 96h LC50 in this study is closely related to the value of 9.35 ml/L obtained in the same species exposed also to WSF of BLCO [16], but is higher than 1.069 ml/L reported for Oreochromis niloticus juveniles exposed to Quai-boe light crude oil [27]. It was however lower than the range value of 32-88 ml/L for Australian crude oil to Menidia berrylina juvenile fish [18]. The impact of petroleum water soluble fraction previously under-reported has in recent times posed critical health concerns to aquatic biota, especially fish [8, 24]. The foregoing gave an indication that petroleum products with high molar mass and greater mean 8PAH, were more carcinogenic and mutagenic compared to lighter petroleum with lower mean 8PAH. Crude oil has shown the ability to cause cancer and changes in the genetic makeup and may damage the genome materials or disrupt cellular metabolic processes of exposed fish to humans alike that consume them [9]. Recent approaches have centered to identify and quantify PAHs in water, soil and air environment, their emission sources through various methods in order to evaluate their carcinogenic and mutagenic effects to human health [7, 21, 3]. The approaches distinguish anthropogenic multiple releases chiefly from petroleum and other sources [20, 9]. BaP is widely accepted as the indicator for measurement of carcinogenicity, thus BaP-equivalent toxicity for other carcinogenic PAHs has been recommended [31, 2] and evaluated for cancer risk assessment [21, 5, 24, 30].The result showed that BLCO contained greater percentage mean of 8PAH than 16PAH and was less carcinogenic than mutagenic to exposed group of C. gariepinus. There is greater need for further investigation of the biochemistry and physiology on the mutagenic effects to fish, animals and humans alike that consume them given its high responses on the experimental fish to BLCO in the present research.
The 96h median lethal concentration of BLCO to the experimental fish fingerlings was 9.6 ml/L. The mean ∑▒ 16PAH gave 6% compared to 11% that of its mean 8PAH. Mean TEQ and MEQ in 8PAH both corresponded to 11% however BaP-TEQ in BLCO ranged from 0% in Chry to 84% in DahA while BaP-MEQ ranged from 0% in Chry to 30% in DahA. The respective Benzo [a] Pyrene carcinogenic and mutagenic equivalents of Clarias gariepinus to BLCO was 2 and 9%.
APHA, (2005). American Public Health Association, American Water works Association and Water Environmental Federation). Standard Methods of Examination of water and Wastewater. 21st ed. APHA Washington DC, pp 20001-23710.
Brown J, Peake B, (2006). Sources of heavy metals and polycyclic aromatic hydrocarbons in urban storm water runoff. Sci. Total Environ. 359:145-155. PMid:16014309
View Article PubMed/NCBIChangsheng Q, Bing L, Haisui W, (2015). Multi-pathway assessment of human health risk posed polycyclic aromatic hydrocacbon, Environ Geochem Health 37 1-15.
Choi H, Harrison R, Komulainen H, Delgado S J, (2010). Polycyclic aromatic hydrocarbons.WHO Guidelines for Indoor Air Quality: Selected Pollutants. Geneva: World Health Organization 2010. Retrieved 2014-12-12.
Durant J, Lafleur A, Busby W, et al. (1996). Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols‖, Mutation Research/Genetic Toxicology and Environmental Mutagenesis, vol. 371, pp 123-157 90103-2
View ArticleEzike CO, Echor FO, Malachy NOA, Vera LM, (2017). Butrylacetycholinesterase activites in liver and plasma, liver glycogen and plasma glucose content, haematology and behaviour of Clariid Catfish Clarias gariepinus to Dichlorvos International Journal of Advanced Fisheries and Aquatic Sciences, 3(1): 90-105.
View ArticleFinney DJ, (1971). Probit Analysis Canbrige University Press London, 1971, 23-125p.
Hylland K, (2006). Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine systems. Journal of Toxicology and Environmental Health, Part A 69 (1-2): 109-123. PMid:16291565
View Article PubMed/NCBIIsioma T, Ozekeke O, Lawrence E, (2017). Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicology Reports, 4: 56-61. PMid:28959625
View Article PubMed/NCBILawal AT, (2017). Polycyclic aromatic hydrocarbons, a review. Environmental Science 3: 1339841.
View ArticleLee RF, (1976). Metabolism of petroleum hydrocarbons in marine sediments. In: Sources, effects and sinks of hydrocarbons in aquatic environment. American Institute Biological Sciences, 333-344.
Li N, Leu HK, (2001). Solid phase extraction of polycyclic hydrocarbons in surface water. J. Chromatogr. A, 921:255-263. 00879-2
View ArticleLi Z.H, Velisek J, Zlabek V, (2001). Chronic toxicity of verapamil on juvenile rainbow trout (Oncorhynchus mykiss): effects on mor-phological indices, hematological parameters and antioxidant responses. J. Hazard Mat 185:870-880. PMid:20970250
View Article PubMed/NCBILonning S, (1977). The effects of crude oil and oil products on marine fish larvae. Astate 10: 37 - 47.
Martinez E, Gros M, Lacorte S, Barcel D, (2004). Simplified procedures for the analysis of polycyclic aromatic hydrocarbons in water sediments and mussels. J. Chromatogr A, 1047:18-188. PMid:15460247
View Article PubMed/NCBIMichael AO, Temitope OS, Adesola O, Osbona FD, Adebayo AO, (2012). Toxicological evaluation and usefulness of lipid peroxidation as biomarker of exposure to crude oil and petroleum products tested against Clarias gariepinus and Cibarus africanus. Nature and Environment 11 (1) 1-6.
Neff JM, (1985). Polycyclic aromatic hydrocarbons. in: Rand G.M. and Petrocelli S.R. (eds.). Fundamentals of aquatic toxicology. Hemisphere Publ. Corp. New York, 416-454p.
Neff JM, Otsazeki S, Gardiner W, Stejskal I, (2000). Effects of weathering on toxicity of the three Australian crude oils and diesel fuel to marine animals. Environmental Toxicology and Chemical 19 (7): 1809-1821.
View ArticleNisbet I.C., LaGoy PK,(1992). Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 16 (1992) 290-300. 90009-X
View ArticlePayne JF, Penrose WR, 1975). Induction of akyl hydrocarbon (benzo (a) Pyrene) hydroxylase in fish by petroleum. Bull Environ. Contam. Toxicol.14: 112-226. PMid:1148422
View Article PubMed/NCBIPeng, C.; Chen, W.; Liao, X.; Wang, M.; Zhiyun, Q.; Jiao, W.; Yang B. Polycyclic aromatic hydrocarbons in urban soils of Bejing: Status, Sources, distribution and potential risk. Environmental Pollution 159 (2011) 802-808. PMid:21159413
View Article PubMed/NCBIRamesh A, Archibong A, Hood DB, Guo Z, Loganathan B G, (2011). Global environmental distribution and human health effects of polycyclic aromatic hydrocarbons. Global Contamination Trends of Persistent Organic Chemicals. Boca Raton FL: CRC Press. Pp. 97-126. PMid:21773776
View Article PubMed/NCBIReed WJ, Burchard Hopson AJ, Jonathan J, Ibraham Y, (1967). Fish and Fisheries of Northern Nigeria. Govt. Press, London, 226pp.
Roubal WT, Collier TK, Malins DC, (1977). Accumulation and metabolism of carbon - 14 labelled benzene, naphthalene and Anthracene by young cohosalmon (Onchorynchus Kisutch) Arch. Environ. Contam. Toxicol. 5: 515-529. PMid:883853
View Article PubMed/NCBISo - Young L, Lee- Yeon I, Han-Seun S, (2015). Evaluation and chemical analysis method and determination of polycyclic aromatic hydrocarbon content in seafood and dairy products. Toxicology Reports, 31(3): 265-271. PMid:26483885
View Article PubMed/NCBITakatsuki S, Susuki S, Sato N, Ushizawa I, (1985). Association of Official Analytica Chemists.Toxicology, 79(2): 221-271.
Udofia U, (2010). Acute toxicity of Qua-Iboe light crude oil on freshwater fish Oreochromis niloticus. Global Journal of Pure and Applied Science 16 (3) 1-5.
View ArticleUnited Nations Environmental Programme (UNEP), (1989). Comparative toxicity of water accommodated fraction of oil and oil dispersants to marine organisms. United Nations Environmental programme. Reference Methods for Marine Pollution Studies 1989, N0 43, 27p.
United States Environmental Protection Agency (USEPA), (1997). Washington, DC, EPA/600/P-95/002F a-c Exposure Factors Handbook (1997 Final Report)| Risk Assessment,
View ArticleVo Thi LH, Nguyen Thi TH, Minora Y, (2016). Human health hazard of polycyclic aromatic hydrocarbon in road dust in Ha Noi metropolis. Journal of Science and Technology.,54 (24) 27-34
View ArticleWorld Health organization (WHO), (2010).WHO Guidelines for indoor air quality:selected pollutants,
View Article