Oyakhilome Gloria Irenosen
Email: gloyaks@yahoo.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 2 ISSUE: 2
Page No: 165-173
Oyakhilome Gloria Irenosen
Email: gloyaks@yahoo.com
1Oyakhilome Gloria Irenosen, 2Ajiwe Vincent Ishmael E.
1, 2 Department of Pure and Industrial Chemistry, Nnamdi Azikiwe University, Awka, Nigeria.
Oyakhilome Gloria Irenosen, Transfer Factor and Bioaccumulation of Heavy metals in Fishes from Rivers Owan and Evbiobe, Edo State, Nigeria(2019)Journal of Aquaculture, Fisheries & Fish Science 2(2)
The Transfer factors and bioaccumulation of heavy metals in three fish species (Clarias gariepinus, Murmyrus rume and Tilapia zilli) were assessed in Rivers Owan and Evbiobe. The availability of Cu, Cr, Pb, Fe, Mn, Cd and Zn in water, sediment, fish and plants samples from the rivers was detected using Atomic absorption spectrophotometer. The result revealed that all the investigated fish species bio-concentrated the trace metals from the rivers water more than the sediment while there was no notable transfer of pollutants from the examined plants to the fish species. The transfer factor of the metals in fishes from water increased in order of Cr > Zn Cu >Pb>Mn> Fe > Cd for Clarias gariepinus and Tilapia zilli with a slight swap between Pb and Mn in Mormyrus rume, all from Owan river. Clarias gariepinus and Tilapia zilli in Evbiobe river had TF values in order of Cr >Pb> Fe > Cd > Zn >Mn> Cu and Cr > Zn> Fe >Pb> Cd > Cu >Mn respectively.
Keywords: Transfer factor, bioaccumulation, fish, Owan and Evbiobe rivers
The aquatic environment makes up a major part of the environment and resources of the interested area. Therefore, its safety is directly related to human health (Ebong et al., 2014). Pollution, loss of biodiversity, and habitat destruction are probably the main environmental threats for aquatic ecosystems. Moreover, the excessive contamination of aquatic ecosystems has evoked major environmental and health concerns worldwide (Abdel Bark, 2011). Pollutants can induce various biological responses in fish, affecting the organisms from the biochemical to the population-community levels (Adefemi, et al, 2004; Bashir et al, 2013), and cause various harmful effects on wildlife (Bashir et al, 2013). Among the various toxic pollutants, trace metals represent a harmful group of elements due to their strong impact on stability of aquatic ecosystems, toxicity persistence, and accumulation tendency (Ekeanyanwu and Etienajirhevwe, 2012). Among trace metals, some are potentially toxic (Cd, Cr, Pb, and Hg), and many are essential (Cu, Zn, Fe, and Mn) (Eno and Etuk, 2015). Even essential metals can produce toxic effects when the metal intake is excessively elevated (Bashir et al, 2013).
Fish are widely used in quality assessment of aquatic environment and as bio indicators of environmental pollution (Biswas et al., 2012). So, field studies on early adverse effects of contaminants, measured directly in organisms in their natural environment, represent one of the main target areas in environmental bio-monitoring programs.
Fishes that are of interest to this study are Clarias gariepinus, Mormyrus rume, and Tilapia spp. Clarias gariepinus, also known as African sharptooth catfish is a species of catfish of the family Clariidae. They are found throughout Africa and the Middle East, and live in freshwater lakes, rivers, and swamps, as well as human-made habitats, such as oxidation ponds or even urban sewage systems. The African sharptooth catfish is a large, eel-like fish, usually of dark gray or black colouration on the back, fading to a white belly, with an average adult length of 1–1.5 m (3 ft 3 in–4 ft 11 in). In Africa, this catfish has been reported as being second in size only to the vundu of the Zambesian waters, although FishBase suggests that African sharptooth catfish surpasses that species in both maximum length and weight (Froese et al., 2014).
These fish have slender bodies, flat bony heads and broad, terminal mouths with four pairs of barbels. They also have large accessory breathing organs composed of modified gill arches. Also, only the pectoral fins have spines (Anoopet al., 2009). It is a nocturnal fish, which feeds on living, as well as dead, animal matter. Because of its wide mouth, it is able to swallow relatively large prey whole. It has been known to take large water-birds such as the common moorhen. Clarias gariepinus is also able to crawl on dry ground to escape drying pool, to survive in shallow mud for long periods of time during the rainy season. Spawning mostly takes place at night in the shallow, inundated areas of the rivers, lakes and streams. Courtship is preceded by highly aggressive encounters between males. Courtship and mating take place in shallow waters between isolated pairs of males and females. The male lies in a U-shape curved around the head of the female, held for several seconds. A batch of eggs is released followed by a vigorous swish of the female's tail to distribute the eggs over a wide area. The pair usually rests after mating (from seconds up to several minutes) and then resume mating (Jansen-van-Rensburget al., 2013).
Parental care for ensuring the survival of the catfish offspring is absent except by the careful choice of a suitable site. Development of eggs and larvae is rapid, and the larvae are capable of swimming within 48–72 hours after fertilization (Anoopet al., 2009). The flesh is tasty and usually consumed as a special delicacy in Africa.
Mormyrusrume
This species belong to the genus Mormyrus, of the familyMormyridae. The Mormyridae is well represented in the local waters with twenty-six different species belonging to six genera. The Mormyrids have the anal fin less than half of the length of the dorsal fin. The have a trunk-like snout and a small mouth. They grow to about a meter in length, usually have a uniform greyish yellow colour on the back and sides and it’s fairly common. Large specimens are capable of giving noticeable electric charges sometimes at the rate of 150-200 pulses per minute (Amoo et al., 2006). They are typical bottom dwellers found in deep, rocky pools in surface water, where the current is not swift. Due to absence of spines and the slender bodies, it is most difficult to catch using any kind of fishing gear in the areas inhabited by Mormyrus rume. The flesh is tasty but contains excess fat hence; preservation by freezing for a long time is difficult. They are therefore generally sold fresh (Froese et al., 2014).
Tilapia spp
Tilapia spp belong to a large family of Cichlidae.Cichlidae include several hundred species of rather generalized freshwater percloid fishes. They are a conspicuous element in the freshwater fauna of Africa and tropical countries. They have a deep laterally compressed body, a double lateral line and their dorsal fins are divided into spines and rays with spot at the base. They have a terminal mouth and truncated or round in shape tail. The habit of Tilapia in guarding their young has given them considerable advantage in the colonization of their choosen habitat (Froese et al., 2014). The three important species that are popular in Nigeria and many other tropical regions include Oreochromis niloticus, Sarotherodongalilaeu sand Tilapia zilli (Jansen-van-Rensburg et al., 2013). Tilapia zilli was used for this study. Tilapia features importantly in many fisheries, and because of their great adaptability, high fecundity and rapid growth rate, they are used extensively for fish culture (Adefemi et al., 2004). Most Tilapia species occur in schools or at least in loose congregation. Such schools may be migratory, but often show a strong tendency to remain within a particular area (Jansen-van-Rensburg et al., 2013).
Transfer Factor (TF) is the ratio of trace metal in the concentrating matrix to the concentration in the ambient matrix under equilibrium conditions. Sediment to fish transfer factor and water to fish transfer factor was computed using the equation given by Muzyed (2011).
Transfer Factor (TF) = Concentration of metals in fish muscle / Concentration of metals in abiotic media
where the abiotic media represent water, sediment and food samples. If the transfer factor is greater than 1.0, then bioaccumulation for metals occur in fish or plant species.
2.1. The Study Area
The areas of study are Rivers Owan and Evbiobe, which transverse through Owan East and OwanWest Local Government Areas of Edo State, Nigeria. It lies within the equatorial climatic region, between longitude 050 40” to 060 45” and latitude 05044” to 070 34”. These rivers serve as drinking water source for most residents in the area, even though Owan river is a washing zone for automobiles of all sorts on daily basis while a major market site is located behind Evbiobe river; thus exposing the rivers to some levels of pollution. Also, fishing activities are practiced in this area as a source of income and livelihood while the major occupation of the people is farming with the use of fertilizer; a practice that could lead to “Eutrophication” (excessive growth of aquatic weed) if the farm land is eroded into the rivers by storm water during the raining season.
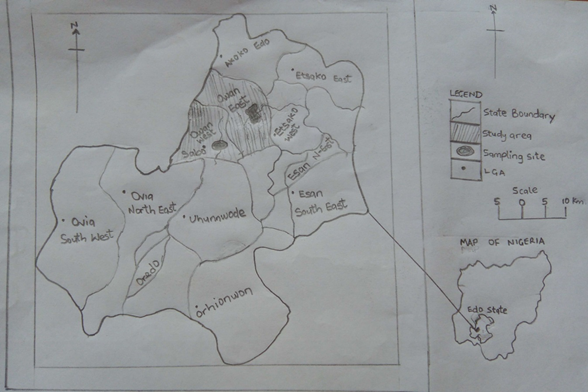
Plot 1: Map of Study area, showing the sampling locat
Live samples of three species of fish, Clarias gariepinus (Catfish),Mormyrus rume and Tilapia zilli were obtained along the sampling route of the rivers with the assistance of local fishermen. The samples were transported live to the laboratory. In the laboratory, the fishes were killed by asphyxiation, weighed and scales removed from Tilapia zilli and then washed with distilled water. Their intestines were later removed, and each fish separated into two parts, the head and the trunk and these were further washed with distilled water and the water allowed drying between two pieces of filter paper (Amoo et al.,2006; Bashir et al, 2013). The samples were kept in the freezer prior analysis.
Each of the powdered samples was further oven- dried at 105oCto a constant weight. Each sample (2.0g) was weighed into a 200ml Kjeldahl digestion flask; 10ml of concentrated HNO3 were added followed by 2ml each of 62% HCLO4 and H2SO4. The flask was heated on an electric heating mantle to almost dryness after the evolution of white dense fumes (Salami and Non, 2002; Bashir et al, 2013). The flask was set aside to cool after which the content was diluted with some quantities of distilled water and then filtered into a 50ml volumetric flask and the solution was made to mark with distilled water. A blank sample was also prepared. All solutions were analyzed for heavy metals using Atomic Absorption Spectrophotometer.
The transfer factors (TF) for all the metals examined in this study for fish/water, fish/sediment, fish/I.aquatica and fish/S.molesta are presented in Tables 1, 2, 3 and 4 respectively.
Table 1: Trace metals’ transter factor for fish/water in the rivers
|
Samples |
Cu |
Cr |
Pb |
Mn |
Fe |
Cd |
Zn |
|
Cg OR-1 |
33.39 |
241.00 |
22.27 |
16.60 |
16.64 |
14.00 |
179.00 |
|
Mr OR-2 |
25.67 |
174.00 |
12.05 |
16.00 |
11.13 |
5.00 |
132.22 |
|
Tz OR-3 |
35.50 |
221.00 |
1.81 |
5.31 |
9.69 |
5.00 |
220.00 |
|
Cg ER-4 |
4.90 |
99.00 |
82.00 |
9.42 |
17.90 |
12.00 |
10.36 |
|
Tz ER-5 |
4.88 |
51.81 |
12.00 |
3.21 |
12.74 |
5.00 |
15.96 |
Where Cg = Clariasgariepinus, Mr = Mormyrusrume, Tz = Tilapia zilli, OR = Owan river
ER = Evbiobe river
Table 2: Trace metals’ transter factor for fish/sediment in the rivers
|
Samples |
Cu |
Cr |
Pb |
Mn |
Fe |
Cd |
Zn |
|
Cg OR-1 |
1.21 |
3.54 |
5.76 |
0.19 |
0.52 |
1.16 |
1.00 |
|
Mr OR-2 |
0.96 |
2.56 |
3.12 |
0.18 |
0.34 |
0.41 |
0.74 |
|
Tz OR-3 |
1.31 |
3.25 |
0.47 |
0.06 |
0.30 |
0.41 |
1.23 |
|
Cg ER-4 |
0.54 |
2.48 |
8.20 |
0.36 |
0.73 |
2.40 |
1.35 |
|
Tz ER-5 |
0.53 |
1.30 |
1.20 |
0.12 |
0.52 |
1.00 |
2.08 |
Where Cg = Clariasgariepinus, Mr = Mormyrusrume, Tz = Tilapia zilli, OR = Owan river
ER = Evbiobe river
Table 3: Trace metals’ transter factor for fish/plant (Ia) in rivers
|
Samples |
Cu |
Cr |
Pb |
Mn |
Fe |
Cd |
Zn |
|
Cg OR-1 |
0.32 |
1.31 |
0.27 |
0.15 |
0.55 |
0.41 |
7.45 |
|
Mr OR-2 |
0.25 |
0.95 |
0.15 |
0.15 |
0.36 |
0.15 |
5.49 |
|
Tz OR-3 |
0.34 |
1.20 |
0.02 |
0.05 |
0.32 |
0.15 |
9.17 |
|
Cg ER-4 |
0.27 |
0.90 |
0.66 |
0.21 |
0.56 |
0.30 |
7.82 |
|
Tz ER-5 |
0.27 |
0.47 |
0.10 |
0.07 |
0.40 |
0.10 |
12.05 |
Where Cg = Clariasgariepinus, Mr = Mormyrusrume, Tz = Tilapia zilli, OR = Owan river
ER = Evbiobe river
Table 4: Trace metals’ transter factor for fish/plant (Sm) in the rivers
|
Samples |
Cu |
Cr |
Pb |
Mn |
Fe |
Cd |
Zn |
|
Cg OR-1 |
0.22 |
0.86 |
0.17 |
0.12 |
0.44 |
0.26 |
13.11 |
|
Mr OR-2 |
0.18 |
0.62 |
0.09 |
0.11 |
0.29 |
0.09 |
9.65 |
|
Tz OR-3 |
0.24 |
0.79 |
0.01 |
0.04 |
0.25 |
0.09 |
16.11 |
|
Cg ER-4 |
0.15 |
0.98 |
0.39 |
0.15 |
0.42 |
0.27 |
13.23 |
|
Tz ER-5 |
0.15 |
0.51 |
0.06 |
0.05 |
0.30 |
0.11 |
20.38 |
Where Cg = Clariasgariepinus, Mr = Mormyrusrume, Tz = Tilapia zilli, R = Owan river,
ER = Evbiobe river
The result revealed that all the investigated fish species bio-concentrated the trace metals from the rivers water more than the sediment while there was no notable transfer of pollutants from the examined plants to the fish species (Table 1). This observation might be associated with the feeding habits of the fish species rather than their dwelling zones, even though the dwelling zones also contributed as these fish species might not be feeding on the examined plants as food. This deduction is further strengthened by the report of Amoo et al. (2006) and Ahmed et al. (2017), who stated that some fish species like Tillapia zilli are filter feeders sipping food particles from the water than the sediment. Although Clarias gariepinus and Murmyrus rume are bottom sediment dwellers, their feeding pattern might have more influence in metal transfer (Amoo et al. 2006; Bashir et al. 2013; Ahmed et al. 2017). Hence, the reason for the higher values of the transfer factor recorded in the water than that of sediment (Tables 2 and 3). This confirmed the earlier assertion that fish bio-accumulates trace metals from water than from sediments and was linked with the feeding behavior of the examined fish (Abdel-Bark et al., 2011). The transfer factors in the different fish species also showed that Clarias gariepinus, followed by Mormyrus rume recorded the highest concentration when compared with Tilapia zilli in both Owan and Evbiobe rivers. This connoted the contributions of dwelling area of each fish species in the aquatic habitants as Tilapia zilli resides mostly on the water shores while Clarias gariepinus and Mormyrus rume that feed on particles from both the water and bottom sediments are more susceptible to accumulate more pollutants from the water bodies as typical bottom sediment dwellers (Adefemi et al,. 2004; Ali et al., 2011; Eno and Etuk, 2015).
The transfer factor of the metals to fish from water increased in order of Cr > Zn> Cu >Pb>Mn> Fe > Cd for Clarias gariepinus and Tilapia zilli with a slight swap between Pb and Mn in Mormyrus rume, all from Owan river. Clarias gariepinus and Tilapia zilli in Evbiobe river had TF values in order of Cr >Pb> Fe > Cd > Zn >Mn> Cu and Cr > Zn> Fe >Pb> Cd > Cu >Mn respectively. It was generally noted that Cd had the least TF values in all the fish samples while Cu and Mn recorded the least values in Clarias gariepinus and Tilapia zilli respectively from Evbiobe river and Cr was the highest concentrated in all the examined fishes from Owan and Evbiobe rivers. However, the transfer factor in water was much greater in magnitude than their corresponding sediment samples.
The ability of fish to accumulate trace metals depends on its ecological needs, exposure duration, the feeding behavior, its metabolism, and degree of pollution in sediment, water, food as well as salinity and temperature of the water (Bashir et al. 2013; Eno and Etuk, 2015). In addition, Tanee and Eshalomi-M (2015) attributed the accumulation of a particular metal to the presence of the metal in the water column, the physiological role of each element and the preference of an element to bind to or replace some element in its tissue. The transfer factor (TF) of the elements in sediment was least concentrated in Mn for all the fish samples from Owan and Evbiobe rivers and Pb had the highest values in Clarias gariepinus and Mormyrus rumefrom both rivers (Table 2). The Table revealed that there was no bioaccumulation of some elements while other elements were bioaccumulated by the fish species. The TF values are in the order of Pb> Cr > Cu > Cd > Zn > Fe >Mn, Pb> Cr > Cu > Zn > Cd > Fe >Mn and Cr > Cu > Zn >Pb> Cd > Fe >Mn in Clarias gariepinus, Mormyrus rume and Tilapia zilli respectively from Owan river while Clarias gariepinus and Tilapia zilli from Evbioebe river recorded values in order of Pb> Cr > Cd > Zn >Fe > Cu >Mn and Zn > Cr >Pb> Cd > Cu > Fe >Mn respectively.
Tables 3 and 4 depict the transfer factor of examined plants (Ipomoea aquatica and Salvinia molesta) to the different fish species. The study showed that there was no appreciable transfer of metals from plants to fish as all the TF values, except Zn were well below one, for all the plant samples. The result for transfer factor in this research is consistent with the findings of many authors (Rasheed, 2001; Abdel-Bark et al., 2011; Eno and Etuk, 2015). Rasheed (2001) computed the transfer factor for some metals from water, sediment, plants and Tilapia nilotica in Nasser Lake and his results indicated that only transfer factors from water for all metals were greater than one. According to Abdel-Bark et al. (2011), if there is no significant accumulation by fish tissues, the presence of the metals in high levels in fish environment may not indicate a toxic risk to fish and man the ultimate consumer of fish. In contrast, bioaccumulation and biomagnification of trace metals via food chain and assimilation by humans might pose a health risk.
Figures1, 2, 3 and 4 present the bioaccumulation (logarithm of the ratio) of investigated metal levels in fish/water, fish/sediment, fish/Ipomoea aquatica and fish/Salvinia molesta respectively.
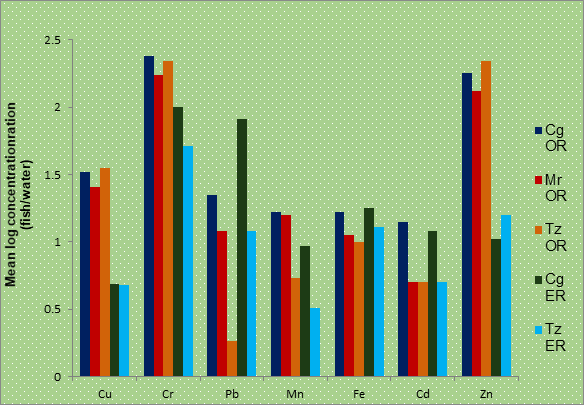
Figure 1: Comparison of mean concentrations of metals in fish and water column
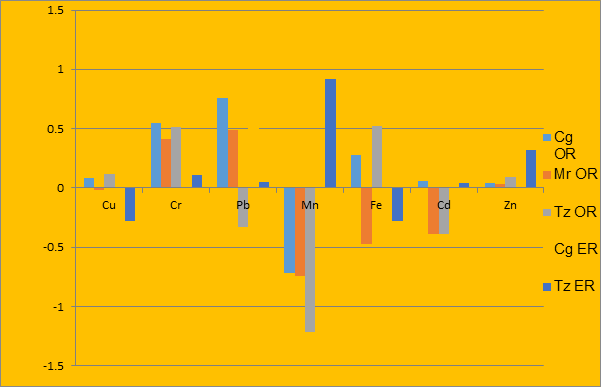
Figure 2: Comparison of mean concentrations of metals in fish and sediment
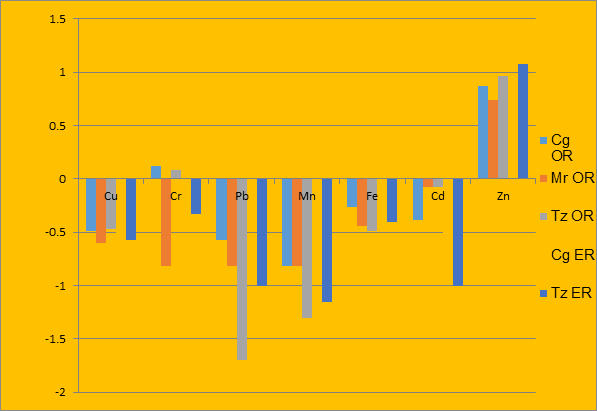
Figure 3: Comparison of mean concentrations of metals in fish and aquatic plant (I. aquatica)
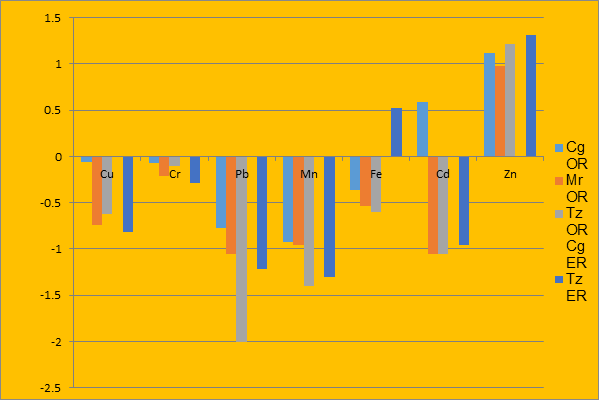
Figure 4: Comparison of mean concentrations of metals in fish and aquatic plant (S. molesta)
It was observed that higher concentrations of the metals in the fish samples than in the water were recorded thus, suggesting bioaccumulation of these metals in the fish body. Similar observations have been reported by many workers in Nigeria (Okoye, 1991; Asaolu, 2003; Obodo, 2004; Adefemi et al., 2004; Amoo et al., 2006; Olaniran et al., 2013) and all over the world (e.g Chale, 2002; Karadede et al.,2004; Al-Weher, 2008; Indrajith et al, 2008; Saha and Hossan, 2011; Dhanakumar et al., 2015).
Cr and Zn had the highest bioaccumulation in the fish samples from Owan river and Pb in Tilapia zilli from Owan river was least bio-accumulated while Clarias gariepinus from Evbiobe river bio-concentrated Pb than other elements. This observation strongly upholds the report of Rasheed (2001) that high concentrations of trace metal in water might not represent the metals’ bioavailability unless it has been proved by that the organism in question has the tendency to bioaccumulate the metals investigated. This was noticed in the higher Pb levels in Fish samples from Owan river than those from Evbiobe river (Tables 3 and 4.) whereas reverse trend of higher bioaccumulations of Pb in Clarias gariepinus from Evbiobe river was observed while the three fish species from Owan river had lower Pb bioaccumulations as recorded in Fig1. Aiyesanmi (2008), attributed such trend to the ability or tendency of each organism to bio concentrate metals and the extent at which an organism can eliminate these metals from its body or level of the excretory mechanism in the organism.
On the other hand, concentrations of Cr and Zn in the sediment were higher than the values of other metals obtained in all the fish samples from both rivers, while Cu and Fe in fish samples from Evbiobe river and Pb and Cd from Owan river did not bio-accumulate in the fish. Others recorded greater concentration in fish than in sediment, possibly due to low deposition of these metals on sediment; or they are more in their exchangeable or labile form in the sediment matrix, thus having low resident time in the sediment; or they are more bio-accumulated in the fish (Aiyesanmi, 2008). However, higher concentrations of some heavy metals in the sediment compared to fish have been reported by Okoye (1991) and Asaolu et al., (1997) for Lagos lagoon and Ondo State coastal waters respectively. Similarly, Mohammed et al. (1982) in their study of heavy metal concentration in fish and sediment samples from AD-Danna sewage outfall area in Saudi Arabia and Boran and Altınok (2010) in their study ofheavy metals in water, sediment and living organisms in the Black Sea also recorded higher concentration of metals.Trace metals are naturally present in all bed sediment samples, but they do not accumulate at the same rate in different fish species and other aquatic fauna (Al-Weher, 2008). Several factors have been proposed to be responsible for the differences in bioaccumulation of metals by fish species and same species in different water bodies. These include speciation, organic matter content, redox state of metals, exposure at the sediment/ water interface, species differences in prey selection, and uptake and elimination kinetics for fish and prey (Saha and Hossan, 2011; Dhanakumaret al., 2015). For example, trace metals are ingested with particulate matter (streambed sediment or suspended sediment in the water column) by benthic organism and can then accumulate and move through the food chain (Adefemi et al., 2004).
The bioaccumulation of these metals in the fish tissues might be beneficial if the metals were essential, but could be harmful in case of highly toxic metals (Aiyesanmi et al., 2010). For example, Fe, Zn, Mn and Cr are essential micronutrient for growth, but their excessive concentration with others such as Cd, Pb and Cu without any significant biological function can be toxic to aquatic organisms. However, the levels of most metals in the fishes of this research are well below the maximum permissible limit (MPL) for fish muscle set by the Us Food and Drug Administration (WHO, 2011). For instance, the highest concentrations recorded for Zn, Cu and Cd in the fishes from this study are 8.40mg/kg, 2.38mg/kg and 0.22mg/kg as against their MPL values of 40.0mg/kg, 30.0mg/kg and 2.0mg/kg respectively. Hence the levels of these trace elements in the fishes may not constitute any health risk, but care should be taken considering the fact that most humans around riverine areas regularly consume large quantity of fish. Consumption of large quantities of fishes that are slightly polluted might result to bioaccumulation and biomagnification of these pollutants in humans exposed to them for a long period of time.
The ratio of fish to aquatic plant (Ipomoea aquatica and Salvinia molesta) in Fig.3 and Fig. 4 respectively showed that there was no significant bioaccumulation by all the examined fish species from the metals except Zn in the plant samples. This might suggest that the fish species do not depend on the examined aquatic plants as food. This further strengthened the fact that the extent of accumulation of metals in a fish is a reflection of the concentration of such metals in the environment as well as the fishes’ interaction (either through feeding, inhalation or dermal contact) with that species of plant in use (Adefemi, 2004; Ali et al., 2011; Eno and Etuk, 2015).
It was generally noted that Cd had the least TF values in all the fish samples from River Owan while Cu and Mn recorded the least values in Clarias gariepinus and Tilapia zilli respectively from Evbiobe river and Cr was the highest concentrated in all the examined fishes from both rivers. However, the transfer factor in water was much greater in magnitude than their corresponding sediment samples as all the fish species bio-accumulated the examined metals from the rivers water.
Abdel-Baki, A. S., Dkhil, M. A. and Al-Quraishy, S. (2011). Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of Wadi Hanifah, Saudi Arabia.African Journal of Biotechnology. 10(13): 2541-2547.
Adefemi, O.S.; Olaofe, O. and Asaolu, S.S. (2004).Concentration of Heavy metals in water sediment and fish parts (Illishaafricana) from Ureje dam, Ado-Ekiti, Ekiti State, Nigeria Journal of Biological and Physical Science. 3: 111 - 114.
Ahmed, M. M., Doumenq P, Syakti, A.D., Asia L, and Chiron, S. (2017). Levelsand sources of heavy metals and PAHs in sediment of Djibouti-city (Republic of Djibouti). Marine pollution bulletin: 120 (1-2), 340-346. PMid:28550950
View Article PubMed/NCBIAiyesanmi, A.F. (2008). Baseline Heavy metals concentration in River Sediments within Okitipupa South East belt of Nigeria bituminous sand field. Journal of Chemical. Society, Nigeria.33 (2): 29 - 41.
Aiyesanmi, A.F., Oguntuase, A.A. and Idowu, G.A. (2010). Investigation on speciation and pollution index of heavy metals in river Ala sediment, Akure, Nigeria.International journal of Biological and Chemical Science. 4 (6) 2348 - 2349.
View ArticleAli, S. Y. Shelly, F. Bibi., (2011) "Peculiarities of mangla reservoir: biodiversity with sustainable use options," Journal of Animal and Plant Sciences, 21 (2): 372-380.
Alia N, Sardar K, Said M, Salma K, Sadia A, Sadaf S, Toqeer A, Miklas S (2015). Toxicity and bioaccumulation of heavy metals in spinach (Spinaciaoleracea) grown in a controlled environment. International Journal of Environmental Resource Public Health, 12:7400-7416. PMid:26133131
View Article PubMed/NCBIAl-Weher, S. M. (2008). Levels of heavy metal Cd, Cu and Zn in three fish species collected from the northern Jordan valley, Jordan. Jordan Journal of Biological Science.1(1): 41 - 46.
Asaolu, S.S. (2003). Determination of some heavy metals in Oreochromis niloticus, Clarias gariepinus and Synodontis spp from the coastal water of Ondo State, Nigeria. Pakistan Journal of Science and Industrial Resource. 45 (1):17 - 19.
Atayese M.O., Eigbadon A.I., Oluwa K.A. and Adesodun J.K. (2009) Heavy metal contamination of amaranthus grown along major highways in Lagos, Nigeria. African Crop Science Journal, 16:135-225.
View ArticleAnoop K.R., Sundar, K.S.G., Khan, B.A. and Lal S. (2009). Common Moorhen Gallinula chloropus in the diet of the African catfish Clariasgariepinus in Keoladeo Ghana National Park, India. Indian Birds. 5(2):22-23.
Biswas S., Prabhu R. K., Hussain K. J., Selvanayagam M., Satpathy K. K. (2012). Heavy metals concentration in edible fishes from coastal region of Kalpakkam, southeastern part of India. Environmental Monitoring and Assessment. 184(8):5097-5104. PMid:21922180
View Article PubMed/NCBIBoran, M.and Altınok, I. (2010) A Review of Heavy Metals in Water, Sediment and Living Organisms in the Black Sea. Turkish Journal of Fishery and Aquatic Science. 10: 565-572.
Chale, F.M.M. (2002). Trace metal concentrations in water, sediment and fish tissues from Lake Tanganyika. Science of Total Environment; 299: 115 - 121. 00252-8
View ArticleDhanakumar, S., Murthy, K.R. and Mohanra, R. (2015). Phosphorousfractionation in surface sediments of the Cauvery Delta region, SoutheastIndia. Environmental Management of River Basin, Pp 477 - 489.
View ArticleEbong G.A, Offiong O.E., and Ekpo B.O. (2014). "Seasonal variations in trace metal levels, speciation and physicochemical determinants of metal availability in dumpsite soils within Akwa Ibom State, Nigeria," Journal of Chemistry and Ecology, pp. 1-15.
View ArticleEfe, S.I., Ogban, F.E., Horsfall, M. Jnr, Akporhonor, E.E. (2005) Seasonal variations of physico-chemical characteristics in water resources quality in Western Niger Delta region, NigeriaJournal of Applied Science and Environmental Management. 9 (1): 191 - 195.
Ekeanyanwu, R.C. and Etienajirhevwe, O.F (2012). In vitro anthelmintic potentials of Xylopiaaethiopica and Monodoramyristica from Nigeria. African Journal of Biochemical Resource. 6:115-120.
View ArticleEno, A. M. and Etuk, B. A. (2015). Human Health Risk Assessment of Trace metals in Water from Qua Iboe River Estuary, Ibeno, Nigeria.Journal Ecotoxicology and environmental safety; 4(3): 150-157.
View ArticleEPA, (2001): Parameters of Water Quality: Interpretation and Standards. Environmental Protection Agency, Ireland. pp133.
Esen E., Kucuksezgin F., Uluturhan E (2010). Assessment of trace metal pollution in surface sediments of Nemrut Bay, Aegean Sea. Environmental Monitoring Assessment. 160:257-266. PMid:19101811
View Article PubMed/NCBIFroese, Rainer, Pauly and Daniel, (2014). "Clariasgariepinus" in FishBase.
Indrajith, H.A.P, Pathiratne, K.A.S and Pathiratne, A. (2008). Heavy metal levels in two fish food species from Negombo eustuary, Sri Lanka: Relationships with the body size. Sri Lanka Journalof Aquatic Science, 13: 63 - 81.
View ArticleJansen van Rensburg, C., van As, J.G. & King, P.H. (2013). New records of digenean parasites of Clariasgariepinus (Pisces: Clariidae) from the Okavango Delta, Botswana.AfricanInvertebrates, 54 (2): 431-446.
View ArticleKaradede, H., Oymak, S. A., & Unlu, E. (2004). Heavy metals in mullet, Liza abu and Cat fish, Silurus triostegus, from the Atatu¨rk Dam Lake (Euphrates), Turkey Environment International., 30: 183-188. 00169-7
View ArticleMuhammed, S., Tahir, H.Z., Amir-ul-Hoda and Atique, A.M. (1982). Heavy metal concentrations in shrimp, crab and sediment. Bull. Environmental Contamination and Toxicology, 29: 313 - 319. PMid:7126923
View Article PubMed/NCBIObodo, G.A. (2004). The bioaccumulation of heavy metals in fish from Anambra River. Journal, Chemical Society of Nigeria, 29 (1): 60 - 62.
Okoye, B.C.O. (1991). Nutrients and selected chemical analysis in the Lagos Lagoon surface waters. International Journal of Environmental Studies, 38: 131 - 135.
View ArticleRashed, M.N. (2001) Monitoring of environmental heavy metals in fish from Nasser lake.Environment International, 27:27-33. 00050-2
View ArticleSaha P.K. and Hossan M.D. (2011). Assessment of Heavy Metal Contamination and Sediment Quality in the Buriganga River, Bangladesh. 22nd International Conference on Environmental Science and Technology, IACSIT Press, Singapore. vol.6.
Tanee,F.B.G. and Eshalomi-M, T.N. (2015). "Heavy Metal Contents in Plants and Soils in Abandoned Solid Waste Dumpsites in Port Harcourt, Nigeria. Research Journal of Environmental Toxicology, 9 (6):342-349.
View ArticleWHO (World Health Organization) WHO Technical Report Series. (2011). Evaluation of certain food additives and contaminants: seventy-third report of the joint FAO/WHO expert committee on food additives.
View Article