Hitoshi Miyasaka
E-mail address: miyasaka@life.sojo-u.ac.jp
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 2
Page No: 245-249
Hitoshi Miyasaka
E-mail address: miyasaka@life.sojo-u.ac.jp
Aoi Koga,1 Yusaku Tani,2 Ken-ichi Ozaki, 2 Takaaki Maki,3 Shuhei Hayashi,1 Shinjiro Yamamoto1, Hitoshi Miyasaka1*
1 Department of Applied Life Science, Sojo University, 4-22-1 Ikeda, Nishiku, Kumamoto 860-0082, Japan
2 Takusui Co. Ltd., Sun heights 708, 3-6-23 Maizuru Chuo-ku, Fukuoka 810-0073, Japan
3 Matsumoto Institute of Microorganisms Co. Ltd., 2904 Niimura, Matsumoto, Nagano 390-1241, Japan
Esteban MA(aesteban@um.es)
Aoi Koga, Yusaku Tani, Ken-ichi Ozaki, Takaaki Maki, Shuhei Hayashi, Shinjiro Yamamoto, Hitoshi Miyasaka, The effects of a marine photosynthetic bacteria Rhodovulum sulfidophilum on the growth and survival rate of Marsupenaeus japonicus (kuruma shrimp)(2021)Journal of Aquaculture, Fisheries & Fish Science 3(2) :245-249
The sustainability of the shrimp aquaculture depends largely on disease control and the health status of shrimp. Probiotics, which make shrimps healthier and more resistant to pathogens, are promising countermeasure for shrimp diseases. In this study, the effects of the marine purple non-sulfur photosynthetic bacterium (PNSB) Rhodovulum sulfidophilum on Marsupenaeus japonicus (kuruma shrimp) growth and survival were examined in 177 m2 aquaria (140 tons of water) for 70 days. The shrimp received feed containing 0.01 % fresh weight (106 colony forming unit/g) of R. sulfidophilum cells. The survival rate significantly improved (P < 0.001) (R. sulfidophilum-fed = 81.9 %; control = 71.5 %), the feed conversion rate improved (R. sulfidophilum-fed = 1.83; control = 2.11), and there was no difference in the shrimp average body weight. The approximate bacterial cell cost was $0.003 to $0.005 per 1 kg feed, indicating that the R. sulfidophilum approach is economically feasible and a promising candidate for probiotic bacteria in shrimp aquaculture.
Keywords: photosynthetic bacteria, Rhodovulum sulfidophilum, Marsupenaeus japonicus, shrimp, probiotics
Shrimp aquaculture is economically important in many countries, and probiotics has attracted attention as an environmentally friendly and cost-effective way to grow healthy shrimps [1]. Probiotic bacteria used in aquaculture are mainly lactic acid bacteria and Bacillus, but purple non-sulfur photosynthetic bacterium (PNSB) is also a promising candidate. Shrimps, such as Litopenaeus vannamei and Penaeus monodon (black tiger), are raised in large quantities in Southeast Asian countries and China, and the PNSB, Rhodopseudomonas palustris, is commonly used as a probiotic and water conditioner [2]. Marsupenaeus japonicus (kuruma shrimp), a high price shrimp, are cultured in southern Japan. PNSB use is not common in Japanese aquaculture, but Rhodobacter sphaeroides (a PNSB) has been used in some shrimp ponds [3]. Rhodopseudomonas and Rhodobacter are terrestrial (freshwater) PNSB strains. Therefore, marine PNSB strains might be a better probiotic for shrimp because most are grown in seawater or brackish water.
Rhodovulum sulfidophilum is a marine PNSB, which is relatively easy-to-culture with various biotechnological applications, such as biohydrogen production [4], biomaterial production [5–7], and bioremediation [8] . Several studies used R. sulfidophilum in aquaculture [9–11], but the cells were added to the feed at relatively high concentrations (> 1 % fresh cell weight) as a nutrient supplement rather than a probiotic. A high dosage of bacterial cells results in high costs, making these approaches economically impractical. Another application of R. sulfidophilum in aquaculture is adding R. sulfidophilum cells to water recycling aquaculture system to improve the water quality and microbial communities [12].
In this study, the effects of a low R. sulfidophilum cell dose (106 colony forming units (cfu)/g feed; 0.01 % fresh cell weight) on Marsupenaeus japonicus growth and survival were examined in 177 m2 aquaria (140 tons of water) for 70 days.
The marine PNSB strain, Rhodovulum sulfidophilum OKHT16 (16S rRNA GenBank/EMBL/DDBJ accession number LC037397), was isolated from Osaka Bay, Japan, seashore sediment as previously described by Yamauchi et al. [13]. This strain is fast-growing (specific growth rate = 0.53 h-1 at 36 °C in light), thermotolerant (up to 48 °C), and can assimilate glycerol [13]. R. sulfidophilum OKHT16 was cultured in glutamate malate (GM) medium [14] with 3 % sodium chloride (NaCl) in light and aerobic conditions.
Two round outdoor aquaria (15 m diameter, 177 m2) located in the Fukuyoshi branch of the Fisheries Cooperative Association of Itoshima, Itoshima, Fukuoka, Japan, were used for the experiments. The aquaria bottoms were covered with sea sand (~20 cm deep), and then filled with sand-filtered seawater; the water depth was 80 cm (~140 tons of water per aquarium). Feed numbers 2, 4, and 5 (juveniles) and P1 (adults) from Hayashikane Sangyo Co., Japan, were used depending on the growth stage. R. sulfidophilum-containing feed (106 cfu/g; 0.01 % fresh cell weight) was prepared by suspending bacterial cells in a 3 % NaCl solution then mixing it with dry feed by shaking. The dosage (106 cfu/g feed) was determined based on previous studies with other kinds of probiotic bacteria [15–17]. The feed amount per day was approximately 5 % of the shrimp body weight. Feed leftovers were checked daily, and the amount was adjusted based on the leftovers. Water was circulated with two paddlewheel aerators, and the temperature, pH, and dissolved oxygen (DO) were recorded daily at 08:00 and 15:00. No water change was performed during the first 30 experimental days, after which approximately 30 % of the water was exchanged with fresh seawater once a week.
The experiment ran for 70 days, and on days 10, 20, 30, and 40, the total body weight of 30 shrimp was measured, and the average body weight was calculated by dividing the total body weight by 30. For days 50, 60, and 70, individual measurements were taken, and the average body weight and standard deviation were calculated. The statistical difference in the number of survivors at the end of experiment between R. sulfidophilum-fed and control was examined by Chi-squared test.
Nine thousand shrimp were initially released into the control and R. sulfidophilum-fed aquaria with an average body weight of 0.03 g; the experiment ran for 70 days (April 28 - July 7, 2017). Figure 1 shows the changes in the average body weights of the control and R. sulfidophilum-fed shrimp; there was no difference.
Figure1: Changes in the average body weights of the control (open circle) and R. sulfidophilium fed (closed circle) shrimps. On days 10, 20, 30, and 40, the total body weight of 30 shrimp was measured, and the average body weight was calculated by dividing the total body weight by 30. For days 50, 60, and 70, individual measurements were taken, and the average body weight and standard deviation were calculated.
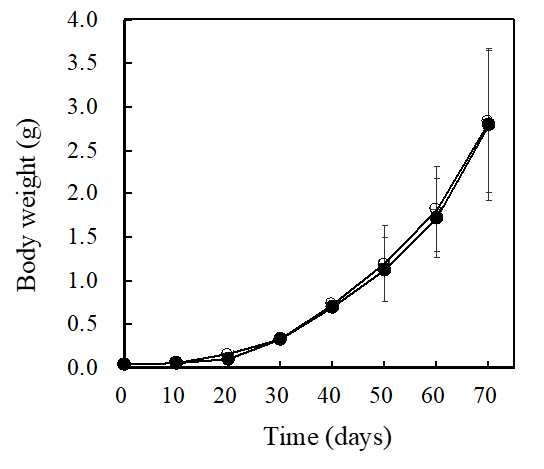
Table 1 shows the shrimp average body weights, number of survivors, survival rates, harvested shrimp total weights, total feed amounts, and feed conversion efficiencies (FCE; total feed amount/harvested total body weight) for the control and R. sulfidophilum-fed shrimp after 70 days. There was no difference in average body weight (2.83 g (control); 2.80 g (R. sulfidophilum-fed)), but the survival rate significantly improved with R. sulfidophilum (71.5 % (6434 of 9000) (control); 81.9 % (7372 of 9000) (R. sulfidophilum-fed); (P < 0.001, Chi-squared test). The improved survival rate in R. sulfidophilum-fed shrimp also increased the total harvested weight (18.2 kg (control); 20.6 kg (R. sulfidophilum-fed)) and improved the FCE value (2.11 (control); 1.83 (R. sulfidophilum-fed)).
Table 1. Average body weights, number of survivors, survival rates, total harvested body weights, total feed amounts, and feed conversion efficiencies (FCE) for control and R. sulfidophilum-fed shrimp.
|
Average body weight (g) |
Final # of shrimp1 |
Survival rate (%) |
Total body weight (kg) |
Total feed amount (kg) |
FCE2 |
|
|
Control |
2.83 |
6434 |
71.5 |
18.2 |
38.5 |
2.11 |
|
R. sulfidophilum-fed |
2.80 |
7372*** |
82.0 |
20.6 |
37.7 |
1.83 |
1 Initial shrimp number = 9000.
2 FCE (feed conversion efficiency) = (total feed amount) / (harvested total body weight)
*** P < 0.001 (Chi-squared test)
The results showed that R. sulfidophilum cells added to M. japonicus feed at 106 cfu/g (0.01 % of fresh cell weight) acts as a probiotic and is economically feasible for aquaculture. In Japan, the price of commercial pure PNSB culture (1 x 109 cfu/mL) is $3 to $5 for 1 L [18]. Adding R. sulfidophilum cells to feed at a concentration of 106 cfu/g is equivalent to 1 mL of PNSB culture (1 x 109 cfu/mL) per 1 kg of feed and costs $0.003 to $0.005; M. japonicus feed is approximately $5 per 1 kg. Therefore, the PNSB cost is 0.06 % to 0.1 % of the feed cost, making R. sulfidophilum a cost-effective and feasible probiotic for shrimp aquaculture.
Rhodovulum sulfidophilum, a marine purple non-sulfur photosynthetic bacterium (PNSB), added to the feed at 106 cfu/g (0.01 % of fresh cell weight) acts as a probiotic, and improves the survival rate, total harvested weight and feed conversion efficiencies (FCE) in M. japonicus aquaculture. The cost for PNSB cells is 0.06 % to 0.1 % of the feed cost, thus R. sulfidophilum is a cost-effective and feasible probiotic for shrimp aquaculture.
We thank the Fisheries Cooperative Association of Itoshima, Itoshima, Fukuoka, Japan, for their help with outdoor aquaria experiments.
We would like to thank Editage (www.editage.com) for English language editing.
Author's contributions
Aoi Koga cultured PNSB and analyzed the data. Yusaku Tani and Ken-ichi Ozaki designed and performed the aquaria experiment. Takaaki Maki designed the procedure for the preparation of shrimp feed containing PNSB. Shuhei Hayashi and Shinjiro Yamamoto revised the manuscript critically. Hitoshi Miyasaka designed the study and wrote the manuscript.
Ninawe AS, Selvin J. Probiotics in shrimp aquaculture: Avenues and challenges probiotics in shrimp aquaculture: Avenues and challenges A.S. Ninawe et al. Crit Rev Microbiol. 2009;35(1):43-66. PMid:19514908
View Article PubMed/NCBIQi Z, Zhang XH, Boon N, Bossier P. Probiotics in aquaculture of China - Current state, problems and prospect. Aquaculture. 2009;290(1-2):15-21.
View ArticleMaki T. Applications of Rhodobacter capsulata in agriculture, stock raising, environmental technologies, and aquaculture. Seibutsu-kogaku kaishi. 2011;89:113-6.
Carlozzi P. Hydrogen photoproduction by Rhodopseudomonas palustris 42OL cultured at high irradiance under a semicontinuous regime. J Biomed Biotechnol. 2012;2012:590693. PMid:22910542
View Article PubMed/NCBIKikuchi Y, Umekage S. Extracellular nucleic acids of the marine bacterium Rhodovulum sulfidophilum and recombinant RNA production technology using bacteria. FEMS Microbiol Lett. 2018;365(3).
View ArticleHiguchi-Takeuchi M, Numata K. Marine Purple Photosynthetic Bacteria as Sustainable Microbial Production Hosts. Frontiers in Bioengineering and Biotechnology. 2019;7:258. PMid:31681740
View Article PubMed/NCBIFoong CP, Higuchi-Takeuchi M, Malay AD, Oktaviani NA, Thagun C, Numata K. A marine photosynthetic microbial cell factory as a platform for spider silk production. Commun Biol. 2020;3(1). PMid:32641733
View Article PubMed/NCBISakpirom J, Kantachote D, Siripattanakul-Ratpukdi S, McEvoy J, Khan E. Simultaneous bioprecipitation of cadmium to cadmium sulfide nanoparticles and nitrogen fixation by Rhodopseudomonas palustris TN110. Chemosphere. 2019;223:455-64. PMid:30784752
View Article PubMed/NCBIAzad S Al, Chong VC, Vikineswary S. Phototrophic bacteria as feed supplement for rearing Penaeus monodon larvae. J World Aquac Soc. 2002;33(2):158-68.
View ArticleLoo PL, Chong VC, Vikineswary S. Rhodovulum sulfidophilum, A phototrophic bacterium, Grown in palm oil mill effluent improves the larval survival of marble goby Oxyeleotris marmorata (Bleeker). Aquac Res. 2013;44(3):495-507.
View ArticleBanerjee S, Azad SA, Vikineswary S, Selvaraj OS, Mukherjee TK. Phototrophic bacteria as fish feed supplement. Asian-Australasian Journal of Animal Sciences. 2000;13:991-4.
View ArticleChang BV, Liao C Sen, Chang YT, Chao WL, Yeh SL, Kuo DL, et al. Investigation of a farm-scale multitrophic recirculating aquaculture system with the addition of Rhodovulum sulfidophilum for milkfish (Chanos chanos) coastal aquaculture. Sustain. 2019;11(7).
View ArticleYamauchi N, Koga A, Okuhata H, Tanaka S, Yamada N, Maki T, et al. Isolation of a marine purple non-sulfur photosynthetic bacterium with a high ability of glycerol assimilation. Int J Plant, Anim Environ Sci. 2019;9(4):214-21.
Sangkharak K, Prasertsan P. Nutrient optimization for production of polyhydroxybutyrate from halotolerant photosynthetic bacteria cultivated under aerobic-dark condition. Electron J Biotechnol. 2008;11(3).
View ArticleBalcázar JL, Rojas-Luna T. Inhibitory activity of probiotic Bacillus subtilis UTM 126 against Vibrio species confers protection against vibriosis in juvenile shrimp (Litopenaeus vannamei). Curr Microbiol. 2007;55(5):409-12. PMid:17680306
View Article PubMed/NCBISadat Hoseini Madani N, Adorian TJ, Ghafari Farsani H, Hoseinifar SH. The effects of dietary probiotic Bacilli (Bacillus subtilis and Bacillus licheniformis) on growth performance, feed efficiency, body composition and immune parameters of whiteleg shrimp (Litopenaeus vannamei) postlarvae. Aquac Res. 2018;49(5):1926-33.
View ArticleWang YC, Hu SY, Chiu CS, Liu CH. Multiple-strain probiotics appear to be more effective in improving the growth performance and health status of white shrimp, Litopenaeus vannamei, than single probiotic strains. Fish Shellfish Immunol. 2019;84:1050-8. PMid:30419396
View Article PubMed/NCBIKoga A, Goto M, Morise T, Tran HTD, Kakimoto T, Kashiyama K, et al. Value-added recycling of distillation remnants of Kuma Shochu: A local traditional Japanese spirit, with photosynthetic bacteria. Waste and Biomass Valorization. 2019;11(12):6717-24.
View Article