John Arron Stride
Email: j.stride@unsw.edu.au
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 1
Page No: 121-126
John Arron Stride
Email: j.stride@unsw.edu.au
1Fatemeh Mirnajafizadeh,2John Arron Stride*
1,2School of Chemistry, University of New South Wales, Sydney, Australia
Narayan Ch Das(ncdas@rtc.iitkgp.ernet.in)
Xiaoting Ji(dingcaifeng2003@163.com)
Maria Goreti Carvalho Pereira(goreti.pereira@ufpe.br)
Hadla S Ferreira(h4sousa@gmail.com)
Fatemeh Mirnajafizadeh, John Arron Stride, A Brief Review on Core/shell Quantum Dots (2020) SDRP Journal of Nanotechnology & Material Science 2(1) pp:121-126
Core/shell QDs are a special class of nanoparticles with unique optical properties such as narrow emission, wide absorption and photo-stability as found in quantum dots, but the specific structure of core/shell QDs promotes their optical properties over simple QDs. This paper details structure, synthesis, properties, classifications and applications of core/shell QDs.
Keywords: Quantum dots, core/shell nanoparticles, aqueous synthesis, quantum yield, Cytotoxicity.
Core/Shell quantum dots are products of further engineering in the structures of quantum dots (QDs). They exhibit improved optical properties over simple QDs due to the shell surrounding the QD core, which improves stability and photoluminescence efficiency [1]. A QD has a high surface area to volume ratio with unsaturated bonds, or dangling bonds, existing on the surface. These under-coordinated atoms make them more active than those in the bulk of the QD materials. Coating of QDs with appropriate materials to form core/shell QDs [2-4] leads them to exhibit higher quantum yields and greater stability than core QDs; the shell growth both confines the excitation to the core and protects the core against oxidation and chemical degradation [5-7].
2. Classification of Core/Shell QDs
Core/shell QDs can be categorized according to the band gap and energy levels of their components into three broad groups: type I, reverse type I and type II [8].
2.1- Type I
In this type of nanoparticle, either the conduction or the valance bands of the core align within the band gap of the shell, such that both the electrons and holes are localized in the core. For example in CdSe/CdS type-I core/shell QDs, the band gap of the CdSe core is 1.74 eV and the band gap of the CdS shell is 2.42 eV. Therefore, both the holes and electrons are confined to the CdSe core [18, 19]. CdSe/ZnS, and InAs/CdSe core/shell QDs are other examples for type-I core/shell nanocrystal systems [9, 10].
2.2. Inverse type I
In inverse type-I materials the band gap of the core is wider than the band gap of the shell and both the conduction and valence bands of the shell are therefore localized within the band gap of core. Consequently, the holes and electrons are confined in the shell [11]. Examples include CdS/HgS, CdS/CdSe and ZnSe/CdSe core/shell QDs [12-14].
2.3. Type II
In type-II systems both the valence and conduction band edges of the core are lower or higher than those in the shell and both the hole and the electron are confined to the core [15-17]. CdSe/ZnSe, CdTe/CdSe and CdS/ZnSe core/shell QDs are some examples of type-II core/shell QDs [15, 18].
3. Synthesis of Core/shell QDs
Core/shell QDs are mostly synthesized in two steps: first, the synthesis of the core QDs and then over-coating of these QDs through shell growth reactions [19].
3.1 Synthesis of Core QDs
QDs can be synthesized using different synthetic methods including physical methods, gas phase syntheses and the liquid phase colloidal synthesis [20-28]. In physical methods, QDs are produced by breaking down bulk semiconductors [20, 21]. Gas phase syntheses are mainly based upon producing QDs by epitaxial growth of thin-films on crystal surfaces to form three dimensional nanoparticles, including QDs. However, the liquid phase colloidal synthesis is a widely used chemical approach to produce various QDs including CdSe, CdSe, ZnSe, ZnS, CdTe, InP and ZnS QDs [22-26]. In the colloidal synthesis approach, uniform QDs can be produced using organometallic synthesis, solvothermal methods, microwave assisted methods, hydrothermal approaches and direct aqueous synthetic methods [27-30]. Fig 1 shows UV emission of CdSe(S) and ZnSe(S) QDs synthesized using an aqueous hydrothermal synthetic method [29, 31].

Fig 1. QDs glowing under UV light: ZnSe(S) QDs (blue) and CdSe(S) QDs (yellow, red and Orange) [29, 31].
3.2 Epitaxial Shell growth
The second step in the formation of core/shell QDs is the coating of QDs with appropriate materials to form the core/shell QDs.
Epitaxial shell growth processes, necessitate the selection of suitable shell materials to allow the formation of core/shell QDs with improved optical properties. For example, ZnS is a suitable shell material for over coating of CdSe core QDs because the small lattice mismatch between ZnS and CdSe prevents a change of crystal structure, minimizes surface-defects and leads to increased quantum yields in CdSe/ZnS core/shell QDs. [32, 33].
In an epitaxial growth process, control of the thickness of the shell over the nanoparticle core is another important parameter as thick shells induce surface defects that result in reduced photoluminescence [34]. Shell growth process can occur in organic or aqueous synthetic methods [35-45].
3.2.1 Organic synthetic approach
Organic shell growth is based upon the addition of organic shell precursors to a solution of colloidal QDs using organic solvents to produce core/shell QDs such as CdSe/CdS, CdSe/ZnS, CdS/ZnS and CdTeSe/CdZnS QDs [35-39]. For instance, Bawendi and co-workers reported the formation of CdSe/ZnS QDs after injection of diethylzinc (ZnEt2) and hexamethyldisilathiane ((TMS)2) as zinc and sulfur precursors in a solution of tricyclophosphine oxide (TOPO), tricyclophosphine (TOP) and CdSe QDs at high temperature. The overcoated QDs showed an improved quantum yield of 50% at room temperature [35]. However, the core/shell QDs synthesized in the organic synthetic route are soluble only in nonpolar solvents such as hexane and they are not soluble in water.
3.2.2. Aqueous Synthesis of core/shell QDs
In addition to producing core/shell QDs via organic routes, core/shell QDs can be formed via aqueous synthetic methods. The process of shell growth in an aqueous route occurs by adjusting experimental parameters such as temperature, pH and reaction time in the presence of QDs by heating, hydrothermal processes or microwave assisted methods, to produce core/shell QDs [40-46]. Fig 2 shows the formation of CdSe(S)/ Fe2O3 QDs via an aqueous synthetic route [47].
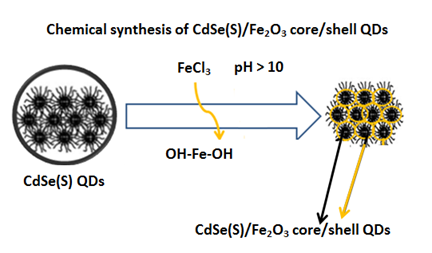
Fig 2. Formation of CdSe(S)/Fe2O3 QDs using an aqueous synthetic approach [47].
As shown in Fig 2, the formation of core/shell QDs in an aqueous media, often consists of over-coating QD cores with metal sulfides, metal oxides or other semiconductors as the shell material [47]. The overgrowth of the shell occurs in an analogous manner to the initial QD growth reactions using the appropriate precursors, adjusting the experimental parameters and using simple thermally-assisted reactions, hydrothermal methods and microwave assisted approaches [48-52].
In all of the QD coating methods, the selection of a shell having an appropriate band gap is very important to form the desired type of QD. Meanwhile, when metal oxides or sulfides are used as the shell material, the resultant shells are amorphous, which prohibits perturbation of the crystalline structure of the cores [48, 52-54].
The products of aqueous synthetic approaches are water soluble core/shell QDs and can be potentially used for bioapplications due to the fact that water solubility is an important parameter in applications of nanoparticles in biological environment [31, 55].
4. Applications of Core/Shell QDs
There are a various applications for core/shell QDs in various industrial fields including lasers, light emitting diodes and photovoltaic devices [1, 56-58]. Core/shell QDs can also be used for bioapplications due to exhibiting lower toxicity than uncoated QDs [59, 60]. As the toxicity of QDs critically depends on free heavy metal ion formation and the oxidation of components involved in the structure of QDs, the presence of the shell around the core QDs protects the core against oxidation, preventing the possible formation of free heavy metal ions particularly when the core contains transition metals such as cadmium [61, 62]. Therefore, core/shell QDs can be used for biological applications.
Core/shell QDs can be produced via epitaxial shell growth on single QDs. The improved optical properties of core/shell QDs including increasing quantum yield and photostability of core QDs highlights the necessity of synthesis of core/shell QDs. Cytotoxicity of QDs can be controlled via further engineering in the structure of nanoparticles such as production of high quality core/shell QDs. The properties of core/shell nanocrystals depend on the individual properties of both the cores and shells; high quality core/shell QDs are naturally obtained by coating highly crystalline and photo stable QDs cores with appropriate shell materials.
Authors would like to thank School of Chemistry University of New South Wales for supporting this study.
Vasudevan, D.; Gaddam, R. R.; Trinchi, A.; Cole, I. Journal of Alloys and Compounds 2015, 636, 395.
View ArticleLiem, N. Q.; Phuong, L. Q.; Thuy, U. T. D.; Chi, T. T. K.; Thanh, D. X. J. Korean Phys. Soc. 2008, 53, 1570.
View ArticleVerma, P.; Pandey, A. C. J. Biomater. Nanobiotechnol. 2011, 2, 409.
View ArticleChen, X.; Lou, Y.; Burda, C. Int. J. Nanotechnol. 2004, 1, 105.
View ArticleHines, M. A.; Guyot-Sionnest, P. The Journal of Physical Chemistry 1996, 100, 468.
View ArticleXie, H.-Y. et al. Preparation and characterization of overcoated II-VI quantum dots. J Nanosci Nanotechnol 5, 880-886 (2005). PMid:16060147
View Article PubMed/NCBIPeng, X.; Schlamp, M. C.; Kadavanich, A. V.; Alivisatos, A. P. J. Am. Chem. Soc. 1997, 119, 7019.
View ArticlePetryayeva, E.; Algar, W. R.; Medintz, I. L. Appl. Spectrosc. 2013, 67, 215. PMid:23452487
View Article PubMed/NCBIYildiz, I.; McCaughan, B.; Cruickshank, S. F.; Callan, J. F.; Raymo, F. i. M. Langmuir 2009, 25, 7090. PMid:19239226
View Article PubMed/NCBIXie, R.; Peng, X. Angew. Chem., Int. Ed. 2008, 47, 7677.
Korsunska, N. E.; Dybiec, M.; Zhukov, L.; Ostapenko, S.; Zhukov, T. Semicond. Sci. Technol. 2005, 20, 876.
View ArticleMews, A.; Eychmueller, A.; Giersig, M.; Schooss, D.; Weller, H. J. Phys. Chem. 1994, 98, 934.
View ArticleTian, Y.; Newton, T.; Kotov, N. A.; Guldi, D. M.; Fendler, J. H. J. Phys. Chem. 1996, 100, 8927.
View ArticleHu, D.; Zhang, P.; Gong, P.; Lian, S.; Lu, Y.; Gao, D.; Cai, L. Nanoscale 2011, 3, 4724. PMid:21989776
View Article PubMed/NCBIJia, G.-Z.; Fei, X.-N.; Wang, J. Chalcogenide Lett. 2010, 7, 181.
Ivanov, S. A.; Piryatinski, A.; Nanda, J.; Tretiak, S.; Zavadil, K. R.; Wallace, W. O.; Werder, D.; Klimov, V. I. J. Am. Chem. Soc. 2007, 129, 11708. PMid:17727285
View Article PubMed/NCBIBalet, L. P.; Ivanov, S. A.; Piryatinski, A.; Achermann, M.; Klimov, V. I. Nano Lett. 2004, 4, 1485.
View ArticleNing, Z.; Yuan, C.; Tian, H.; Fu, Y.; Li, L.; Sun, L.; Aagren, H. J. Mater. Chem. 2012, 22, 6032.
View ArticleCai, Z.; Shi, B.; Zhao, L.; Ma, M. Spectrochim. Acta, Part A 2012, 97, 909. PMid:22902934
View Article PubMed/NCBISalas, G.; Costo, R.; Morales, M. d. P. In Frontiers of Nanoscience; Jesus, M. d. l. F., Grazu, V., Eds.; Elsevier, 2012, 4, 35.
View ArticlePark, J.; Joo, J.; Kwon, S. G.; Jang, Y.; Hyeon, T. Angew. Chem., Int. Ed. 2007, 46, 4630. PMid:17525914
View Article PubMed/NCBIJun, Y.-w.; Choi, J.-s.; Cheon, J. Angew. Chem., Int. Ed. 2006, 45, 3414. PMid:16642516
View Article PubMed/NCBIRatsch, C.; Zangwill, A. Surf. Sci. 1993, 293, 123. 90250-N
View ArticleMa, C.; Ding, Y.; Moore, D.; Wang, X.; Wang, Z. L. J Am Chem Soc 2004, 126, 708. PMid:14733532
View Article PubMed/NCBIZhang, Y.; Wang, L.; Liu, X.; Yan, Y.; Chen, C.; Zhu, J. J Phys Chem B 2005, 109, 13091. PMid:16852628
View Article PubMed/NCBIPan, Z. W.; Dai, Z. R.; Xu, L.; Lee, S. T.; Wang, Z. L. J. Phys. Chem. B 2001, 105, 2507.
View ArticleMurray, C. B.; Sun, S.; Gaschler, W.; Doyle, H.; Betley, T. A.; Kagan, C. R. IBM J. Res. Dev. 2001, 45, 47.
View ArticlePeng, Z. A.; Peng, X. J. Am. Chem. Soc. 2001, 123, 183. PMid:11273619
View Article PubMed/NCBIMirnajafizadeh, F.; Ramsey, D.; McAlpine, S.; Wang, F.; Reece, P.; Stride, J. A. Mater. Sci. Eng., C 2016, 64, 167. PMid:27127041
View Article PubMed/NCBIMirnajafizadeh, F and Stride, J. A. International Journal of Advanced in Science Engineering and Technology, 2018, 6, 1, 56.
F. Mirnajafizadeh, D. Ramsey, S. McAlpine, F. Wang, J.A. Stride, , Nanomaterials 2019, 9, 465. PMid:30897752
View Article PubMed/NCBISamarth, N.; Luo, H.; Furdyna, J. K.; Qadri, S. B.; Lee, Y. R.; Ramdas, A. K.; Otsuka, N. Applied Physics Letters 1989, 54, 2680.
View ArticleYoun, H. C.; Baral, S.; Fendler, J. H. J. Phys. Chem. 1988, 92, 6320.
View ArticleReiss, P.; Protiere, M.; Li, L. Small 2009, 5, 154. PMid:19153991
View Article PubMed/NCBIDabbousi, R. O.; Rodriguez-Viejo, J.; Mikulec, F. V.; Heine, J. R.; Mattoussi, H.; Ober, R.; Jensen, K. F.; Bawendi, M. G. J. Phys. Chem. B 1997, 101, 9463.
View ArticleHuang, G.-W.; Chen, C.-Y.; Wu, K.-C.; Ahmed, M. O.; Chou, P.-T. Journal of Crystal Growth 2004, 265, 250.
View ArticleMalik, M. A.; O'Brien, P.; Revaprasadu, N. Chemistry of Materials 2002, 14, 2004.
View ArticlePons, T.; Lequeux, N.; Mahler, B.; Sasnouski, S.; Fragola, A.; Dubertret, B. Chemistry of Materials 2009, 21, 1418.
View ArticleManna, L.; Scher, E. C.; Li, L.-S.; Alivisatos, A. P. J. Am. Chem. Soc. 2002, 124, 7136. PMid:12059239
View Article PubMed/NCBIAldeek, F.; Balan, L.; Medjahdi, G.; Roques-Carmes, T.; Malval, J.-P.; Mustin, C.; Ghanbaja, J.; Schneider, R. l. The Journal of Physical Chemistry C 2009, 113, 19458.
View ArticleZhan, H.-J.; Zhou, P.-J.; He, Z.-Y.; Tian, Y. Eur. J. Inorg. Chem. 2012, 2012, 2487.
View ArticleWang, J.; Han, H. J. Colloid Interface Sci. 2010, 351, 83. PMid:20692669
View Article PubMed/NCBIAldeek, F.; Mustin, C.; Balan, L.; Medjahdi, G.; Roques-Carmes, T.; Arnoux, P.; Schneider, R. Eur. J. Inorg. Chem. 2011, 794.
View ArticleFu, T.; Qin, H.-Y.; Hu, H.-J.; Hong, Z.; He, S. J. Nanosci. Nanotechnol. 2010, 10, 1741. PMid:20355568
View Article PubMed/NCBIHe, Y.; Lu, H.-T.; Sai, L.-M.; Su, Y.-Y.; Hu, M.; Fan, C.-H.; Huang, W.; Wang, L.-H. Adv. Mater. (Weinheim, Ger.) 2008, 20, 3416.
View ArticleDatta, A.; Panda, S. K.; Chaudhuri, S. The Journal of Physical Chemistry C 2007, 111, 17260.
View ArticleMirnajafizadeh, F.; Wang, F.; Reece, P.; Stride, J. A. J. Mater. Sci. 2016, 51, 5252.
View ArticleSchumacher, W.; Nagy, A.; Waldman, W. J.; Dutta, P. K. The Journal of Physical Chemistry C 2009, 113, 12132.
View ArticleWang, J.; Han, H. J. Colloid Interface Sci. 2010, 351, 83. PMid:20692669
View Article PubMed/NCBILaw, W.-C.; Yong, K.-T.; Roy, I.; Ding, H.; Hu, R.; Zhao, W.; Prasad, P. N. Small 2009, 5, 1302. PMid:19242947
View Article PubMed/NCBIFu, T.; Qin, H.-Y.; Hu, H.-J.; Hong, Z.; He, S. J. Nanosci. Nanotechnol. 2010, 10, 1741. PMid:20355568
View Article PubMed/NCBIAldeek, F.; Mustin, C.; Balan, L.; Medjahdi, G.; Roques-Carmes, T.; Arnoux, P.; Schneider, R. Eur. J. Inorg. Chem. 2011, 794.
View ArticleJia, G.; Hao, B.; Lu, X.; Yao, J. Int. J. Electrochem. Sci. 2013, 8, 8167.
Xie, H.-Y.; Liang, J.-G.; Liu, Y.; Zhang, Z.-L.; Pang, D.-W.; He, Z.-K.; Lu, Z.-X.; Huang, W.-H. J Nanosci Nanotechnol 2005, 5, 880. PMid:16060147
View Article PubMed/NCBINajafi Zadeh, F. M.; Stride, J. A. International Conference on Nanoscience and Nanotechnology 2014, p 13.
Blanco-Canosa, J. B.; Medintz, I. L.; Farrell, D.; Mattoussi, H.; Dawson, P. E. J. Am. Chem. Soc. 2010, 132, 10027. PMid:20597509
View Article PubMed/NCBIKostic, R.; Stojanovic, D. Optoelectron. Adv. Mater., Rapid Commun. 2012, 6, 121.
Li, Y.; Rizzo, A.; Mazzeo, M.; Carbone, L.; Manna, L.; Cingolani, R.; Gigli, G. J. Appl. Phys. 2005, 97, 113501/1.
View ArticlePeng, Z. A.; Peng, X. J. Am. Chem. Soc. 2001, 123, 183. PMid:11273619
View Article PubMed/NCBIPradhan, N.; Efrima, S. J Am Chem Soc 2003, 125, 2050. PMid:12590524
View Article PubMed/NCBIPan, Z. W.; Dai, Z. R.; Xu, L.; Lee, S. T.; Wang, Z. L. J. Phys. Chem. B 2001, 105, 2507.
View ArticleMurray, C. B.; Sun, S.; Gaschler, W.; Doyle, H.; Betley, T. A.; Kagan, C. R. IBM J. Res. Dev. 2001, 45, 47.
View Article