Justin J. Turcotte PhD, MBA
Email: jturcotte@aahs.org
2000 Medical Parkway Suite 503 Annapolis, MD 21401
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 2
Page No: 188-198
Justin J. Turcotte PhD, MBA
Email: jturcotte@aahs.org
2000 Medical Parkway Suite 503 Annapolis, MD 21401
Justin J. Turcotte PhD MBA, Mary E. King, Sohail N. Zaidi MD MBA
Luminis Health Anne Arundel Medical Center, Department of Surgery, Annapolis, Maryland
Justin Turcotte, PhD, MBA, Mary E. King, Sohail N. Zaidi MD MBA, Risk Factors for Rescue Neuromuscular Blockade Reversal Using Sugammadex (2022) Journal of Anesthesia & Surgery 4(2) p:188-198
Background: Previous studies have demonstrated the superiority of sugammadex over neostigmine for reversal of nondepolarizing neuromuscular blockade (NMB) reversal. However, its increased cost over neostigmine remains a barrier to use in many institutions.
Aims: The purposes of this study were to compare the outcomes of patients receiving neostigmine alone vs. patients receiving neostigmine and sugammadex and to identify the risk factors for patients requiring sugammadex as a rescue neuromuscular blockade reversal agent.
Methods: A retrospective observational study of general anesthesia cases using rocuronium or vecuronium for NMB at a single regional medical center from January 1, 2019 to March 30, 2021 was performed. Demographics, surgical details, and outcomes of patients receiving neostigmine only (N) were compared to those of patients receiving neostigmine followed by sugammadex (NS). A conditional logistic regression model was developed to identify predictors of patients requiring a rescue dose of sugammadex for NMB reversal.
Results: A total of 7104 patients were included (N=6684, NS=420). In comparison to patients in the N group, those in the NS group experienced shorter duration from last NMB administration to first reversal, longer PACU recovery times, longer length of stay, and higher rates of reintubation. After risk adjustment, patients receiving NS were more likely to be female, of non-white race, have increased BMIs, and a greater comorbidity burden than those requiring N only. In comparison to patients undergoing general surgery, those undergoing thoracic surgery are at increased risk for requiring NS. A model for predicting which patients would receive NS was generated with an AUC of 0.681 (95% CI: 0.654-0.708), sensitivity of 78% and specificity of 48%.
Conclusion: These findings may assist anesthesiologists in identifying which patients are likely to require sugammadex for rescue NMB reversal after use of neostigmine and are therefore suitable candidates for using sugammadex as a first-line therapy.
Key Words : Neuromuscular blockade reversal, sugammadex, neostigmine, residual neuromuscular blockade, predictive model
Core Tip: After risk adjustment, patients who were female, of non-white race, have increased BMIs, have a greater comorbidity burden, or undergo thoracic surgery are at increased risk for requiring sugammadex after neostigmine. A model for predicting which patients would receive neostigmine and sugammadex was generated with an AUC of 0.681 (95% CI: 0.654-0.708), sensitivity of 78% and specificity of 48%. These findings may assist anesthesiologists in identifying which patients are likely to require sugammadex for rescue NMB reversal after use of neostigmine and are therefore suitable candidates for using sugammadex as a first-line therapy.
Neostigmine and sugammadex are commonly used pharmacologic agents for reversal of nondepolarizing neuromuscular blockades (NMB). Neostigmine, an acetylcholinesterase inhibitor, has limitations such as indirect mechanism of reversal, limited and unpredictable efficacy, inability to reverse deep NMB, and undesirable autonomic response.1 Sugammadex is designed for rapid reversal and has potential benefits such as fast and predictable reversal of any degree of block, patient safety, reduced incidence of residual block during recovery.1 The drug works through a direct mechanism of action by selectively binding rocuronium or vecuronium, which reverses their neuromuscular blocking action.2 Its 1:1 binding allows for reversal of any depth of block—an advantage over neostigmine which is ineffective in deep blockades. Previous randomized controlled trials have shown that in comparison to neostigmine, sugammadex is faster in reversing NMB, more likely to be associated with higher train-of-four ratio values at extubation, and lowers risk of residual curarization after extubation, global adverse events, respiratory adverse events and cardiovascular adverse events.3 While the efficacy of sugammadex is well established, its increased cost over neostigmine remains a barrier to use in many institutions, as the drug is approximately five times more expensive than neostigmine.4
Neostigmine and sugammadex can be used in combination when there is not adequate reversal of the neuromuscular blockade from neostigmine alone.5 The combination of both medications has been found to decrease recovery time and can also reduce the dosage of sugammadex needed, which can reduce costs in some cases. Previous studies have shown that using neostigmine alone results in a higher chance of systematic muscarinic side effects, such as hypertension, compared to using sugammadex alone. Using both medications may increase the risk of these side effects.5 Because of the potentially increased side effect profile of combination therapy and the increased cost associated with using both agents, it is important that the profile of appropriate patients who should receive sugammadex as a first line NMB reversal agent be defined.
The purposes of this study were to compare the outcomes of patients receiving neostigmine alone vs. patients receiving neostigmine and sugammadex and to identify the risk factors for patients requiring sugammadex as a rescue neuromuscular blockade reversal agent. We suggest that identifying the profile of this at-risk population may assist anesthesiologists in preoperatively selecting the most appropriate patients to receive sugammadex for first line NMB reversal.
This study was deemed institutional review board exempt by the institution’s clinical research committee. A retrospective observational study of patients undergoing surgical procedures with general anesthesia at a single regional medical center from January 1, 2019 to March 30, 2021 was performed. Patients that were ≥ 18 years old at the time of surgery and received vecuronium or rocuronium for NMB along with a NMB reversal agent were included in this study. Only patients receiving neostigmine alone or neostigmine and sugammadex were included. Patients undergoing cesarean section or those receiving only succinylcholine for NMB, sugammadex-only for NMB reversal, or no reversal agent were excluded. The decision for NMB reversal was made based on anesthesiologist judgment. At our institution qualitative train of four count (TOF-C) measurement is performed at prior to extubation when NMB agents are used. TOF-C measurement was performed by delivering four successive supramaximal stimuli at 2Hz to the ulnar or facial nerves and monitoring response twitch frequency. The last TOF-C prior to neostigmine administration and first TOF-C after neostigmine administration were recorded and analyzed. Prior to extubation, patients were clinically assessed for normothermia, spontaneous breathing, head lift, tidal volume, muscle strength, agitation, and ability to follow commands.
The electronic health record was queried to extract patient demographics, comorbidities, the type of surgery performed, and perioperative outcomes. Comorbidities were extracted using International Classification of Disease 10th Edition (ICD-10) codes present in the patients’ chart at the time of surgery. The coding definitions are presented in the Appendix. Surgery types were defined based on the specialty of the primary surgeon. Perioperative outcomes evaluated included minutes in the operating room (OR), minutes in the post anesthesia care unit (PACU), the first and last oxygen saturation (SpO2) documented in the PACU, minutes from last NMB agent administration to administration of the first reversal agent, length of stay (measured in days and hours), and rates of reintubation.
2.1 Statistical Analysis
Univariate statistical analysis was performed to compare differences in demographics, comorbidities, surgeries performed, and outcomes between patients receiving neostigmine (N) and neostigmine followed by sugammadex (NS). Use of sugammadex after neostigmine was defined as rescue use. Chi-square tests were performed to compare binary data points, with Fisher’s exact test used as indicated. Two-sided independent samples t-tests were performed to compare continuous measures. A multiple backwards conditional logistic regression model was then created to evaluate predictors of patients receiving NS. All independent variables were entered into the model, and over 16 steps all variables not affecting overall model fit with the endpoint were eliminated. A receiver operating characteristics (ROC) curve was then generated to assess overall model performance and the optimal sensitivity and specificity were calculated. All statistical analysis was performed in SPSS version 27 (IBM, Armonk, NY). P-values of <0.05 were treated as statistically significant.
In comparison to patients requiring N only, those receiving NS had significantly higher BMIs (N: 30.9 ± 7.7 kg/m2 vs. NS: 33.6 ± 8.9, p<0.001) and were more likely to be of non-white race (N: 23.6% vs. NS: 30.8%, p<0.001). No differences in age or gender were observed between groups. Evaluation of comorbidities highlighted that those receiving NS had higher rates of sleep apnea, other respiratory diseases, diabetes, chronic kidney disease, hypertensive disease, and pulmonary hypertensive disease or pulmonary circulatory disease (all p<0.05). This was reflected by a higher proportion of patients with an American Society of Anesthesiologists (ASA) score ≥ 3 in the NS group (N: 46.0% vs. NS: 55.4%, p<0.001). Finally, significant differences in the types of surgeries performed were observed (p<0.001). In the N group, the most common surgeries performed were general (44.0%), orthopedics (22.4%), and obstetrics & gynecology (11.2). In the NS group the three most common surgeries were general (60.2%), orthopedics (9.3%), and thoracic (9.0%) (Table 1).
Table 1. Patient Demographics, Comorbidities, and Surgeries Performed
|
Characteristic |
Neostigmine (N=6684) |
Neostigmine and Sugammadex (N=420) |
P-Value |
||
|
Demographics |
|
|
|
||
|
|
Age (yrs.) |
56.8 ± 16.5 |
56.5 ± 16.4 |
0.699 |
|
|
|
BMI (kg/m2) |
30.9 ± 7.7 |
33.6 ± 8.9 |
<0.001 |
|
|
|
BMI ≥ 30 |
3129 (46.9) |
248 (59.3) |
<0.001 |
|
|
|
BMI ≥ 35 |
1712 (25.7) |
162 (38.8) |
<0.001 |
|
|
|
Female |
3994 (59.8) |
270 (64.3) |
0.066 |
|
|
|
Non-White Race |
1469 (23.6) |
119 (30.8) |
0.001 |
|
|
Comorbidities |
|
|
|
||
|
|
ASA ≥ 3 |
3066 (46.0) |
232 (55.4) |
<0.001 |
|
|
|
Sleep Apnea |
997 (14.9) |
84 (20.0) |
0.005 |
|
|
|
COPD |
358 (5.4) |
31 (7.4) |
0.077 |
|
|
|
Other Respiratory Disease |
1188 (17.8) |
108 (25.7) |
<0.001 |
|
|
|
Diabetes |
1110 (16.6) |
88 (21.0) |
0.021 |
|
|
|
Chronic Kidney Disease |
474 (7.1) |
48 (11.4) |
0.001 |
|
|
|
Liver Disease |
321 (4.8) |
26 (6.2) |
0.201 |
|
|
|
CHF |
202 (3.0) |
19 (4.5) |
0.086 |
|
|
|
Hypertensive Disease |
3406 (51.0) |
252 (60.0) |
<0.001 |
|
|
|
Ischemic Heart Disease |
658 (9.8) |
42 (10.0) |
0.917 |
|
|
|
Pulmonary Hypertensive Disease or Pulmonary Circulatory Disease |
57 (0.9) |
13 (3.1) |
<0.001 |
|
|
|
Other Heart Disease |
805 (12.0) |
52 (12.4) |
0.837 |
|
|
|
Neoplasm |
1511 (22.6) |
94 (22.4) |
0.915 |
|
|
|
Nicotine Dependence |
690 (10.3) |
42 (10.0) |
0.833 |
|
|
Surgery Type |
|
|
<0.001 |
||
|
|
General |
2942 (44.0) |
253 (60.2) |
|
|
|
|
Breast |
192 (2.9) |
6 (1.4) |
|
|
|
|
Other |
61 (0.9) |
4 (1.0) |
|
|
|
|
Obstetrics & Gynecology |
748 (11.2) |
27 (6.4) |
|
|
|
|
Neurosurgery |
333 (5.0) |
15 (3.6) |
|
|
|
|
Orthopedics |
1495 (22.4) |
39 (9.3) |
|
|
|
|
ENT |
92 (1.4) |
2 (0.5) |
|
|
|
|
Plastics |
109 (1.6) |
9 (2.1) |
|
|
|
|
Thoracic |
195 (2.9) |
38 (9.0) |
|
|
|
|
Urology |
306 (4.6) |
20 (4.8) |
|
|
|
|
Vascular |
211 (3.2) |
7 (1.7) |
|
|
|
|
P-values <0.05 in bold Data presented as average ± standard deviation or n (%) ASA- American Society of Anesthesiologists Physical Status Classification System COPD- chronic obstructive pulmonary disease CHF – congestive heart failure ENT - otololyrngology
|
||||
During the perioperative period, patients receiving NS experience longer recovery times in PACU (N: 144.8 ± 96.2 min. vs. NS: 155.8 ± 94.0, p=0.023). These patients also had a shorter time from the last dose of NMB agent to first dose of reversal medication (N: 73.2 ± 42.1 min. vs. NS: 54.3 ± 64.8, p<0.001) and experienced higher rates of re-intubation (N: 6.3% vs. NS: 9.3%, p=0.015). No differences in first or last SpO2 were observed between groups. Overall, patients receiving NS experienced longer hospital stays than those receiving only N (N: 1.88 ± 3.32 days vs. NS: 2.7 ± 4.39, p<0.001) (Table 2).
Table 2. Perioperative Outcomes
|
Outcome Measure |
Neostigmine (N=6684) |
Neostigmine and Sugammadex (N=420) |
P-Value |
|
Minutes in OR |
138.1 ± 66.9 |
132.8 ± 80.3 |
0.190 |
|
Minutes in PACU |
144.8 ± 96.2 |
155.8 ± 94.0 |
0.023 |
|
First PACU SpO2 |
96.8 ± 3.5 |
96.5 ± 3.2 |
0.130 |
|
Last PACU SpO2 |
96.9 ± 2.3 |
96.7 ± 2.6 |
0.290 |
|
Minutes from Last NMB to First Reversal Agent |
73.2 ± 42.1 |
54.3 ± 64.8 |
<0.001 |
|
LOS Days |
1.88 ± 3.32 |
2.75 ± 4.39 |
<0.001 |
|
LOS Hours |
50.76 ± 79.00 |
71.72 ± 104.62 |
<0.001 |
|
Reintubation |
419 (6.3) |
39 (9.3) |
0.015 |
|
P-values <0.05 in bold |
|||
|
Data presented as average ± standard deviation or n (%) |
|||
|
OR – operating room |
|||
|
PACU – post anesthesia care unit |
|||
|
SpO2 – oxygen saturation |
|||
|
NMB – neuromuscular blockade |
|||
|
LOS – length of stay |
|||
TOF-C was documented prior to neostigmine administration in 2,146 (19.8%) cases and after neostigmine administration in 907 (8.4%) cases. Prior to neostigmine administration the average TOF-C was 2.9 ± 1.4, and the average TOF-C was 4.0 ± 0.3 after neostigmine administration. In patients receiving both neostigmine and sugammadex the average TOF-C after neostigmine administration was 3.7 ± 0.8.
In the final backward conditional multivariate logistic regression model, 14 independent variables were retained as predictors of patients requiring NS. All variables were independently significant predictors with the exception of neurosurgery (p=0.052) and ENT surgery (p=0.069). Females, non-white patients, and those with a BMI ≥ 35 kg/m2 were at increased risk for requiring NS. Of the comorbidities examined, respiratory disease, chronic kidney disease, hypertensive disease, and pulmonary hypertensive disease or pulmonary circulatory disease were all independent predictors of increased risk of receiving NS. The strongest relationship observed was between pulmonary hypertensive disease or pulmonary circulatory disease and risk for receiving NS (OR: 3.155, 95% CI: 1.645-6.048, p=0.001). For analysis of surgery types, general surgery was used as the basis of comparison for all groups. In comparison to patients undergoing general surgery, those undergoing breast surgery, obstetric & gynecologic surgery, orthopedic surgery, and vascular surgery were at significantly decreased risk of receiving NS, while those undergoing thoracic surgery were more likely to receive NS (OR: 2.329, 95% CI: 1.530-3.544, p<0.001) (Table 3). When applied to the data set, the model generated an area under the curve (AUC) of 0.681 (95% CI: 0.654-0.708), indicating moderate predictive value. At the optimal cutoff point, patients receiving NS were able to be identified with a sensitivity of 78% and specificity of 48% (Figure 1).
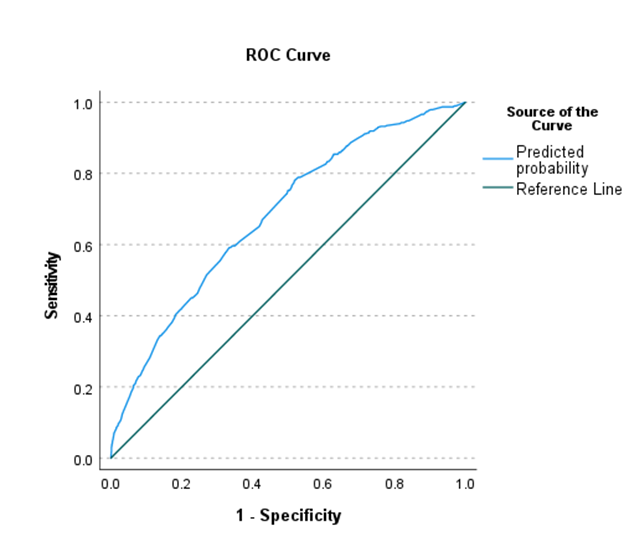
Area under the curve = 0.681 (95% CI: 0.654-0.708)
Figure 1. Model Performance in Predicting Neostigmine and Sugammadex
Table 3. Multiple Logistic Regression: Predictors of Patients Requiring Neostigmine and Sugammadex
|
Independent Variable |
Odds Ratio (OR) |
OR 95% CI |
P-Value |
|
Female |
1.277 |
1.012-1.611 |
0.039 |
|
Non-White Race |
1.309 |
1.036-1.654 |
0.024 |
|
BMI ≥ 35 |
1.582 |
1.259-1.988 |
<0.001 |
|
Other Respiratory Disease |
1.303 |
1.006-1.687 |
0.045 |
|
Chronic Kidney Disease |
1.622 |
1.142-2.302 |
0.007 |
|
Hypertensive Disease |
1.303 |
1.042-1.629 |
0.020 |
|
Pulmonary HD or Pulmonary Circulatory Disease |
3.155 |
1.645-6.048 |
0.001 |
|
Breast Surgery |
0.338 |
0.137-0.837 |
0.019 |
|
Obstetric/Gynecologic Surgery |
0.451 |
0.297-0.684 |
<0.001 |
|
Neurosurgery |
0.586 |
0.342-1.004 |
0.052 |
|
Orthopedic Surgery |
0.300 |
0.209-0.431 |
<0.001 |
|
ENT Surgery |
0.269 |
0.065-1.110 |
0.069 |
|
Thoracic Surgery |
2.329 |
1.530-3.544 |
<0.001 |
|
Vascular Surgery |
0.354 |
0.163-0.772 |
0.009 |
|
P-Values <0.05 in bold |
|||
|
HD – hypertensive disease |
|||
|
ENT - otolaryngology |
|||
In the present study, we observed significant differences in demographics, comorbidity burden, and types of surgeries performed in patients receiving neostigmine alone versus those requiring neostigmine and sugammadex for NMB reversal. Overall, after risk adjustment, patients receiving neostigmine and sugammadex were more likely to be female, of non-white race, have increased BMIs, and a greater comorbidity burden than those requiring neostigmine only. In comparison to patients undergoing general surgery, those undergoing thoracic surgery are at increased risk for requiring both reversal agents. These findings may assist anesthesiologists in identifying which patients are likely to require sugammadex for rescue NMB reversal after use of neostigmine and are therefore suitable candidates for using sugammadex as a first-line therapy.
The safety and efficacy of sugammadex have been well described by previous studies. In a 2017 meta-analysis, 4206 patients from 41 studies were reviewed.1 For moderate NMB, sugammadex 2mg/kg was compared to neostigmine 0.05mg/kg. Time from second twitch to TOF ratio >0.9 was found to be 6.6 times faster in patients receiving sugammadex. Examination of deep NMB reversal compared sugammadex 4 mg/kg to neostigmine 0.07 mg/kg. Sugammadex was found to be 16.8 times faster than neostigmine in in reversing NMB from post‐tetanic count (PTC) 1 to 5 to TOFR > 0.9. As secondary outcomes, any dose of sugammadex and neostigmine were compared to evaluate rates of adverse and serious adverse events. Significantly fewer composite adverse events in the sugammadex group compared with the neostigmine group. Risk of adverse events was 28% in the neostigmine group and 16% in the sugammadex group. Regarding specific adverse events, patients receiving sugammadex were at significantly less risk of bradycardia, postoperative nausea and vomiting (PONV) and overall signs of postoperative residual paralysis. Both sugammadex and neostigmine were associated with serious adverse events in less than 1% of patients, and data showed no differences in risk of serious adverse events between groups.1
A unique aspect of our study design is our specific examination of risk factors for patients requiring sugammadex as a second line NMB reversal agent after failed reversal with neostigmine. Other studies have examined risk factors for residual NMB that should be considered in addition to the factors presented in the current study when targeting appropriate candidates for receipt of sugammadex. Most commonly defined as a TOF ratio of <0.9 at admission to PACU,6 complications of residual NMB include skeletal and upper airway muscular weakness, which may result in partial or complete airway obstruction, concurrent hypoxemia, and respiratory failure requiring reintubation.7,8 Surgical risk factors that must be considered include emergent surgery, duration of surgery and type of procedure, while anesthetic considerations include the use of opioids, type and dose of NMB agent—and the resulting depth of blockade, and type of general anesthetic used. Specific factors under the control of the anesthesiologist include increased risk of residual NMB in patients receiving a minimum alveolar concentration of inhaled agents >1, hypothermia upon arrival to PACU, and an interval of less than 30 minutes from administration of an NMB agent to reversal.9 Previously described patient-specific risk factors for residual NMB include chronic obstructive pulmonary disease (COPD), diabetes, obesity, advanced age, and male sex.10-12 Despite the extensive literature investigating risk factors for residual NMB, few studies have attempted to develop predictive models for evaluating perioperative risk. One such study, performed a retrospective review of 2144 adult noncardiac surgical patients and assessed the accuracy of a model for predicting residual NMB against the true presence of a TOF ratio of <0.9 upon admission to PACU. The authors identified ten independent predictors of residual paralysis: hepatic failure, neurological disease, high-neostigmine dose, metastatic tumor, female sex, short time between neuromuscular blocking agent administration and extubation, aminosteroidal neuromuscular blocking agent, BMI more than 35, absence of nurse anesthetist and having an experienced surgeon. The model generated an AUC of 0.63 (95% CI of 0.60-0.66).13 While this study provides a valuable tool for assessing perioperative risk, its inclusion of NMB reversal agent as an independent predictor limits its applicability when attempting to pre or intraoperatively target appropriate patients for use of sugammadex as a first line reversal agent. We suggest the current study builds upon these results to address this limitation by including only preoperatively available information in the predictive model. The clinical utility of the current study’s results is further supported by a recent study by Gilbertson et al. examining patterns of rescue reversal before and after the introduction of sugammadex to a single institution in 2016.14 This retrospective review of 24,027 cases demonstrated that the rate of rescue reversal, defined as a second NMB reversal dose, increased from 6% of neostigmine cases prior to the introduction of sugammadex to 18% in 2018. In contrast, only 2.5% of cases using sugammadex for first-line reversal required rescue doses. Using predictive models such as the one presented in the current study hold promise for proactively identifying those patients that may derive the greatest benefit from the lower rates of residual NMB observed with first-line sugammadex use.
Given the clinical benefits of sugammadex over neostigmine for NMB reversal, its increased cost over neostigmine continues to be the primary factor limiting its routine use. In a recent analysis, Thilen et al. concluded that routine adoption of sugammadex for 30 million patients per year would add a drug acquisition cost of over 3 billion dollars to institutions, translating to actual cost to insurers and patients of at least twice this amount.15 The authors expand on this to highlight that sugammadex may be an expensive solution that is not addressing the widespread failure to use quantitative monitoring to assess residual paralysis—which was recommended for routine use in a 2018 consensus statement—resulting in potential overuse in patients with no need for pharmacologic reversal.16 Despite the high direct costs of sugammadex, other studies have found it to be cost-effective based on its ability to facilitate OR throughput and decrease complications related to incomplete NMB reversal. The economic value derived from reducing postoperative pulmonary complications (PPCs) using sugammadex was evaluated by Jiang et al. In a one-year decision analytic model of a hypothetical cohort of 100,000 patients, sugammadex was estimated to reduce PPC events by 12%, leading to a savings of $309 per procedure—a 10.9% reduction in total cost.17 In a 2020 study by Hurford et al, the authors combined data from a local hospital system and previously published results to evaluate the modeled cost of using sugammadex, neostigmine, or no reversal drug. They concluded that routine reversal with sugammadex is preferable to both alternatives when accounting for potential savings in OR time if institutions value this resource at over $8.60/minute. When OR time was not considered, routine use of sugammadex was not recommended solely on the basis of an increased ASA score of ≥ 3 or as a strategy to reduce PONV.4 The results of this study are refuted by those of Deyhim et al, who examined 640 cases at a single tertiary hospital and found no significant difference between cases using sugammadex and those using neostigmine/glycopyrrolate after adjusting for confounding factors. Based on the increased cost of $178.20 for acquisition of sugammadex, and no correlation with OR time savings or increased workflow capacity, restrictive use of sugammadex based on clinical relevance was recommended.18 If the assertion that routine use of sugammadex is cost effective based on potential OR time savings is to be accepted, further study is required to evaluate whether these time savings actually translate to additional surgical cases or performance of other productive clinical activities by staff, which has not been suitably evaluated to date.19,20 Within our cohort, using institutional costs a direct savings of $9987.60 ($20.50 per dose of neostigmine + $3.28 per dose of glycopyrrolate x 420 patients) can be estimated by eliminating the use of neostigmine/glycopyrrolate in the 420 patients that ultimately required sugammadex to achieve complete NMB reversal. Although a modest economic effect, the use of risk based algorithms such as the model presented in this work may enhance the true cost effectiveness of sugammadex by avoiding the cost of multiple medications rather than relying on potentially unrealizable gains in throughput.
This study does have multiple limitations. As a single center retrospective study, it is possible that our population is not representative of the broader population. Second, this study is subject to significant potential for selection bias, as the primary endpoint was the clinical decision of which NMB reversal agent or combination thereof to use. Similarly, although we suggest the use of rescue dosing of sugammadex as a proxy for residual NMB is warranted based on clinical experience, we cannot confirm the specific reason a second NMB reversal agent was used and whether appropriate indications for use were met in each case. Additionally, given the inconsistent documentation of TOF-C values we were unable to use this more objective measure of residual NMB as our primary endpoint. Third, we were unable to include a variety of potentially significant aspects of the anesthetic management into our predictive model. Important other aspects such as NMB agent dosing and the specific types of inhaled anesthetics and concomitant intraoperative medications received were not extractable from our medical record system. Opportunity to build upon and enhance the accuracy of our model by including these data exists. Fourth, due to the large sample size, we were unable to perform manual chart review to confirm the validity of the coded comorbidities, which has been previously described as variable.21,22 Finally, while our model generated an AUC of 0.681, in alignment with previously reported predictive models, this level of predictive accuracy is modest. However, we suggest that the relatively low risk of unnecessarily administering sugammadex in false positive cases warrants its use as a clinical decision support aid. NMB reversal agent selection must continue to be made based on the anesthesiologist’s clinical judgement on a patient specific basis.
Patients requiring sugammadex for rescue residual NMB reversal after receiving neostigmine were more likely to be female, of non-white race, have increased BMIs, and have increased rates of other respiratory disease, chronic kidney disease, hypertensive disease, and pulmonary hypertensive disease or pulmonary circulatory disease, and more likely to be undergoing thoracic surgery procedures. Based on these risk factors, a model for predicting which patients would receive both neostigmine and sugammadex was generated with an AUC of 0.681, sensitivity of 78% and specificity of 48%. These findings may assist anesthesiologists in identifying which patients are likely to require sugammadex for rescue NMB reversal after use of neostigmine and are therefore suitable candidates for using sugammadex as a first-line therapy.
Abbreviations
NMB – neuromuscular blockade
ICD-10 – International Classification of Disease 10th Edition
OR – operating room
PACU – post-anesthesia care unit
SpO2 – oxygen saturation
N – neostigmine
NS – neostigmine and sugammadex
ROC – receiver operating characteristics
ASA – American Society of Anesthesiologists
BMI – body mass index
AUC – area under the curve
TOF – train-of-four
PTC – post-tetanic count
PONV – postoperative nausea and vomiting
COPD – chronic obstructive pulmonary disease
PPC – postoperative pulmonary complication
Hristovska AM, Duch P, Allingstrup M, Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev. 2017(8). PMid:28806470
View Article PubMed/NCBISchaller SJ, Fink H. Sugammadex as a reversal agent for neuromuscular block: an evidence-based review. Core Evid. 2013;8:57-67. PMid:24098155
View Article PubMed/NCBICarron M, Baratto F, Zarantonello F, Ori C. Sugammadex for reversal of neuromuscular blockade: a retrospective analysis of clinical outcomes and cost-effectiveness in a single center. Clinicoecon Outcomes Res. 2016;8:43-52. PMid:26937203
View Article PubMed/NCBIHurford WE, Welge JA, Eckman MH. Sugammadex versus neostigmine for routine reversal of rocuronium block in adult patients: A cost analysis. J Clin Anesth. 2020;67:110027. PMid:32980763
View Article PubMed/NCBIde Menezes CC, Peceguini LA, Silva ED, Simoes CM. Use of sugammadex after neostigmine incomplete reversal of rocuronium-induced neuromuscular blockade. Rev Bras Anestesiol. 2012;62(4):543-547. 70153-8
View ArticleRaval AD, Uyei J, Karabis A, Bash LD, Brull SJ. Incidence of residual neuromuscular blockade and use of neuromuscular blocking agents with or without antagonists: A systematic review and meta-analysis of randomized controlled trials. J Clin Anesth. 2020;64:109818. PMid:32304958
View Article PubMed/NCBIAbrishami A, Ho J, Wong J, Yin L, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database Syst Rev. 2009(4):Cd007362. PMid:19821409
View Article PubMed/NCBIBrull SJ, Kopman AF. Current Status of Neuromuscular Reversal and Monitoring: Challenges and Opportunities. Anesthesiology. 2017;126(1):173-190. PMid:27820709
View Article PubMed/NCBIKhamtuikrua C SS, Rhoopanwong S, Sangsab P, Chaikittisilpa N, Bormann B. . Risk Factors for Residual Neuromuscular Blockade after General Anesthesia. J Med Assoc Thai. 2017;100(75).
Cammu G. Residual Neuromuscular Blockade and Postoperative Pulmonary Complications: What Does the Recent Evidence Demonstrate? Current anesthesiology reports. 2020:1-6. PMid:32421054
View Article PubMed/NCBIPedersen T, Viby-Mogensen J, Ringsted C. Anaesthetic practice and postoperative pulmonary complications. Acta Anaesthesiol Scand. 1992;36(8):812-818. PMid:1466220
View Article PubMed/NCBIKarcz M, Papadakos PJ. Respiratory complications in the postanesthesia care unit: A review of pathophysiological mechanisms. Canadian journal of respiratory therapy : CJRT = Revue canadienne de la therapie respiratoire : RCTR. 2013;49(4):21-29.
Rudolph MI, Ng PY, Deng H, et al. Comparison of a novel clinical score to estimate the risk of REsidual neuromuscular block Prediction Score and the last train-of-four count documented in the electronic anaesthesia record: A retrospective cohort study of electronic data on file. Eur J Anaesthesiol. 2018;35(11):883-892. PMid:30020144
View Article PubMed/NCBIGilbertson LE, Wolf F, Lynde GC. Patterns of rescue following reversal with neostigmine and sugammadex: A longitudinal observational study. Perioperative Care and Operating Room Management. 2021;24:100187.
View ArticleThilen SR, Weigel WA. Sugammadex: A Costly Simple Solution That Is Not Really Solving the Problem. Anesth Analg. 2020;131(2):e73-e74. PMid:32243289
View Article PubMed/NCBINaguib M, Brull SJ, Kopman AF, et al. Consensus Statement on Perioperative Use of Neuromuscular Monitoring. Anesth Analg. 2018;127(1):71-80. PMid:29200077
View Article PubMed/NCBIJiang Y, Bash LD, Saager L. A Clinical and Budgetary Impact Analysis of Introducing Sugammadex for Routine Reversal of Neuromuscular Blockade in a Hypothetical Cohort in the US. Adv Ther. 2021;38(5):2689-2708. PMid:33871823
View Article PubMed/NCBIDeyhim N, Beck A, Balk J, Liebl MG. Impact of Sugammadex Versus Neostigmine/Glycopyrrolate on Perioperative Efficiency. Clinicoecon Outcomes Res. 2020;12:69-79. PMid:32099426
View Article PubMed/NCBICammu G. Sugammadex: Appropriate Use in the Context of Budgetary Constraints. Current anesthesiology reports. 2018;8(2):178-185. PMid:29904285
View Article PubMed/NCBIPaton F, Paulden M, Chambers D, et al. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: a systematic review and economic evaluation. Br J Anaesth. 2010;105(5):558-567. PMid:20935005
View Article PubMed/NCBIO'Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40(5 Pt 2):1620-1639. PMid:16178999
View Article PubMed/NCBIStoresund A, Haugen AS, Hjortås M, et al. Accuracy of surgical complication rate estimation using ICD-10 codes. Br J Surg. 2019;106(3):236-244. PMid:30229870
View Article PubMed/NCBI