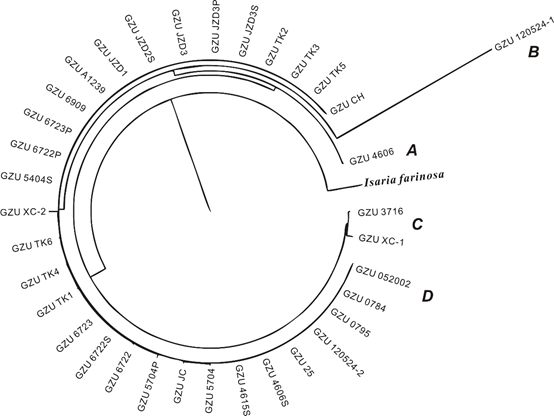
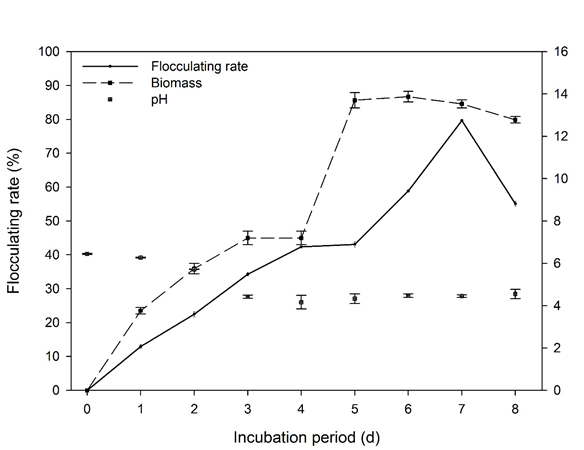
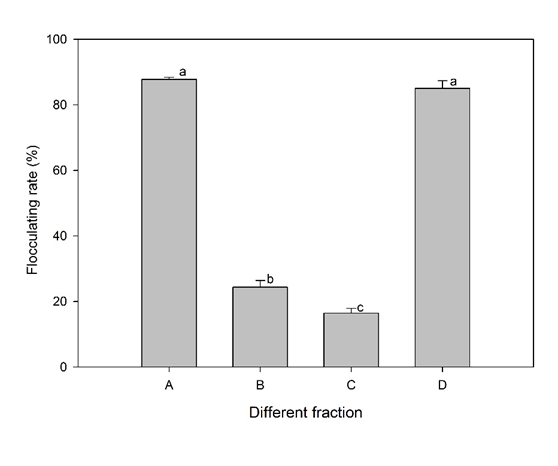
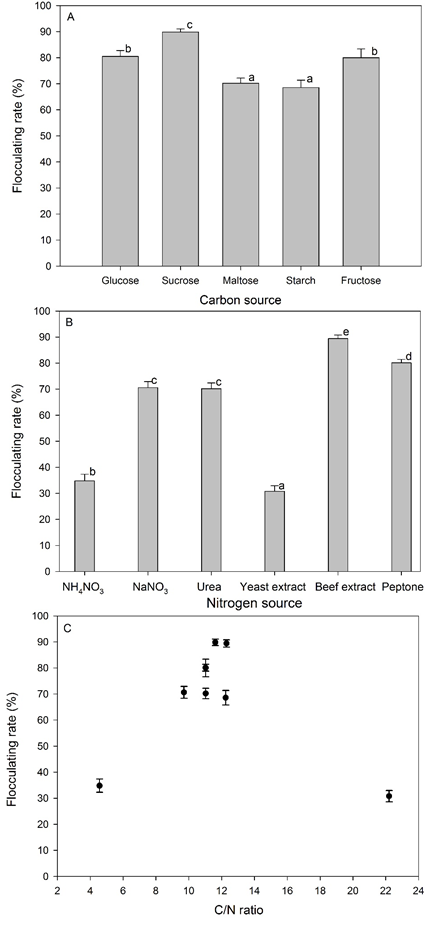
Yanlong Jia 2, Tangfu Xiao 3, Fanli Meng 4, Maosheng Wang 5, Zengping Ning 6
1 Institute of Fungal Resources, Guizhou University, Guiyang 550081, China
2 School of Resources and Environmental Engineering, Guizhou Institute of Technology, Guiyang 550001, China
3 Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education; School of Environmental Science and Engineering, Guangzhou University, Guangzhou 510006, China
4 Guizhou Institute of Environmental Science and Design, Guiyang 550081, China
5 School of Food and Pharmaceutical Engineering, Guizhou Institute of Technology, Guiyang 550001, China
6 State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang 550081, China