Qunshan Wei
Tel.: +86 02167792557; Fax: +86 02167792557;
E-mail: qswei@dhu.edu.cn
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 5 ISSUE: 4
Page No: 110-120
Qunshan Wei
Tel.: +86 02167792557; Fax: +86 02167792557;
E-mail: qswei@dhu.edu.cn
Bilal a, Qunshan Wei *a, b, Muhammad Noman a, Zhemin Shen c, Kokab Saba Ali d, Sajid Ullah e, Faisal khan f, Kashif Ali Panhwar a, Hamidova Emiliya g, Rabia Tasleem h, Javed Ahmad h, Izhar Ul Haq h, Muhammad Subhanullah h, Zakir Ullah i
a College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
b State Environmental Protection Engineering Center for Pollution Treatment and Control in Textile Industry, Donghua University, Shanghai 201620, China
c School of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
d Department of Geospatial Sciences (GIS/RS), National Centre of Excellence in Geology (NCEG), University of Peshawar, Peshawar, Khyber Pakhtunkhwa 25120, Pakistan
e School of Environmental Science and Engineering, East China University of Science and Technology, Shanghai 200237, China
f Department of Environmental Sciences, University of Swabi, Swabi, Khyber Pakhtunkhwa 23430, Pakistan
g Landscape-Azerbaijan State Pedagogical University, Baku, Azerbaijan AZ1000
h Department of Environmental Sciences, Abdul Wali Khan University Mardan, Mardan, Khyber Pakhtunkhwa 23200, Pakistan
i Faculty of Water Resource and Hydroelectric Engineering (Program of Environmental Science and Engineering), Xi’an University of Technology, Xi’an, Shannxi Province 710048, China
Bilal, Qunshan Wei, Muhammad Noman, Zhemin Shen, Kokab Saba Ali, Sajid Ullah, Faisal khan, Kashif Ali Panhwar, Hamidova Emiliya, Rabia Tasleem, Javed Ahmad, Izhar Ul Haq, Muhammad Subhanullah, Zakir Ullah.(2020) Phytoremediation of contaminated soil Lead and Cadmium by Brassica júncea (L.) Czern plant. Journal of Earth Sciences & Environmental Studies 5(4) :110-120
Many remediating strategies are used for polluted soils, however, but mostly the essential phytoremediation is a less expensive, organically satisfying technique that is generally reasonable for various countries. Pot tests were managed to dissect the Brassica júncea plant biomass cultivated on Pb as well as Cd polluted soils as well to survey its ampleness for the evacuation of Pb and Cd. Samples of picked plants developed at a blend of alluvial soil and sand were moved with vessel of pots the earth finishing extents as well allowed make with time regenerative development. Through acid digestion, Pb and Cd extraction was settled from the plant.
Consequently, they were collected and afterwards examined for chosen metals through utilizing Atomic Absorption Spectrometry (AAS). Generally, the current examination results demonstrated that no hyperaccumulators of Pb as well Cd were recognized in the region. Body parts of the plant were categorized as Pb low accumulators, moderate accumulators and excluder, as well as Cd low accumulator, excluder. Additionally, Cd concentration was high up than the allowable range in species of plant. In plants, allowable range of Pb and Cd is 0.2 - 20 and 0.1 -2.4 mg kg – 1. In Brassica júncea plant the Pb as well Cd both were no hyperaccumulators. Hence, this local plant had the suitable ability to use for phytoremediation of contaminated soils around the Hayatabad Industrial area, Peshawar. All experimental Results demonstrated that from the medium of soil by Brassica júncea (L.) Czern plant the maximum lead and cadmium removals were 94 % and 94.26 %, respectively in the open environment, while in the control environment this removal was 82 % for Pb and 93.16 % for Cd .The present research work observes that brassica júncea (L.) Czern plant was more helpful for Cd take-up contrasted with Pb, and thus it is capacity we suggest Pb as well Cd for remediation from polluted soils.
Keywords: Lead, Cadmium, Contaminated soil, Removal
Earth contamination through heavy metals was extended profoundly toward the start of the twentieth century, in light of current furious and nonsensical population advancement, introducing significant common and human clinical issues far and wide [1]. Few debase origins polluted colossal zones everywhere throughout the globe, i.e., spreads through residues from mining, smelting industry, emissions from waste incinerators, car exhaust, military activities and from the agriculture amendments of use (mineral fertilizer, pesticides, sludge and fertilizer of urban) [2,3]. Defilement is an offshoot witnessed in this evolutionary world. It suggests air contamination, water bodies and soil in light of mixing of undesired substances coming about as various result tasks in mechanical division, basic techniques, impetuses, urbanization and waste treatment plants [4, 5]. Effluents from various anthropocentric sources and enterprises are showing to significant impacts on the new water bodies, for instance, the groundwater, saline waters, streams, and lakes [6]. They lead to difficult nature the biological system and good antagonistic effects on the neighborhood population. Majority parts of the wastes of industries are untreated and dumped into the environment without hazardous treatments. They lead to unbalanced nature in the organic framework and hostile prosperity effects on the local population. The majority part of the losses of enterprises is untreated and dumped into the earth without unsafe medications [7]. Interestingly natural pollutants of metals are not biodegradable, as well as fundamentally animal stresses to earth and to living through their movement development of malignant, which causing mutagenic blends [8]. Concentration through inflicting increased toxic effects on plants and human health, i.e. Causes skin lesions, damage the respiratory system, renal system, cancer, chromosomal aberrations, damage of DNA [9, 10, 11]. Availability of metals of high concentration quantity in soils affected agriculture productivity also, the development of different species of plant [12]. Herbs are diverse inside protection from substantial metals, in many cases, a huge segment through the developing plants are not hyper gathered for substantial metals because of internally impacts on plant bedchamber [13, 14]. Among particular, Heavy metals, for instance, (Cr, Cd, Cu, Hg, Pb, As, Ni, Al, Mn) [15, 16], moreover, Zn demonstrating the most generally perceived heavy met-al contaminant [17]. However, the metals mentioned above cannot make conveniently corrupt with toxicant goods, for instance, CO2, as well as clean-up ordinarily depend upon ejection [8]. A couple of invigorating developments arise open for handling overwhelming met-ales sullied loams. Normally, the invigorating developments as it may be categorized acquainted noteworthy ternary sorts specifically, bodily, i.e., loam renewal, loam repression as well consolidate progress [18, 19], substance, condensation, alteration/fossilization and loam cleaning developments [1, 20, 21], moreover, natural., bio sorbent as well met-al take-up at microbes [22]. Most of coming from invigorating progressions are exorbitant, require genuine exertion as well it may be making tremendous pollutants in general condition [23, 24], then again may be possible to provoke horrible effects on natural pursuits, loam composition as well vanity issues [25]. Along these lines, nearby exist a necessity about a progressively reasonable as well common all-around displayed clean-up method. Currently, the association between plants, creatures, and metals was polluted in much thought due to physiological ability to remove metals through microorganisms direct from debased soil as well possible occupation through microorganisms in propelling herbs-development in polluted soils [26]. The technology of Bioremediation is an advancement wherein hazardous pollutions are corrupted under controlled conditions to an innocuous state or to underneath obsession level acceptable reach or killed normally which set up by authorities [27]. From water and soils, for metals removal, phytoextraction is used as an effective technique. For contaminated soils cleaning, different species of plant use is known as phytoremediation. For phytoremediation purpose, the capability of many plant species to accumulate metals has been studied [28]. Phytoremediation is an economically and promising effective tool that uses different species of plant for to decontaminate terrestrial sites and contaminated aquatic sites through heavy metals [29, 30]. However, this procedure exists a fundamental, cost effective as well as biologically inviting with immaterial, natural interference. Phyto-remediation advancements are incorporating Phyto-extraction, Phyto-adjustment and Phyto-volatilization courses for particle removal of metals from defiled loam through the parenchyma of herbs [31], along these lines, it alludes to be adequately and safely handled with as well reused, for aching, treating the soil or arid [32]. Metals fall from immaterial segments through the growth of herbs, as well in loam might start gash due to internally high obsessions, as same like cause passing of herbs at an incredibly significant quantity of metals fixation metals [33]. Tainting soil through substantial metals has enhanced an essential regular go into on the quantity of its internally negative environmental impacts.
Contaminants of soil due to its availability of widespread are considered toxic metals and their chronic as well acute effect of such soils at the development of plants species [34]. Plants are various in heavy metals gathering capability [35, 36]. However different species of plants for this purpose chooses for heavy metals of phytoextraction depends on biomass of the selected plant as well ability of tolerant capacity [37]. Huge renewable of plant species, massive particles detached from the evaluated loam. Species of some herbs can accumulate heavy metals in tissues as well less biomass production as well plants are slow-development, which can make it unworkable for these species of plants in phytoremediation, i.e. Selvamand Wong (2008) production biomass of B. napus decline which is developed at Cd polluted soil. So, production of biomass of chooses species of the herb for hyperaccumulator is significant for factor controlling phytoremediation technique. A phytoremediation is a suitable tool for various retrieve treatment due to non-interfere with the ecosystem. It needs human resources lightly, so therefore it is not expensive compared with traditional physicochemical techniques. It has obtained increasing attraction as clean, effective and cheap technology. Phytoremediation has the benefit of being inexpensive as well as not destructive or disturbing the structure of soils physically, chemically and biologically [38]. There are many types of phytoremediation technology which are including, Phyto stabilization, phytovolatilization, Phyto filtration, phytodegradation and phytoextraction [39, 40].
In Pakistan open dumping of stores, mining wastes, mechanical sewages, pesticides and rustic wastes are un-treadled flown into the streams and like this into the water supplies and soil. Heavy metals grouping is predicted in plants species and soil of the examination districts and causing significant issues for the local system similarly herbs as well animals of chooses research work areas. So, the research work is planned to inquire about convergence through substantial metals poisonous, inside the loam and herbs to best suggest phytoremediation frameworks for hereafter probability.
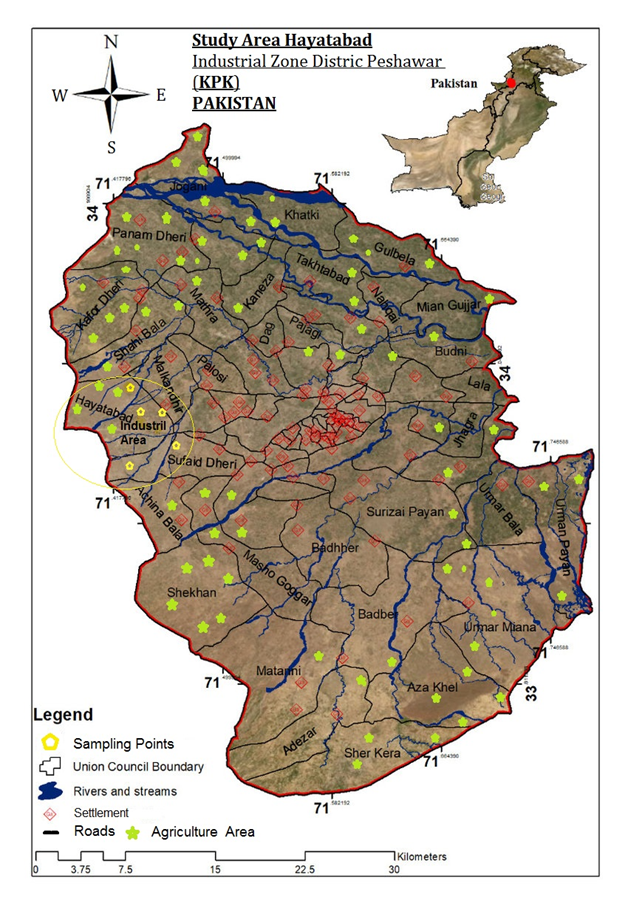
Figure 1. Study Area Map
2.1 Study area description
District Peshawar is a study area, which Khyber Pakhtunkhwa capital. Peshawar in the sixteenth century was set up during the time of Mughal by Akbar. The interactions were different cultures and the main business center between Central Asia, South Asia, and Middle Asia. It is one of the most ancient, political, cultural, commercial and economic capital of Pakistan. In Peshawar, relatively cold winters and climate is a desert with more warm summers. In Peshawar, winter month season starts in November as well ends in March, while winter season sometimes increases into through the month of mid-April, while summer start from May- mid to mid of September. In the month of hottest, the minimum temperature is 25 °C, and maximum temperature of summer surpasses 40 °C. While the maximum temperature is 18.3 °C and during the coldest month, the minimum temperature is 4 °C. The annual average rainfall of district Peshawar is 15 inches (38cm). Mostly in Peshawar rain falls from July to September month during the summer monsoon. As well Peshawar also a moderate winter rainy season experience. Peshawar weather is delightful and moderate. Its humidity is 79%, and the average temperature is 82 F. For tourists and travelling weather is nice and pleasant. City Peshawar is enclosed with sediments of gravel, clay, sands and silt of current times of geographical. The areas between Nala of Budni and River Kabul are the plains/zones of the flood. On Northwest through Southwest at the upper northern portion of city flood plain wander openly. At the city of Northwest River Kabul enters, at Peshawar city plain, Kabul river is categorized in many waterways. The main two waterways are the River of Adizai Eastward fallings with a boundary through city Charssadda, while the 2nd branching waterway from the right site is the shah Alam of Naguman area river that is interring further in the East with River Nauman. In the country, Peshawar is the best agriculture zone known. For the cultivation of tobacco and sugarcane, its soil is very suitable and fertile. Wheat, maize, tobacco, sugarcane, mustard and rapeseed etc., are significant crops which grow in the city. While vegetables and fruits are also grown. Orange, pear, rare mango, apricot, plum, peach and apple are the important fruits [41].
2.2 Sampling of soil
Samples of soil were collected randomly (n=5),1.5 kg up to (20-25 cm) depth at the bottom from the plants at study and surrounding areas. All the samples were taken in plastic bags, and for identification, all the samples in the field area were explicitly marked. Then transported to the laboratory.
2.3 Preparation of Stock solutions
Lead and Cadmium standard solution of high analytical grade through high purity solutions of the standard have utilized the origin of stock solutions of Cadmium and Lead. The required mixture was made with analytical reagent standard double distilled water. Through every survey, metal was utilized to polluted these soils with Cd as well Pb at various concentration of (0), (10), (20), (40), (80), (100), as well 200mkg-1 a single stock standard solution.
2.4 Determination and preparation of metals in samples of soil
At room temperature, all soil samples in the laboratory were air-dried. By hand, first, all pebbles and organic materials were removed. Then sieved through a mesh <2mm and for analysis further stored in kraft paper. By using of Fritsch Analysette 3 PRO sieve machine (Germany), soil texture was measured. First, a sample of soil was weighed 30g and then passed through the following tracery of (0.02, 0.05), as well <0.02 mm size. According to [42], in each mesh, the remaining soil was weighted and in percentage particle size for (sand), (silt), as well (clay). Sample of soil in indigestion tube o.5 g was taken for massive metal extraction and then to the sample 15 ml mixture of HNO3, H2SO4 as well (HCLO4) was added and then mask with cork glass that tube of digestion. Then leave were overnight all the mixtures. After 24 hours then the digestion tube was heated 80 ℃ carefully and the temperature increased gradually up to 160 ℃ till the yellowish clouds of NO2 have finished. Then perchloric acid of three ml (HCLO4) was added after cooling of the solution. Again, samples were heated until a mixture of a little amount remained. After digestion completion, all the samples were cooled and filtered. 50 ml of volume finally was made with deionized water (DDW). By using (AAS, Perkin-Elmer, A 700), the concentration of metals was determined in the Central Research Laboratory (CRL) Peshawar University, Peshawar, Khyber Pakhtunkhwa, Pakistan. The heavy metal slandered reference recovery rates were satisfactory. In triplicates, every sample was analyzed, and for additional interpret-ion mean value was taken.
2.5 Pot experiments
In plastic pots which have sand fertile soil mixture the seeds of Brassica júncea (L.) Czern were grown and then spraying through deionized water (DDW) for two weeks. Seeds of two weeks then were kept through various metals concentration, as well only one left under control. Every container was kept for 70℃ holding capability of water to decide the pots per week for two times. Two weeks after the seeds were lighted to two herbs per pot and then was kept it for 56 days. Then Hoagland's nutrient and tap water solution were added, which as needed. Then replicates three times each treatment, and in the greenhouse, pots were arranged randomly.
2.5.1 Plant analysis, preparation and harvesting
From all the container pots species of plants were removed gently after eight weeks of plant cultivation.
The weight and length were determined, while shoots and roots were separated.
Roots as well shot of plant species at (70 ℃) dried, rinsed and were cleaned with dirty water (DDW); also, the dry matter was measured. Using (AAS) the denseness of Pb as well Cd both were determined at the digest tube with help through HCL/HNO3 3:1, v/v w through a digested mixture with (0.5g) portion of sieved plant matter and plant materials were ground firstly.
2.5.2 Translocations and accumulations of Heavy Metals
Broadly was used of the factor of translocation (TF.), a factor of bioaccumulation, (BAF) to evaluate in development tissues of plants species, the translocation factor, (TF.) through metals. In this research soil to roots, Pb and Cd Bioaccumulation factor (BF) by multiplying in the tissue of plant species roots were measured contented of all determining metal through the chosen metal in the loam of total contents. Accordant with [31, 43, 44], the factor of translocation (TF) was calculated from soil to plant. According to [45], shoot root Translocation Factor, (TF) was observed which defines that the ratio between metal accumulation in plant roots as well accumulation in plant shoots.
2.6 Statistical analysis
By using Excel 2010 (Microsoft Office), all tables and figures were plotted. For location map, Arc Geographic Information System (Arc-GIS) was used.
Recently, industrial activities in developing countries caused environmental damage. However, the condition has increased the problem of disposal of wastes, in cities, untreated of hazardous wastes coming from various factories which are mostly discharged in inland water which causing become oily nature, greasy, discoloration, as well stench of different bodies of water [46]. In the developing countries, one of the significant reasons for environmental degradation is the industrial wastewater effluents, because of the lack of wastewater treatment facilities [47]. The estimate shows land (20 million hectares) at about 50 countries, partially treated wastewater is irrigated with or raw [48]. Moreover, wastewater uses for agriculture purpose without any treatment are deteriorating groundwater quality and affecting human health [49].
Wastewater has been enormously influenced by anthropogenic impacts in quality. It consists of liquid waste discharged by domestic properties, commercial residences, agriculture and industry and can include an extensive range of contaminants [50]. The continuous release of heavy metals into water bodies can cause toxicity and several diseases in humans/animals; such as high blood pressure, kidney failure, renal disorders, ulcer, cancer, metabolic acidosis and harm or damage stomach of the rodents [51]. There are some trace and heavy metals that are essential for water. In wastewater purification method the essential metals are Cu, Cd, Cr, Pb, Mn, Na, Zn, Mo, Ca, K, As, Co, Fe, Mg, Hg, Ni, Se and V. Some of these metals (Ca, Na, Mg, Fe, Cr, K, Cu, Ni, Co, Zn and Mn) these all are significant for the development of living organisms with varying quantity (micro or macro). Non-essential metals are such as Cr, Pb, Fe, Cu, Fe, Mn, Hg, Se, Zn, As, have no biological role [52]. Impair the quality of food and water and toxic by heavy metals; therefore, among environmental contaminants, they are globally concern [53].
3.1 Soil contamination due to industrial wastewater effluents
Mostly in developing countries, industrial and municipal wastewater use commonly for agriculture purpose [54]. Use of inadequately/untreated wastewater is practiced for agriculture irrigation purpose in developing countries. Urban areas, due to the unavailability of suitable water, the farmer uses wastewater for growing fodder and vegetables. Wastewater is loaded with heavy metals, diseases causing microorganisms and salts. Soil degradation result from the continuous use of wastewater for growing vegetables. A toxic of human wellbeing result from the inhale of metals in excessive quantity. Wastewater of all types can be treated to use for agriculture irrigation [55]. The study carried out in the industrial area of India by [56] shows that industrial wastewater having variable pH can increase the number of metals in groundwater from rocks, sediment and soil by leaching toxic heavy particles.
Many species of herbs presented to heavy metals at massive results focuses on extreme harm with different metabolism exercises driving; therefore, with passing through herbs species. The introduction of metals to species of herbs subdues physiologically and special useful enzymes [57], photosystems disband [58], also, damage of mineral processing [59]. Referenced over, the picked plant's biomass through remediation progress is crucial to sure an enormous dispatch pace of malicious metals. Yield (Brassica júncea (L.) Czern plant) was picked to their high biomass in this study, fast progress concentration similarly, their capability different metals through polluted soils [60]. At Pakistan, there is no proper treatment facility available for the treatment of industrial and city wastes. Therefore, the effluents cause' surface and groundwater pollution and lowering the production of agriculture. In this study, we used Brassica júncea plant for removal of selected metals such as Pb and Cd from the soil with heavy metals. Collected soil samples show a higher degree concentration of contamination with Pb and Cd. All experiments of the study showed that the Brassica júncea plant is useful for removal of selected metals from study area soil. Explanation of these metals is given in the oncoming section.
3.2 Metals concentrations in the soil
The heavy metals take-up was analyzed in different parts of Brassica júncea (L.) Czern. Accumulation of metals in various parts was starting late studied by different researchers as [61, 62, 63]. The reason behind the presence of heavy metals in the region were household effluents, different industries, construction of work and hotels. Localize people are confronting different clinical issues while getting orally from them concerning the quality of water. Disease of kidneys, stomach related issues and cancer are prevalent in the region. Various heavy metals accumulating plants, mainly the plantation of Alnus nitida will help with reducing the environmental pollution.
Table 1. In soil selected metals concentrations
|
|
Control Environment |
Open Environment |
||
|
Samples |
Pb (mg/Kg) |
Cd (mg/Kg) |
Pb (mg/Kg) |
Cd (mg/Kg) |
|
Soil |
10 |
10.5 |
10 |
10.5 |
|
Root |
2.1 |
2.6 |
1.9 |
2.3 |
|
Stem |
1.6 |
1.8 |
1.3 |
1.9 |
|
Leaves |
1.1 |
0.4 |
0.7 |
1.4 |
The Pb concentrations found in the soil from 1.1 mg/kg to 2.1mg/kg, however while the concentration of Cd found 0.4 mg/kg to 2.6 mg/kg under (control environment). While under the open environment, Pb concentrations found from 0.7mg/kg to 1.9 mg/kg in the soil while the concentration of Cd found from 1.4 mg/kg to 2.3 mg/kg. Detail of these all metals is given Table 1. As clear from the Table, the soil is contaminated with these metals, so their removal from the soil is necessary to use the soil for agriculture or other stated purposes. For this reason, for the removal of these metals from the soil, we select the Brassica júncea plant.
3.3 Removal of heavy metals (Pb & Cd) from contaminated soil
3.3.1 (Pb) removal from contaminated soil
Brassica júncea was grown in the same soil. The plant was uptakes heavy metals and accumulate in its different parts such as root, stem and leaves. Therefore, different parts of the selected pants were analyzed for these metals. After assessment of tests and collection, it was seen that Pb and Cd are accessible in all of them in any case, its aggregate varies in plant parts and besides. The detail of the Cd is shown in Table 2. Pb is available in all of them regardless of its all-out changes in plant parts. Pb highest value (3.3 mg/kg) was observed in the plant root (under control environment) while the lowest value of Pb (0.7 mg/kg) is in plant leaves under open environment. Regular appraisals of Pb contents in different models uncovered that the plant showed beneficial take-up of Pb and moved it in various plant tissues. The order of Pb accumulation in plant body parts was in order followed, Root>shoot>leaves>seeds. All outcomes by then were differentiated, and the previous works the metal storing up in various parts were starting late focused by various investigation scientists as [61, 62, 63]. The [64] examined accumulations of metals in plant roots of the Alnus nepalensis. The watched decline in dry issue creation because of metal weight is in concurrence with that revealed prior in plants other than Brassica júncea plant [65, 66].
Table 2. Pb and Cd concentration in various body parts of plants
|
|
Control Environment |
Open Environment |
||
|
Samples |
Pb (mg/Kg) |
Cd (mg/Kg) |
Pb (mg/Kg) |
Cd (mg/Kg) |
|
Soil |
10 |
10.5 |
10 |
10.5 |
|
Root |
3.3 |
3.8 |
3.1 |
3.5 |
|
Stem |
2.8 |
3.6 |
2.5 |
3.1 |
|
Leaves |
2.4 |
1.6 |
1.9 |
2.6 |
|
Seeds |
0.9 |
0.6 |
0.7 |
0.9 |
3.3.2 (Cd) removal from contaminated soil
Cd highest value (3.8 mg/Kg) was observed in the plant root under control environment, while the lowest value of Cd (0.4 mg/kg) is in the plant leaves. Standard evaluations of Cd contents in different models uncovered that the plant appeared to be productive take-up of Cd and move it in various tissues of the plant. Accumulation of Cd in plant parts were ordered in followed Root>Shoot>leaves>seeds. All results were also compared with the other research work.
Preparing and improvement of sunflower were influenced capably by the pressure metals, for Pb and Cd. The concentration of Pb and traditionalist circle acknowledged forestalled headway, lessened biomass creation and passed on apparent brand impacts like those depicted through other laboratories at various species of plant [67, 68]. We conduct study both under control and natural conditions. The experimental results showed that under control conditions, there was the highest adsorption as compared to natural conditions. This may be due to the variability in environmental conditions. The comparisons of both these metals under both conditions are detailed in Table 2.
The percentage absorption were found to be :root 33 for Pb and 36. 20 % for Cd, Stem 24 for Pb and 34. 28 % for Cd, leaves 24 for Pb and 15. 22 % for Cd, and seeds 9 for Pb and 8. 56 % for Cd, in the control environment while in the open environment the percentage absorption were found to be :root 31 for Pb and 33. 32 % for Cd, stem 25 for Pb and 29. 52 % for Cd, leaves 19 % for Pb and 24. 76 % for Cd and seeds 7 for Pb and 5. 56 % for Cd ( Fig.2).
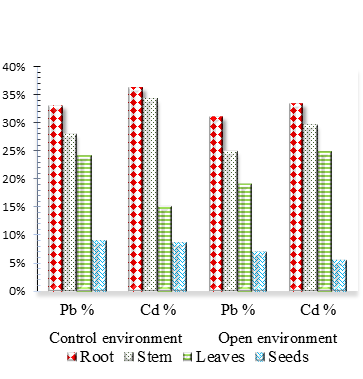
Figure 2. Absorbed (Pb) and (Cd) percent composition of Brassica juncea by parts from soil
3.4 Accumulation, Translocation, Bioaccumulation factors of Pb and Cd in plants tissues
The plant was observed and thus suggest that Brassica júncea (L.) Czern could prove useful for Phyto- stabilization for Pb as well for Cd as revealed by the values (<1) of bioconcentration factor (BCF) in plant shoots, in plant roots as well also translocation factor (TF.) Details are given in Table 3. The plant various body parts bioconcentration factor, (BCF) as well Biological Absorption Coefficient, (BAC) both were under <1 of Pb.
Table 3. BAC, BCF and TF values of Brassica júncea (L.) Czern
|
|
|
Control Environment |
|
Open Environment |
|
|
Pb (mg/Kg) |
Cd (mg/Kg) |
Pb (mg/Kg) |
Cd (mg/Kg) |
|
BAC |
0.28 |
0.36 |
0.25 |
0.31 |
|
BCF |
0.33 |
0.38 |
0.31 |
0.35 |
|
TF |
0.84 |
0.94 |
0.80 |
0.88 |
However, this shows in soil that Lead concentration was more than various body parts of the plant. From present analysis and work, it is revealed that plant species showed suitable for Phyto stabilization of metals. An explanation about availability expanded of metals in the soil test when compared with others [64, 69]. High values of TF of plants species, were viewed as fitting for the phytoremediation that all-around needs movements of metals of inadequately harvestable plant various body organs, i.e. Shoots [70], The plant was considered to be aggregator as divulging by the attributes (<1) of BCF in plant Stem, BCF in the plant of the roots, too in like manner in the TF. Similarly, comparable results show that BCF and the BAC of all plant species were under < 1 focus in the dirt for Cd and Lead was more than tissues of the plant species. [71] examined that plants, which had low metal bioaccumulation in their primary roots and high TF, could recognize essential for the removal of metals from phytoextraction.
This research work expulsion was represented to recognize the capacity of Brassica júncea plant for Pb as well as Cd removal from polluted soil of heavy metals. All results which were obtained indicated that Brassica júncea (L.) Czern has the capability in its tissues (roots as well shoots) to accumulate Pb as well Cd, in plant roots, Cd accumulation was better suitable than Pb. The BAF value of Pb as well Cd for all treatments were less than 1. Generally, all results indicated that for Pb and Cd, no hyperaccumulators were recognized in that place. Plant body parts were categorized as excluder or low Pb accumulator and moderate accumulator. Concentrations of Cd in the plant was higher than its standard range, in plants, the Cd average value was 0.1 -2.4 mg kg – 1. This research work concludes that Brassica júncea (L.) plant species was very well suitable for uptake of Cd than contrast with Pb, so thus we recommend their capability for phytoremediation of Lead as well as Cadmium polluted soils.
This work was supported in part by China National Critical Project for Science and Technology on Water Pollution Prevention and Control (No. 2017ZX07202005-005), the National Key Research and Development Program of China (No. 2019YFC0408304) and the National Natural Science Foundation of China (NSFC) (No. 21876025).
Abdelhafez, A.A., Li, J., 2014. Geochemical and statistical evaluation of heavy metal status in the region around Jinxi River, China. Soil Sediment. Contam. 23, 850–868.
View ArticleAbdelhafez, A.A., Abbas, H.H., Abd-El-Aal, R.S., Kandil, N.F., Li, J., Mahmoud, W., 2012. Environmental and health impacts of successive mineral fertilisation in Egypt. Clean 40, 356–363.
View ArticleAbou-Shanab, R.A.I., 2011. Bioremediation: new approaches and trends. In: Khan, M.S. (Ed.), Bio management of Metal-Contaminated Soils, Environmental Pollution. Springer Publications, NY, USA, pp. 65–94.
View ArticleVillaseñor-Basulto, D. L., Astudillo-Sánchez, P. D., del Real-Olvera, J., & Bandala, E. R. (2018). Wastewater treatment using Moringa oleifera Lam seeds: a review. Journal of Water Process Engineering, 23, 151-164.
View ArticleZhu, J., Wang, Q., Yu, H., Li, M., & He, N. (2016). Heavy metal deposition through rainfall in Chinese natural terrestrial ecosystems: evidence from national-scale network monitoring. Chemosphere, 164, 128-133.
View ArticleAsfaram, A., Ghaedi, M., & Ghezelbash, G. R. (2016). Biosorption of Zn 2+, Ni 2+ and Co 2+ from water samples onto Yarrowia lipolytica ISF7 using a response surface methodology and analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES). RSC advances, 6(28), 23599-23610.
View ArticleRaman, G. (2013). Biodegradation of Nicotine by A Novel Nicotine-Degrading Bacterium, Pseudomonas Plecoglossicida Tnd35 And Characterization of Its New Biotransformation Metabolites (Doctoral dissertation).
View ArticleWu, W., Wu, P., Yang, F., Sun, D., Zhang, D., Yi, Zhou, 2018. Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Sci. Total Environ. 630, 53–61.
View ArticleChoudhary, B., & Paul, D. (2018). Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. Journal of Environmental Chemical Engineering, 6(2), 2335-2343.
View ArticleOrozco, A. M. F., Contreras, E. M., & Zaritzky, N. E. (2008). Modelling Cr (VI) removal by a combined carbon-activated sludge system. Journal of hazardous materials, 150(1), 46-52.
View ArticleFernández, P. M., Viñarta, S. C., Bernal, A. R., Cruz, E. L., & Figueroa, L. I. (2018). Bioremediation strategies for chromium removal: current research, scale-up approach and future perspectives. Chemosphere, 208, 139-148.
View ArticleRoy, S., Labelle, S., Mehta, P., 2005. Phytoremediation of heavy metal and PAH contaminated brown field sites. Plant Soil 272, 277–290.
View ArticleHall, J.L., 2002. Cellular mechanism of heavy metal detoxification and tolerance. J. Exp. Bot. 53, 1–11.
View ArticlePeixoto, P.H.P., Cambria, J., Sant Anna, R., Mosquim, P.R., Moreira, M.A., 2001. Aluminium effects on fatty acid composition and lipid peroxidation of a purified plasma membrane fraction of root apices of two sorghum cultivars. J. Plant Nutr. 24, 1061–1070.
View ArticleUllah, A., Heng, S., Munis, M. F. H., Fahad, S., & Yang, X. (2015). Phytoremediation of heavy metals assisted by plant growth-promoting (PGP) bacteria: a review. Environmental and Experimental Botany, 117, 28-40.
View ArticleThekkudan, V. N., Vaidyanathan, V. K., Ponnusamy, S. K., Charles, C., Sundar, S., Vishnu, D., ... & Subramanian, S. (2016). Review on nano adsorbents: a solution for heavy metal removal from wastewater. IET nanobiotechnology, 11(3), 213-224.
View ArticleYan-de, J., Zhen-li, H., Xiao, Y., 2007. Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J. Zhejiang Univ. Sci. 8 (3), 197–207.
View ArticleDellisanti, 2016. In-field remediation of tons of heavy metal-rich waste by Joule heating vitrification. Int. J. Miner. Process. 93, 239–245.
View ArticleDouay, F., Pruvot, C., Roussel, H., Ciesielski, H., Fourrier, H., Proix, N., Waterlot, C., 2008. Contamination of urban soils in an area of northern France polluted by dust emissions of two smelters. Water Air Soil Pollut. 188, 247–260.
View ArticlePark, B., & Son, Y. (2017). Ultrasonic and mechanical soil washing processes for the removal of heavy metals from soils. Ultrasonics sonochemistry, 35, 640-645.
View ArticleUcaroglu, S., & Talinli, I. (2012). Recovery and safer disposal of phosphate coating sludge by solidification/stabilization. Journal of environmental management, 105, 131-137.
View ArticleFauziah, S.H., Jayanthi, B., Emenike, C.U., Agamuthu, 2017. Remediation of heavy metal contaminated soil using potential microbes isolated from a closed disposal site. Int. J. Biosci. Biochem. Bioinf. 7, 230–237.
View ArticleHaque, N., Peralta-Videa, J. R., Jones, G. L., Gill, T. E., & Gardea-Torresdey, J. L. (2008). Screening the phytoremediation potential of desert broom (Baccharis sarothroides Gray) growing on mine tailings in Arizona, USA Environmental Pollution, 153(2), 362-368.
View ArticleMarques, APGC, Oliveira, R.S., Rangel, AOSS, Castro, P.M.L., 2008. Application of manure and compost to contaminated soils and its effect on zinc accumulation by Solanum nigrum inoculated with arbuscular mycorrhizal fungi. Environ. Pollut. 151, 608–620.
View ArticlePulford, I. D., & Watson, C. (2003). Phytoremediation of heavy metal-contaminated land by trees—a review. Environment International, 29(4), 529-540. 00152-6
View ArticleDeng, Z., Cao, L., Huang, H., Jiang, X., Wang, W., Shi, Y., & Zhang, R. (2011). Characterization of Cd-and Pb-resistant fungal endophyte Mucor sp. CBRF59 isolated from rapes (Brassica Chinensis) in a metal-contaminated soil. Journal of Hazardous Materials, 185(2-3), 717-724.
View ArticleKumar, A., Bisht, B.S., Joshi, V.D. and Dhewa, T., 2011. Review on bioremediation of polluted environment: a management tool. International journal of environmental sciences, 1(6), pp.1079-1093.
View ArticleBader, N., Alsharif, E., Nassib, M., Alshelmani, N., & Alalem, A. (2019). Phytoremediation potential of Suaeda vera for some heavy metals in roadside soil in Benghazi, Libya. Asian Journal of Green Chemistry, 3(1. pp. 1-124), 82-90.
View ArticleSharma, S., Singh, B., & Manchanda, V. K. (2015). Phytoremediation: role of terrestrial plants and aquatic macrophytes in the remediation of radionuclides and heavy metal contaminated soil and water. Environmental Science and Pollution Research, 22(2), 946-962.
View ArticleAbou, R. A. E. A. I. (2011). Bioremediation: new approaches and trends. In Bio management of metal-contaminated soils (pp. 65-94). Springer, Dordrecht.
View ArticleAbbas, M. H., & Abdelhafez, A. A. (2013). Role of EDTA in arsenic mobilization and its uptake by maize grown on an As-polluted soil. Chemosphere, 90(2), 588-594.
View ArticleLasat, M. M. (2002). Phytoextraction of toxic metals. Journal of environmental quality, 31(1), 109-120.
View ArticleOves, M., Saghir Khan, M., Huda Qari, A., Nadeen Felemban, M., & Almeelbi, T. (2016). Heavy metals: biological importance and detoxification strategies. Journal of Bioremediation and Biodegradation, 7(2), 1-15.
View ArticleYadav, S. K. (2010). Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany, 76(2), 167-179.
View ArticleKacálková, L., Tlustoš, P., & Száková, J. (2015). Phytoextraction of risk elements by willow and poplar trees. International journal of phytoremediation, 17(5), 414-421.
View ArticleNouri, J., Khorasani, N., Lorestani, B., Karami, M., Hassani, A. H., & Yousefi, N. (2009). Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environmental Earth Sciences, 59(2), 315-323.
View ArticleRezania, S., Taib, S. M., Din, M. F. M., Dahalan, F. A., & Kamyab, H. (2016). A comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. Journal of hazardous materials, 318, 587-599.
View ArticleMidhat, L., Ouazzani, N., Esshaimi, M., Ouhammou, A., & Mandi, L. (2017). Assessment of heavy metals accumulation by spontaneous vegetation: Screening for new accumulator plant species grown in Kettara mine-Marrakech, Southern Morocco. International journal of phytoremediation, 19(2), 191-198.
View ArticleGhosh, M., & Singh, S. P. (2005). A review on phytoremediation of heavy metals and utilization of its by-products. Asian J Energy Environ, 6(4), 18.
View ArticleChibuike, G. U., & Obiora, S. C. (2014). Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2014: 12.
View ArticleJan, F. A., Ishaq, M., Khan, S., Ihsanullah, I., Ahmad, I., & Shakirullah, M. (2010). A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). Journal of Hazardous Materials, 179(1†"3), 612-621.
View ArticleLiu, W., Xu, W., Han, Y., Wang, C., & Wan, S. (2007). Responses of microbial biomass and respiration of soil to topography, burning, and nitrogen fertilization in a temperate steppe. Biology and fertility of soils, 44(2), 259-268.
View ArticleBazała, M. A., Gołda, K., & Bystrzejewska-Piotrowska, G. (2008). Transport of radiocesium in mycelium and its translocation to fruitbodies of a saprophytic macromycete. Journal of environmental radioactivity, 99(7), 1200-1202.
View ArticleEhlken, S., & Kirchner, G. (2002). Environmental processes affecting plant root uptake of radioactive trace elements and variability of transfer factor data: a review. Journal of Environmental Radioactivity, 58(2-3), 97-112. 00060-1
View ArticleGupta, A. C., Srivastava, A. K., & Wiita, P. J. (2008). Periodic oscillations in the intra-day optical light curves of the blazar S5 0716+ 714. The Astrophysical Journal, 690(1), 216.
View ArticleAkaninwor, J. O., Anosike, E. O., &Egwim, O. (2007). Effect of Indomie industrial effluent discharge on Microbial properties of new Calabar River. Scientific Research and Essays, 2(1), 001-005.
View ArticleMubin, S., Gul, S., Khokhar, M. I. A., & Ashraf, M. (2002). Statistical solution for the industrial waste problem. Journal of Drainage and Water management, 6(2), 55-68.
View ArticleScott, C. A., Faruqui, N. I., &Raschid-Sally, L. (2004). Wastewater use in irrigated agriculture: Management challenges in developing countries: In: (UK. Scott (ed.) Wastewater use in irrigated agriculture: confronting the livelihood and environmental realities. CABI International, UK.
View ArticleNasir, A., Arslan, C., Khan, M. A., Nazir, N., Awan, U. K., Ali, M. A., et al. (2012). Industrial waste water management in destrict Gujranwala of Pakistan- Current status and future suggestions. Pakistan Journal of Agricultural Sciences, 49(1), 79-85.
View ArticleIslam, R., Al Foisal, J., Rahman, M., Lisa, L.A. and Paul, D.K., 2016. Pollution assessment and heavy metal determination by AAS in waste water collected from Kushtia industrial zone in Bangladesh. African Journal of Environmental Science and Technology, 10(1), pp.9-17.
View ArticleBahadir, T., Bakan, G., Altas, L., &Buyukgungor, H. (2007). The investigation of lead removal by biosorption: An application at storage battery industry wastewaters. Enzyme and Microbial Technology, 41(1), 98-102.
View ArticleHussein, H., Farag, S., Kandil, K., &Moawad, H. (2005). Tolerance and uptake of heavy metals by Pseudomonads. Process Biochemistry, 40, 955 – 961.
View ArticleShah, M. T., Ara, J., Muhammad, S., Khan, S., & Tariq, S. (2012). Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohmand agency, northern Pakistan. Journal of Geochemical Exploration, 118(0), 60-67.
View ArticleAhmad, M., Bajahlan, A. S., & Al-Hajery, K. A. (2010). Potential impacts of industrial reclaimed water on landscape irrigation. International Journal of Agriculture and Biology, 12, 707-712.
View ArticleJamil, M., Zia, M. S., &Qasim, M. (2010). Contamination of Agro–Ecosystem and Human Health Hazards from Wastewater used for Irrigation. Journal of Chemical Society of Pakistan, 32(3).
View ArticleShivkumar, K., & Biksham, G. (1995). A statistical approach for the assessment of water pollution around the industrial area. Environ Monitoring and Assessment 36, 229-249.
View ArticleGadd, G. M. (2007). Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycological research, 111(1), 3-49.
View ArticleSandmann, G., &Böger, P. (1980). Copper-induced exchange of plastocyanin and cytochrome c-533 in cultures of Anabaena variabilis and Plectonemaboryanum. Plant Science Letters, 17(4), 417-424. 90128-5
View ArticleJanas, K.M., Zielińska-Tomaszewska, J., Rybaczek, D., Maszewski, J., Posmyk, M.M., Amarowicz, R. and Kosińska, A., 2010. The impact of copper ions on growth, lipid peroxidation, and phenolic compound accumulation and localization in lentil (Lens culinaris Medic.) seedlings. Journal of Plant Physiology, 167(4), pp.270-276.
View ArticleForte, J., &Mutiti, S. (2017). Phytoremediation potential of Helianthus annuus and Hydrangea paniculata in copper and lead-contaminated soil. Water, Air, & Soil Pollution, 228(2), 77.
View ArticleLi DZ & Pritchard HW (2009). The science and economics of ex situ plant conservation. Trends in Plant Sci 14(11): 614-621.
View ArticleLin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY &Kuo HP (2004). The impact of delirium on the survival of mechanically ventilated patients. Critical Care Med 32(11): 2254-2259.
View ArticleTurner AP & Dickinson NM (1993). Survival of Acer pseudoplatanus L (sycamore) seedlings on metalliferous soils. New Phytologist 123(3): 509-521.
View ArticleShin MN, Shim J, You Y, Myung H, Bang KS, Cho M & Oh BT (2012). Characterization of lead resistant endophytic Bacillus sp. MN3-4 and its potential for promoting lead accumulation in metal hyperaccumulator Alnus firma. J of Hazardous Materials 199: 314-320.
View ArticleRyser, P., & Sauder, W. R. (2006). Effects of heavy-metal-contaminated soil on growth, phenology and biomass turnover of Hieraciumpiloselloides. Environmental Pollution, 140(1), 52-61.
View ArticlePandey, N., Pathak, G. C., & Sharma, C. P. (2006). Zinc is critically required for pollen function and fertilization in lentil. Journal of Trace Elements in Medicine and Biology, 20(2), 89-96.
View ArticleTewari, P.K. and Singh, A.K., 2002. Preconcentration of lead with Amberlite XAD-2 and Amberlite XAD-7 based chelating resins for its determination by flame atomic absorption spectrometry. Talanta, 56(4), pp.735-744. 00606-3
View ArticleGajewska, E. and Skłodowska, M., 2007. Relations between tocopherol, chlorophyll and lipid peroxides contents in shoots of Ni-treated wheat. Journal of plant physiology, 164(3), pp.364-366.
View ArticleGunatilake SK (2015). Methods of removing heavy metals from industrial wastewater. Methods 1(1): 14.
View ArticleYoon, J., Cao, X., Zhou, Q. and Ma, LQ, 2006. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Science Total Environment, 368: 456 -464.
View ArticleEbrahimi, M. and Madrid Díaz, F., 2014. Use of Festuca ovina L. in chelate assisted phytoextraction of copper contaminated soils. Jour. Rangeland Science, 4(3): 171 -181.
View Article