Jiting Wang
Email: jtwang@sdau.edu.cn
ORCID: https://orcid.org/0000-0003-1483-333X
Tel: +86 538 8242593 ext 8107, Fax: +86 538 8241419.
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 6 ISSUE: 2
Page No: 148-161
Jiting Wang
Email: jtwang@sdau.edu.cn
ORCID: https://orcid.org/0000-0003-1483-333X
Tel: +86 538 8242593 ext 8107, Fax: +86 538 8241419.
Yanli Wang ab1, Qi Li ab1, Xiao Yun ab, Jie Zhou ab, Jiting Wang abc*
a Lab of aquatic ecology;
b Lab of Aquatic Animal Nutrition & Environmental Health;
c Shandong Provincial Key Lab. of Animal Biotechnology and Disease Control and Prevention; Shandong Agricultural University, 61 Daizong Street, Taian City, Shandong Province, 271018, China.
1 Both authors contributed equally to this work.
Yanli Wang, Qi Li, Xiao Yun, Jie Zhou, Jiting Wang, A review on the ecotoxicological effects of heavy metals on aquatic organisms (2022) Journal of Earth Sciences & Environmental Studies 6 (2) :148-161
In recent years, with the rapid development of the global economy, heavy metals have been widely used in industrial production and daily life because of their unique properties. This, however, has simultaneously led to heavy metal pollution due to various reasons. After entering the aquatic environment, heavy metals are not easily decomposed by microorganisms and become toxic when they reach a certain concentration. Heavy metals can easily enter the liver and other vital organs of aquatic organisms, where they accumulate and severely affect the growth and reproduction of these organisms. They can also threaten human health through the food chain. Therefore, heavy metal pollution has potential ecological and health risks. This paper summarizes the sources and hazards of heavy metals in water, the pattern of enrichment of heavy metals in aquatic organisms, the toxic effects of heavy metals on aquatic organisms, the tolerance mechanism of aquatic animals to heavy metals, and the factors affecting the toxicity of heavy metals. The authors put forward three feasible suggestions for the study of ecotoxicological effects of heavy metals on aquatic organisms in the future. The results of this study have considerable significance for aquaculture and environmental management and even for humans.
Keywords: Heavy metal, Aquatic environment, Aquatic organisms, Toxicity, Ecosystems
Water is one of the most important resources for the natural environment, industrial production, and life. However, at present, all major river basins in China have been affected by different types of water pollution, especially pollution due to heavy metals (Harguinteguy et al. 2014). Pollution by heavy metals has become one of the major environmental problems because of their persistence in the environment, biological toxicity, nondegradability, and ability to enter the food chain. They can also induce redox reactions with some substances and convert them into more toxic pollutant compounds (Yohannes et al., 2013; Fu et al., 2016). Aquatic organisms such as aquatic animals, aquatic plants, and aquatic microorganisms can accumulate heavy metals in their body (Rose et al., 2015). However, when the concentration and toxicity of heavy metals in water exceed the regulatory capacity for aquatic organisms, it will have a severe negative impact on their related functions and even life activities and can lead to genetic alteration and changes in species diversity. Heavy metals in the aquatic environment can be transmitted to humans through various food chains, and hence, heavy metal pollution poses a serious threat to human health. For example, heavy metals cannot be easily eliminated from the human body. Once they exceed the physiological regulatory limits for the human body, they will cause damage to the physiological system of the body, resulting in acute or chronic harm. Heavy metals such as zinc (Zn), cadmium (Cd), mercury (Hg), selenium (Se), and nickel (Ni) are teratogenic. In an environment with a high concentration of heavy metals, excessive intake of heavy metals by animals will cause poisoning and even serious consequences (Zhao, 2010)
2. Pollution status of heavy metals in water
The pollution of heavy metals in water has become a global environmental problem and is also a very prominent issue in China. The results of water quality monitoring in recent years show an increase in heavy metal pollution of major lakes and rivers in China. For example, in the Pearl River of China, approximately 3000 tons of Pb, 15000 tons of Zn, 300 tons of Cd, and 1000 tons of As are discharged into the waters every year, mainly through ship maintenance, metal corrosion, and pesticide application (Yang, 2015). The Yellow River basin, Jiulong River, Poyang Lake, and other aquatic systems have also been polluted to varying degrees (Fan et al., 2008; Li et al., 2010; Yang et al., 2011). The distribution pattern of different heavy metals in the sediments of Poyang Lake is lake area > inlet area > outlet area, indicating that heavy metals are concentrated in the sediments of the lake area. The content of different heavy metals in Poyang Lake is much lower than that in its sediments, indicating that heavy metals in water can easily settle and accumulate in sediments (Li et al., 2010). Cr, Cd, Pb, Cu, and Zn have also been detected in waters near Kosovo. The content of Cr exceeds European water quality standards, especially in sediments (Maloku et al., 2015). From the results of the investigation of heavy metal pollution in water in China and abroad, it was found that the pollution of heavy metals in water occurred mainly as composite pollution due to the coexistence of several heavy metals. Moreover, heavy metals in water are easily adsorbed on the sediments, thereby making sediments a pollutant source that releases heavy metals for a long time. Consequently, there is long-term persistence of heavy metal pollution in water.
3. Sources and hazards of heavy metals in water
Heavy metals can be generally divided into essential metals (copper, zinc, iron, magnesium, nickel, etc.) and nonessential metals (aluminum, cadmium, mercury, tin, lead, etc.). The sources of heavy metals in water include industrial sources, agricultural sources, and urbanization sources. A large number of heavy metals in the waste gas generated by chemical, electroplating, mining, and metal smelting industries enter the atmosphere and then enter the water bodies through rainfall. Heavy metals such as Cu, As, Hg, and Pb enter the soil through the application of fertilizers and pesticides and then enter the water bodies through rainwater leaching. With the acceleration of urbanization, heavy metals from waste incineration and automobile exhaust can also enter the water bodies through dry and wet deposition (Zhang et al., 2018). Tables 1 and 2 list the sources, hazards, maximum contaminant level (MCL) of some heavy metal pollutants, and the limits of GB 3838–2002 environmental quality standard for surface water (Class III).
Table 1. Sources of some heavy metals in water environment
|
Heavy metals |
Sources |
|
As |
Pesticides, fungicides, sedimentary rocks, geothermal water and weathered volcanic rocks, human activities such as mining, manufacturing, gold treatment and wood preservation. |
|
Pb |
Paint, pesticide, smoking, automobile exhaust, coal combustion, etc. |
|
Hg |
Mineral resources, fossil fuels, ores, pesticides, batteries, paper industry. |
|
Cd |
Steel and plastics industry, cooling tower, metal electroplating and coating operation, nickel cadmium battery, cadmium film, solar cell, pigment, galvanized pipe, welding, fertilizer and nuclear emission device. |
|
Cr |
Industrial wastewater is discharged into the environment, cooling tower blowdown, electroplating and metal electroplating and coating operations. |
|
Cu |
Pesticide industry, mining, metal pipeline, chemical industry. |
|
Zn |
Brass mapping, wood pulp production, grinding and newsprint production, iron and steel plants with zinc lines, zinc and brass metal products, refineries and pipelines. |
|
Se |
Water loss, rock weathering, rainfall and decomposition of organisms, combustion of coal and oil, agricultural irrigation, use of phosphate pesticides and fertilizers. |
|
Ni |
Battery manufacturing, alloy production, zinc base casting, printing, electroplating, silver refinery. |
Table 2. Potential toxic effects of heavy metals and the maximum harmful concentration levels (MCL) and GB3838-2002 in China
|
Heavy metals |
Hazards |
MCL (mg/L) |
GB 3838-2002 In China (mg/L) |
References |
|
As |
Cancer of the skin, lungs, bladder, and kidneys; cancer and other internal tumor diseases; vascular diseases and diabetes; infant mortality and weight loss of newborns; hearing loss; developmental abnormalities and neurobehavioral disorders; reproductive toxicity; blood diseases; nervous system diseases. |
0.05 |
0.05 |
Baskan et al., 2011; Ntim et al., 2012 |
|
Pb |
Anemia; cancer; kidney disease; neurological impairment; mental retardation; mental impairment and behavioral problems in children. |
6×10-3 |
0.05 |
Salem et al., 2011; Qu et al., 2013 |
|
Hg |
Damage to the immunity of the renal reproductive system; damage to the blood, cardiovascular and respiratory systems and the brain. |
3×10-5 |
0.0001 |
Shamsijazeyi et al., 2010; Natale et al., 2011 |
|
Cd |
Renal cancer damage; bronchiolitis; chronic obstructive pulmonary disease; emphysema; fibrotic bone damage |
0.01 |
0.005 |
Arias et al., 2002; Vukovi et al., 2010 |
|
Cr |
Severe diarrhea; vomiting; pulmonary congestion; liver and kidney damage. |
0.05 |
0.05 |
Liu et al., 2011; Ihsanullah et al., 2016 |
|
Cu |
Increased blood pressure and breathing; kidney and liver damage; convulsions, spasms, vomiting. |
0.25 |
1.0 |
Awual et al., 2013, Al-Rashdi et al., 2013 |
|
Zn |
Gastric nausea; skin irritation; spasms; vomiting and anemia. |
0.8 |
1.0 |
Deliyanni et al., 2007 |
|
Se |
Gastrointestinal discomforts; hair and nail loss; fatigue; cardiac arrhythmia and nerve injuries. |
— |
0.01 |
Li et al., 2017 |
|
Ni |
Dry coughing; bone, nose, and lung cancer; shortness of breath; chest tightness and chest pain; nausea and vomiting; dizziness and headache. |
0.2 |
— |
Reddad et al., 2002; Yavuz et al., 2003 |
4. Pattern of enrichment of heavy metals in aquatic organisms
Heavy metals in water cannot be easily metabolized by aquatic organisms, and therefore, these metals tend to get enriched in organs such as the liver and kidney. Studies have shown that heavy metals can enter aquatic animals through their gills or during feeding and bind with metallothioneins and other substances in the body (Yang, 2015). The pattern of enrichment of heavy metals, however, varies according to the enrichment ability of different aquatic species. In the aquatic environment of the Pearl River Delta of China, the content of Pb in tilapia muscle was found to be higher than that of other species (Leung et al., 2014). At the same concentration, the accumulation of Pb in crucian carp tissues showed the following trend: viscera > gills > fins > muscles, and the content of Pb was significantly higher in viscera (Wang et al., 2015). Zheng et al. (2014) found that the enrichment level of the essential trace elements Fe, Zn, and Cu in the hepatopancreas of fish was higher than that in other tissues. In addition, in the Meizhou area of Guangzhou, the pollution level of heavy metals in fish showed the following trend: Pb > Cr > Cd > Cu. These findings show that fish species encounter the problem of composite pollution of heavy metals rather than pollution of a single metal element.
5. Toxic effects of heavy metals on aquatic organisms
Heavy metals in water enter aquatic organisms in three ways. First, aquatic animals absorb heavy metal ions in water through gill tissues and then transport them to various tissues of the body. Second, aquatic animals absorb heavy metals into their bodies by ingesting food contaminated with heavy metals. Third, heavy metals enter the bodies of aquatic animals by osmotic exchange through subcutaneous absorption (Long et al., 2016). Figure 1 shows the toxicological effects of heavy metal pollution on organisms at different levels.
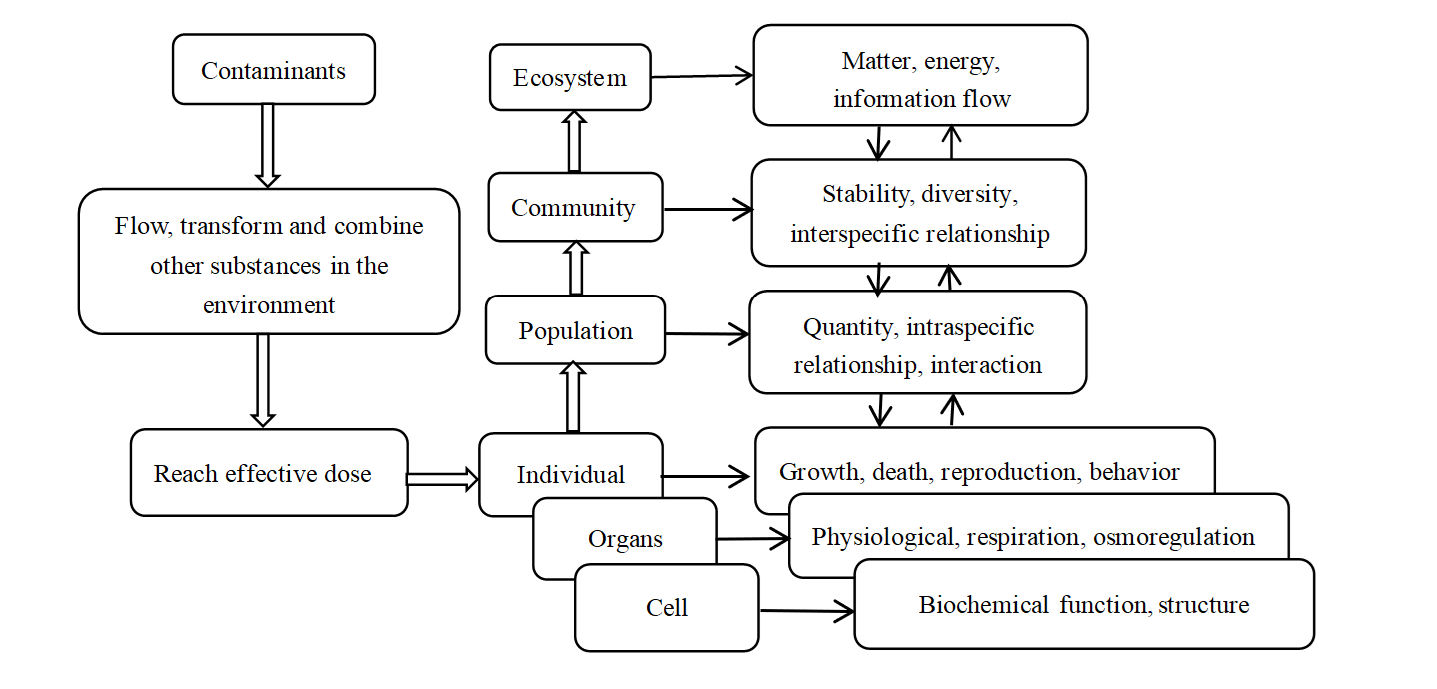
Fig. 1. The toxicological effects of heavy metal pollution on organisms at different levels
Some heavy metals entering aquatic animals can promote the growth, metabolism and enzyme activity of aquatic animals within an appropriate concentration range. However, with the extension of exposure time, due to the accumulation effect of metal ions on aquatic animals, when its concentration reaches a certain threshold, it also produces a series of toxicological effects on the growth, physiology and biochemistry, genetic gene expression, behavior, metabolism and other processes of aquatic animals. Moreover, the potential risks of heavy metals to different kinds of aquatic animals are also different. Du et al. (2013) compared the sensitivity of marine vertebrates and invertebrates to eight kinds of heavy metals and the acute ecological risk of different heavy metals, indicating that the ecological risk of heavy metals to crustaceans is greater than that to fish. Bian et al. (2016) showed that aquatic animals of benthic oligochaetes are more sensitive to heavy metal pollution, followed by leeches, gastropods and insects.Generally speaking, the enrichment of heavy metals by aquatic animals is related to specific factors such as age, geographical distribution, season, species differences and the form of heavy metals. Moreover, the distribution of different kinds of heavy metals in different tissues of aquatic animals is also different, so it will cause different ecotoxicological effects on aquatic animals.
5.1 Bioaccumulation of heavy metals
Once heavy metals enter the aquatic organisms, they are difficult to be decomposed, metabolized, and excreted and are thus very easily enriched in animal organs such as the liver and kidney. Olivares et al. (2016) confirmed that binding with metal binding proteins such as metallothioneins is the main mechanism of heavy metal accumulation in organisms. Rainbow et al. (1992) found that the process of heavy metal intake by organisms does not require energy expenditure and that heavy metals are not easily excreted and thus show the cumulative effect of toxicity. Qin et al. (2015) studied the acute toxic effects of six types of heavy metals on three species of mariculture organisms and found that the content of heavy metals in three species increased significantly after 96 h of treatment. Sun et al. (2015) investigated the enrichment characteristics of heavy metals in sediments and marine organisms in the sea area of Zhanjiang port and found that except for Cd, the contents of other heavy metals in sediments were higher than those in marine organisms. Cu, Zn, and Cd were highly enriched in organisms in the Zhanjiang port basin. Mollusks and crustaceans showed the same accumulation ability for Hg and Pb; mollusks exhibited a stronger enrichment ability for Sn and Cd and a weaker enrichment ability for Cu than crustaceans. Rzymski et al. (2014) showed that bivalves have a high accumulation ability for Cu and Cd. Topcuoglu et al. (2002) reported the highest enrichment of Pb, Cd, and Cr and the lowest enrichment of Fe and Zn in mollusks and shellfish from the Black Sea, Turkey.
5.2 Effect of toxicity of heavy metals on early biological development
The early embryonic and larval development stages of fish are prone to be affected by heavy metals. After the heavy metal pollutants enter the larvae of aquatic organisms, they react with nucleic acids, enzymes, vitamins, hormones, and other substances in the organisms; alter their chemical structure and biological activity; and then disrupt the functions of multiple systems such as the endocrine and central nervous systems, resulting in various diseases and even death (Tolins et al., 2014; Jakubowski et al., 2015; Meng et al., 2018). Guo et al. (2015) studied the toxicity of heavy metals in water samples from the Three Gorges Reservoir area on the embryonic development of zebrafish (Danio rerio). The results showed that after seven days of exposure, although there was no significant difference in the hatching rate, relative survival rate, and deformity rate in all zebrafish embryos, there was a significant decrease in the expression levels of genes related to reproduction and neurodevelopment in juveniles. Zhang et al. (2010) measured the toxic effects of Cu and Cd on embryonic development by using zebrafish early embryonic development technology; the authors found that both Cu and Cd had toxic effects on zebrafish embryos in terms of death and inhibition of embryo hatching at 24 h and 72 h, respectively. García et al. (2014) noted that a certain concentration of Ag ions significantly increased the mortality and distortion rate of eel (Nereis succinea) embryos. Munley et al. (2013) found that the embryonic growth of aquatic snails was inhibited after 28 days of exposure to Co, Cu, Pb, and Ni, and the oviposition was inhibited after 56 days. Cu, Pb, and Zn can also significantly interfere with the development of early biological embryos, resulting in delayed embryonic development (Salvaggio et al., 2016).
5.3 Immunotoxicity of heavy metals to aquatic organisms
Low concentrations of heavy metals or short-term exposure to heavy metals can stimulate the immune response of organisms and enhance the phagocytic activity of blood cells (Rickwood et al., 2004; Hannam et al., 2009). However, high concentrations of heavy metals or long-term exposure will significantly reduce the phagocytic capacity of body cells, mainly because heavy metals change the fluidity of the cell membrane and the permeability of ion pumps on the cell membrane and reduce the stability of the cell membrane, thereby leading to a decrease in phagocytic activity (Grundy et al., 1996; Camus et al., 2002). Paul et al. (2014) studied the immunotoxicity of Pb in freshwater fish and found that Pb ions significantly reduced the level of tumor necrosis factor in serum. Qin et al. (2012) reported that Cd exposure affected the enzyme activity in river crab (Potamidae). Vijayavel et al. (2009) showed that Ni exposure promoted hemolymph phagocytosis of green crab and significantly inhibited the activity of phenoloxidase. Chandurvelan et al. (2013) revealed that Cd exposure increased the level of basophils and eosinophils in mussel blood, resulting in DNA damage.
5.4 Heavy metals affect gene mutation and variation
After the heavy metal pollutants from the environment enter the organisms, they tend to accumulate in various organs of the organisms. When their concentration exceeds a certain level or after long-term exposure to these heavy metals, they cause damage to the tissue, induce the production of a high amount of reactive oxygen species (ROS) and electrophilic metabolites, and then combine with DNA molecules; consequently, the cells are subjected to oxidative attack from the external environment, resulting in multiple reactions of lipids in the organism, such as peroxidation, alterations in genetic material, and ribose oxidation, which leads to cell death or carcinogenesis (Waisberg et al., 2003; Pan et al., 2005; Thomas et al., 2007). Hix et al. (1999) revealed that DNA is highly methylated when organisms are attacked by a large number of methyl radicals induced by Fe ions. Leszkowicz et al. (1987) also showed that heavy metal ions can significantly inhibit the activity of methyltransferase. For example, Pb, Cu, and Zn ions can inhibit the activity of 5-methyltransferase. Rossiello et al. (1991) also reported that Pb can cause low methylation of DNA. Tang et al. (2013) found that both single and combined toxicity of Cu and Pb caused DNA damage in the eggs of Misgurnus anguillicaudatus, thus showing these metals induced genotoxic effects. Xing et al. (2016) also showed that Pb and Cr exerted genotoxic effects on loach in varying degrees, and the toxic effects increased with the increase in treatment concentration and treatment time under certain conditions; moreover, the toxic effects were inhibited after reaching a certain concentration and time.
5.5 Endocrine-disrupting toxicity of heavy metals
One of the mechanisms of metabolic diseases induced by heavy metal exposure is the disruption of the endocrine system by heavy metals. In vivo, heavy metals can interfere with hormone synthesis and secretion and induce endocrine-disrupting toxicity; for example, Cd, Mn, and Cr were found to increase the incidence rate of metabolic disorders. Exposure to metal endocrine disruptors can increase the risk of oxidative stress and induce mitochondrial dysfunction (Rafa et al., 2010). Exposure to sublethal concentrations of Pb and Cd caused a significant increase or decrease in blood steroid concentration, ovarian steroid secretion activity, and ovarian development. Mu (2017) showed that Cd exposure greatly interfered with thyroid hormone levels in the plasma of carp (Cyprinus carpio). Luo (2015) found that Cd increased the level of estradiol in serum but had no effect on the level of testosterone. At the gene level, Cd exposure increased the expression of estrogen receptor (ER) in the ovary but inhibited the production of vitellogenin. In the testis, Cd inhibited the development of spermatids and sperm by increasing the expression of the glucocorticoid receptor. Li et al. (2014) found that after minnow larvae were exposed to Hg for four days, the gene expression of the adrenocorticotropic hormone-releasing hormone, thyroglobulin, and thyroid receptors α and β were significantly induced, and the contents of thyroxine T3 and T4 increased.
6. Tolerance mechanism of aquatic animals to heavy metals
Living in the environment stressed by heavy metal ions for a long time, some aquatic animals can adjust some physiological and biochemical indexes to improve their stress and tolerance to heavy metal ions. For example, after Daptmia magna was exposed to high-dose Zn2+ for two generations, the reproduction and growth of larvae slowed down, but after three generations of exposure, the growth and reproduction of larvae did not decrease significantly, indicating that multi generations of heavy metal exposure tended to increase the tolerance of Daphnia magna to heavy metals (Vandegehuchte et al., 2010). In aquatic animals, the antioxidant enzyme system composed of SOD and CAT forms the first defense line against heavy metal toxicity, which can alleviate the oxidative damage caused by heavy metals; The second antioxidant damage defense line composed of glutathione and glutathione related enzymes plays an important role in cell metabolism and free radical scavenging. SOD can disproportionate superoxide anion free radicals to produce H2O2, which can remove harmful free radicals; CAT can catalyze H2O2 to produce harmless H2O and O2, and also cooperate with SOD and POD to remove excess free radicals and peroxides in the body; GPX can convert H2O2 into H2O and O2, so as to reduce the damage of H2O2 to body tissues.
Metallothionein widely exists in aquatic animals. Because it is rich in cysteine, the sulfhydryl group on the side chain of cysteine residues can strongly chelate with toxic metal ions, and can combine with 18 metal ions to form non-toxic or low toxic complexes, so as to play the role of detoxification, alleviate the toxicity of heavy metals and protect against oxidative stress (Ryvolov et al., 2011;Sheng et al.,2014). Mao et al. (2012)analyzed the MT gene sequence and protein structure of Charybdis japonica, detected the temporal and spatial expression pattern of MT mRNA during spermatogenesis, and found that Charybdis japonica MT had more non conservative Cys at the C-terminal, which may be helpful to resist heavy metal pollution.The antioxidant enzyme system and antioxidant non enzyme system in aquatic animals will also play a series of detoxification action when aquatic animals are stressed by heavy metals. Kim et al. (2014) found that when Tigriopus japonicus is exposed to water polluted by a certain concentration of Ag, As, Cd, Cu and Zn ions, its antioxidant enzyme system can be effectively activated and can alleviate the damage of reactive oxygen species to itself. Liu et al. (2008) found that low concentration of Ce 3+ can increase the contents of SOD, CAT, GSH-Px, vitamin and GSH, reduce the accumulation of reactive oxygen species and inhibit lipid peroxidation. Some aquatic animals secrete abundant mucus under heavy metal stress, which can improve their tolerance to metals to a certain extent. For example, the acute toxicological test of striped bass by Tripathi et al. showed that when stressed by heavy metal Cd2+, rich mucus played a good protective role against the harm of toxic heavy metals (Tripathi et al., 2012). Rhee et al. (2013) showed that exposure of Daphnia japonicus to water polluted by a certain concentration of Ag, As, Cd and Cu would significantly increase its GST sigma protein, thus improving its ability to detoxify heavy metals and adapt to the environment. At the same time, the physiological protection mechanism of some marine shellfish and the antagonism between metal ions can also reduce the damage of heavy metal ions to shellfish (Sun et al., 2015). In addition, heat shock protein (HSP) and transferrins (TF) are also one of the important tolerance mechanisms against heavy metal stress (Ding et al., 2012; Yang et al., 2015).
Damaged DNA can be self repaired by organisms under normal physiological conditions to ensure the stability of genetic genes and reduce the probability of gene mutation. However, when the concentration of heavy metals in the body exceeds a certain threshold or the retention time of heavy metals in the body exceeds a certain time, the repair function will be damaged, resulting in gene mutation; In addition, heavy metals can also interfere with the normal repair process of DNA damage, so that DNA can not be repaired in time after damage, resulting in the destruction of body tissues. The results showed that DNA damage of digestive gland and gill tissue cells increased with the increase of Cd2+ concentration and exposure time within 12 hours; However, within 24 hours, the degree of DNA damage in both tissues decreased in varying degrees, showing a certain repair function to DNA; However, at 96 h, the damage degree of high concentration group began to increase, and at the same concentration, the DNA damage degree of gill tissue was higher than that of digestive gland cells (Lu et al., 2011).
7. Physical and chemical factors affecting the toxicity of heavy metals
Many factors affect the biological toxicity of heavy metals, such as temperature, pH, hardness of water, dissolved oxygen, light, and salinity. In addition, the existing form of heavy metals, the existence of other metals or contaminants, and biological conditions play an important role in affecting the toxicity of heavy metals.
7.1 Temperature
Temperature is one of the regulatory factors of metal ion concentration in the environment and metal toxicity to algae. Generally, the toxicity of heavy metals to algae is positively correlated with temperature. Klotz et al. (1981) found that the toxicity of Cu to Scenedesmus decreased below the optimum temperature but increased at the optimum temperature. The toxicity of Zn to Nitzschia linearis and Cyclotella meneghiniana increased with the increase in temperature from 22 °C to 30 °C, while the opposite was true for Scenedesmus quadricauda and Chlamydomonas. The same results were obtained in the acid sulfate ion toxicity test. The maximum toxicity occurred at the temperature with the most vigorous metabolism. This may be because the increase in temperature enhances respiratory activity, which promotes the absorption of metals by algae. Klotz et al. (1981) showed that temperature change significantly affected the toxicity of Cu to Scenedesmus and Chlorella and the toxic effect of Cu on algal cell division. The division rate of Chlorella cells treated with 0.15 ppm Cu decreased greatly at 6 °C.
7.2 pH and redox potential
pH and redox potential are two important physical and chemical factors that affect the migration and transformation of heavy metals in water. They play a decisive role in the migration and transformation of Zn, Fe, Mn, and Cu. Under acidic and reducing conditions, Fe and Cu exist as easily soluble compounds of Fe2+ and Cu2+, while under alkaline or near-neutral and oxidizing conditions, insoluble Fe3+ and Mn4+ oxides and hydroxides are formed. Rai et al. (1990) showed that heavy metals induce high toxicity under acidic conditions, but their toxicity is reduced under alkaline conditions. Their analysis showed that metals exist as free ions at acidic pH, while at alkaline pH, they form insoluble carbonate, phosphate, sulfate, oxide, or hydroxide precipitates. The precipitation of different metals requires different potential and pH values, especially for those metal cations with more than one valence state. The valence state, morphology, and bioavailability of heavy metal ions are greatly restricted by environmental pH and redox potential. Therefore, at acidic conditions, most metals are free cationic, organic matter can be most utilized and toxic; Under alkaline conditions, the opposite is true (Les et al., 1984). Peterson et al. (1990) showed that the toxicity of Cd to crescent algae increased by eight times for each unit of pH increase, but the change in the toxicity of Cu was smaller. At pH 6, the toxicity of Cd was 500 times lower than that of Cu, while at pH 10, the toxicity of Cd was twice that of Cu.
7.3 Dissolved oxygen
When the temperature and contaminant concentrations are constant, the toxicity of toxic substances tends to increase with the decrease in dissolved oxygen concentration. This is because when the level of dissolved oxygen is insufficient, to obtain adequate oxygen, the respiratory and circulatory systems increase their activity, and the amount of water flowing through the gill filaments increase; consequently, the amount of toxins entering the body increases, and these toxins are transported to each sensitive organ through the blood, resulting in enhanced toxicity. Dissolved oxygen also has an important effect on the redox state of water, which will significantly change the presence of chemical elements with variable valence in water, thus affecting their biological effectiveness. For example, the concentration of heavy metals in sediment interstitial water with very low dissolved oxygen content and anaerobic microorganisms is ten times higher than that in upper clear water, resulting in greater toxicity to aquatic organisms residing in the sedimental region.
7.4 Hardness of Water
It is generally believed that water hardness affects the toxicity of heavy metals through the formation of insoluble carbonate or calcium carbonate absorption. However, the increase in Ca2+ and Mg2+ plasma concentration can reduce the toxicity of heavy metals; hence, it is considered to have a protective function. Pellegrini et al. (1993) systematically studied the detoxification of Ca2+ on Cd, Cu, and Zn in brown algae. They designed a series of experiments and found that Ca2+ had a protective effect in all experiments, but the detoxification mechanism was not clear. Bjerselius et al. (1993) conducted the acute toxicity test of Atlantic salmon to Cu and found that when the concentration of Ca2+ and Mg2+ increased, the toxicity of Cu greatly decreased. Jayaraj et al. (1992) studied the effects of Ca, Mg, and Fe on the toxicity of Cu, Cd, and Ni in algae. They found that the increase in Ca2+ and Mg2+ concentration reduced the toxicity of these heavy metals before their concentration reached 100 mg/L, but the opposite situation occurred when the concentration continued to increase further. Although it has been suggested that the effect of common ions such as Ca and Mg on heavy metal toxicity is related to the competitive reaction of metal ions at the active site, this view needs to be further discussed.
7.5 Organic matter
Many studies have shown that organic complexes can greatly reduce the toxicity of heavy metal pollutants because the combination of heavy metals and organic complexes greatly reduces the concentration of free metal ions. Oikari et al. (1992) found that humic acid (HA) increased the toxicity of Cd and Cr to Daphnia magna. Stackhouse and Benson (1989) reported that when 0.5 mg/L HA was added to water, Daphnia magna could enrich more Cd, while the metal enrichment decreased when HA concentration was higher (5–50 mg/L). This shows that the effect of HA on metal toxicity is multifaceted, and its mechanism remains to be discussed. The biological toxicity of heavy metal complexes has also been reported, such as the secretion of copper and algae, copper and citric acid, and ethylenediamine complexes. Tubbing et al. (1993) confirmed that regardless of how high the EDTA concentration is, a small amount of copper can cause changes in the structure and function of bacteria and algae. All these findings show that the EDTA complex copper is also toxic to bacteria and algae.
7.6 Biological factors
Biological factors that affect the toxicity of heavy metal ions in water include biological age, size, weight, growth period, and tolerance. For example, the 96-h 50% lethal concentration of Cd is 2, 5, and 7 µg/L for carp younger than 10 days, carp aged 10–20 days, and carp aged more than 20 days, respectively.
When heavy metals enter the natural water environment, they can only migrate and transform among various forms through adsorption-desorption, dissolution-precipitation and oxidation-reduction, and finally stay in the water environment for a long time in one or more forms, resulting in permanent potential harm (Zhou et al.,2005). With the change of environmental conditions, the toxicity of some heavy metals may be strengthened or weakened. For example, the granular heavy metals deposited in the sediment will be re released into the water body when the redox potential changes, resulting in secondary pollution (Daniele et al., 2008). The toxicity of heavy metals to aquatic organisms is a very complex process, and the combined toxicity between heavy metals and between heavy metals and other organics is even more complex. Therefore, the research on the toxicity of heavy metals to aquatic organisms has a long way to go; At the same time, the research on the tolerance mechanism of aquatic organisms will also be of far-reaching significance in the prevention of heavy metal pollution.
With the development of science and technology and the deepening of ecotoxicological research on heavy metals in water, it is believed that some new tolerance mechanisms of aquatic organisms to heavy metals will be put forward. Especially in the low-dose and long-term exposure of heavy metals, it is of great significance to deeply understand the toxic mechanism of heavy metals and the health status of aquatic animals. The following suggestions are put forward for the future research on the ecotoxicological effects of heavy metals on aquatic organisms: (1) Due to the significant internal relationship between sediment particles and suspended particles in water and the enrichment, migration and bioavailability of heavy metals, the research on the bioaccumulation model of heavy metals in sediment and suspended particles in water can be strengthened and further study, predict and understand the response and change of organisms to heavy metals in water environment. (2) Because the harmful effects of some aquatic organisms are the result of the long-term effects of toxic heavy metals, the concentration of heavy metals in the water environment is usually lower than the acute poisoning dose and is difficult to detect, but even low concentration levels can have a significant impact on the growth, reproduction and survival of aquatic organisms (Sfakianakis et al., 2015). Therefore, we should carry out comprehensive research on acute toxicology test, subacute toxicology test and chronic toxicology test, analyze and establish a database of various diseases caused by aquatic organisms over time, so as to provide more basis for formulating water environmental sanitation standards. (3) Strengthen multi-disciplinary cooperation, carry out in-depth research on the ecotoxicological effects of different forms of heavy metals and compound pollution coexisting with other pollutants on aquatic organisms and the tolerance mechanism of aquatic organisms themselves, and accurately locate the key factors of heavy metal injury and biological tolerance. It is of great significance for the formulation of environmental quality standards for heavy metals, the establishment of a complete and reliable water quality risk assessment system, the remediation and treatment of heavy metal pollution in water, biological evolution and improvement, etc.
This work was supported by the National Natural Science Foundation of China (Grant No. 31472288) to Jiting Wang; the Shandong Science and Technology Development Plan Project to Jiting Wang (Grant No. 2014GGH210010); the Key R&D Projects of Shandong Province to Jiting Wang (Grant No. 2019GNC106078); and Funds of Shandong “Double Tops” Program.
Author contribution statement
Yanli Wang: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Qi Li: Conceptualization,Writing – original draft,Writing – review & editing. Xiao Yun: Resources, Writing – review & editing. Jie Zhou: Resources, Writing – review & editing. Jiting Wang: Conceptualization, Methodology, Writing – review & editing, Project administration, Supervision.
Declaration of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Al-Rashdi, B. A. M., Johnson, D. J., Hilal, N., 2013. Removal of heavy metal ions by nanofiltration. Desalination 315: 2-17.
View ArticleAntonio, S., Fabio, M., Marco, A., Roberta, P., Giuseppina, C., Daniele, T., Vincenzo, B., Lombardo, B. M., Salvatore, S., Veronica, M., 2016. Toxic effects of zinc chloride on the bone development in Danio rerio (Hamilton, 1822). Front. Physiol. 7, 56.
View ArticleArias, M., Barral, M. T., Mejuto, J. C., 2002. Enhancement of copper and cadmium adsorption on kaolin by the presence of humic acids.Chemosphere 48, 1081-1088. 00169-8
View ArticleAwual, M. R., Ismael, M., Yaita, T., El-Safty, S. A., Shiwaku, H., Okamoto, Y., Suzuki, S., 2013. Trace copper(Ⅱ) ions detection and removal from water using novel ligand modified composite adsorbent.Chem. Eng. J. 222, 67-76.
View ArticleBaskan, M. B., Pala, A., 2011. Removal of arsenic from drinking water using modified natural zeolite.Desalination 281, 396-403.
View ArticleBian, B.,Zhou, Y., Fang, B., 2016. Distribution of heavy metals and benthic macroinvertebrates:Impacts from typical inflow river sediments in the Taihu Basin.,China. Ecol. Indic. 69, 348-359.
View ArticleBjerselius, R., Winberg, S., Winberg, Y., Zeipel, K.,1993. Ca2+ protects olfactory receptor function against acute Cu(II) toxicity in Atlantic salmon. Aquat. Toxicol. 25,125-137. 90024-U
View ArticleCamus, L., Jones, M. B., Børseth, J. F., Grøsvik, B. E., Regoli, F., Depledge, M. H., 2002. Total oxyradical scavenging capacity and cell membrane stability of haemocytes of the Arctic scallop, Chlamys islandicus,following benzo (a) pyrene exposure. Mar. Environ. Res. 54 (3-5), 425-430. 00140-X
View ArticleChandurvelan, R., Marsden, I., Gaw, S., Glover, C., 2013. Waterborne cadmium impacts immunocytotoxic and cytogenotoxic endpoints in green-lipped mussel, Perna canaliculusAquat. Toxicol. 142-143C, 283-293. PMid:24077184
View Article PubMed/NCBIDaniele, M.,Maria, F. B., Angela, L., Pini, S., 2008.Heavy metals in sediments from canals for water supplying and drainage:Mobilization and control strategies.J. Hazard. Mater. 161(2), 723-729. PMid:18486335
View Article PubMed/NCBIDeliyanni, E. A., Peleka, F., Matis, K. A., 2007. Removal of zinc ion from water by sorption onto iron-based nanoadsorbent. J. Hazard. Mater. 141, 176-184. PMid:16916577
View Article PubMed/NCBIDing, W. Q.,Liu, D. Q.,Ge, F.,Chen, C. Y., Rao, J., Zhou, A. T., 2012. The molecular mechanism by heavy metal stress in fish. Chin. J. Biol. 29(2), 84-87 (in Chinese).
Du, J. G.,Zhao, J., Chen, B.,Chen, M., Zhou, T., Yu, W., Ma, Z., Hu, W., 2013. Assessing eological risks of heavy metals to marine organisms by species sensitivity distributions.Asian J. Ecotoxicol. 8(4), 561-570 (in Chinese).
Fan, Q., He, J., Xue, H., Lv, C., Sun, Y., Shen, L., 2008. Heavy metal pollution in the Baotou section of the Yellow River, China. Chem. Spec. Bioavailab. 20, 65-76.
View ArticleFu, H. X., Liu, Y., Dong, Z. Y., Li, Y., 2016. Research Progress on ecotoxicological effects of compound pollution of antibiotics and heavy metals.Environ. Eng. 34, 60-63.
García-Alonso, J., Rodriguez-Sanchez, N., Misra, S. K., Jones, E. V., Croteau, M. N., Luoma, S. N., Rainbow, P. S., 2014. Toxicity and accumulation of silver nanoparticles during development of the marine polychaete Platynereis dumerilii. Sci. Total Environ. 476-477, 688-695. PMid:24514586
View Article PubMed/NCBIGrundy, M. M., Moore, M. N., Howell, S. M., Ratcliffe, N. A., 1996. Phagocytic reduction and effects on lysosomal membranes by polycyclic aromatic hydrocarbons, in haemocytes of Mytilus edulis.Aquat. Toxicol. 34 (4), 273-290. 00044-5
View ArticleGuo, Y., Hua, J., Yang, L., Zhou, B., 2015. Content of heavy metals in water samples from the Three Gorges Reservoir area and its toxicity to zebrafish embryonic development. Acta Hydrobiologica Sinica 39, 885-892.
Hannam, M. L., Bamber, S. D., Sundt, R. C., Galloway, T. S., 2009. Immune modulation in the blue mussel Mytilus edulis exposed to North Sea produced water. Environ. Pollut. 157, 1939-1944. PMid:19217195
View Article PubMed/NCBIHarguinteguy, C. A.,Cirelli, A. F., Pignata, M. L., 2014. Heavy metal accumulation in leaves of aquatic plant Stuckenia filiformis and its relationship with sediment and water in the Suquía river (Argentina). Microchem. J. 114,111-118.
View ArticleHix, S., Augusto, O., 1999. DNA methylation by tert-butyl hydroperoxideiron (Ⅱ): a role for the transition metal ion in the production of DNA base adducts.Chem.-Biol. Interact. 118 (2), 141-149. 00079-4
View ArticleIhsanullah, Al-Khaldi, F. A., Abu-Sharkh, B., Abulkibash, A. M., Qureshi, M. I., Laoui, T., 2016. Effect of acid modification on adsorption of hexavalent chromium (Cr(Ⅵ)) from aqueous solution by activated carbon and carbon nanotubes. Desalin. Water Treat. 57, 7232-7244.
View ArticleJakubowski, Marek., 2015. Handbook on the Toxicology of Metals [M].4 Edition.
Jayaraj, Y. M., Aparanji, M., Nimbargi, P.M., 1992. Amelioration of heavy metal toxicity on primary productivity of aquatic ecosystems by calcium, magnesium and iron. Environ. Ecol. 10, 667-676.
Jie, L., Ma, L.,Yin, S., Hong, F., 2008. Effects of Ce 3+ on conformation and activity of superoxide dismutase. Biol. Trace Elem. Res. 125(2), 170-178. PMid:18521547
View Article PubMed/NCBIKim, B. M.,Rhee, J. S.,Jeong, C. B.,Seo, J. S., Park, G. S., Lee, Y. M., Lee, J. S., 2014.Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopusjaponicus.Comp. Biochem. Phys. C 166(1), 65-74. PMid:25058597
View Article PubMed/NCBIKlotz, R. L., 1981. Algal response to copper under riverine conditions. Eviron. Pollut. Series A. 24, 1-19. 90118-5
View ArticleLes, A., Walker, R. W., 1984. Toxicity and binding of copper, zinc, and cadmium by the blue-green alga, Chroococcus paris. Wat. Air Soi1 Pollut. 23, 129-139.
View ArticleLeszkowicz, A., Baldacini, O., Keith, G., Dirheimer, G., 1987. Stimulation of rat kidney, spleen and brain DNA- (cytosine-5) -methyltransfby divalent cobalt ions. Biochimie 69, 1235-1242. 90151-9
View ArticleLeung, H., M., Leung, A, O., Wang, H. S., Ma, K. K., Liang, Y., Ho, K. C., Cheung, K. C., Tohidi, F., Yung, K. K. L., 2014. Assessment of heavy metals /metalloid (As,Pb,Cd,Ni,Zn,Cr, Cu,Mn) concentrations in edible fish species tissue in the Pearl River Delta (PRD), China. Mar. Pollut. Bull. 78, 235-245. PMid:24239097
View Article PubMed/NCBILi, M., Liu, Q. J., 2010. Characteristics and assessment of heavy metals pollution in Poyang Lake.J. Nanchang Univ. 5, 486-494.
Li, R., Yang, J. Q., Sun, X. J., Shi, K. L., Wu, W. S., 2017. Analytical methods of selenite(Ⅳ)/selenate(Ⅵ) in environmental water samples: A review. Environ. Chem. 36, 939-950.
Li, Z. H., Chen, L., Wu, Y. H., Li, P., Li, Y. F., Ni, Z. H., 2014. Alteration of thyroid hormone levels and related gene expression in Chinese rare minnow larvae exposed to mercury chloride.Environ. Toxicol. Pharmacol. 38, 325-331. PMid:25064382
View Article PubMed/NCBILiu, S. S., Chen, Y. Z., Zhang, L. D., Hua, G. M., Xu, W., Li, N., Zhang, Y., 2011.Enhanced removal of trace (Cr (Ⅵ)) ions from aqueous solution by titanium oxide-Ag composite adsorbents.J. Hazard. Mater. 190, 723-728. PMid:21514991
View Article PubMed/NCBILong, Y., Luo, Y., Xiao, J., Guo, Z., Chen, L., Xiao, Y., 2016. Research progress on the effects of heavy metal stress on fish. J. Southern Agr. 47, 1608-1614.
View ArticleLu, H. X., Xu, Y. J., 2011.Effects of cadmium on antioxidant enzyme activity and DNA damage in Sinonovacula constricta.Mar. Environ. Sci. 30(1), 96-101 (in Chinese).
Luo, Y. J., 2015. Study on toxic effect and fecundity of cadmium on Tilapia [D]. Nanjing, Nanjing Agricultural University.
Maloku, F., Ahmeti, A., Kopali, A., Doko, A., Sule, S., 2015. Water and sediment heavy metal pollution in Ereniku River of Kosovo. Albanian J. Agricult. Sci. 14, 137-148.
Mao, H.,Tan, F. Q.,Wang, D. H., Zhu, J. Q., Yang, W. X., 2012.Expression and function analysis of metallothionein in the testis of stone crab Charybdis japonica exposed to cadmium.Aquat. Toxicol. 124-125(12), 11-21. PMid:22885795
View Article PubMed/NCBIMeng, D., Wang, X., Wang, S., Han, G., Qian, Y., 2018. Review on combined toxicity of typical heavy metals and perfluorinated compounds in water environment on aquatic organisms. Asian J. Ecotoxicol.13, 13-22.
View ArticleMoiseenko, T.I., 2008. Aquatic Eeotoxicology: Theoretical principles and practical application. Water Resour. 35, 30-541.
View ArticleMu, W. N., 2017. Effects of combined exposure of low-dose tributyltin and cadmium on thyroid axis and antioxidant indexes of common carp [D]. Wuhan, Huazhong Agricultural University.
Munley, K. M., Brix, K. V., Panlilio, J., Deforest, D. K., Grosell, M., 2013. Growth inhibition in early life-stage tests predicts full life-cycle toxicity effects of lead in the freshwater pulmonate snail,Lymnaea stagnalis.Aquat. Toxicol. 128-129, 60-66. PMid:23274352
View Article PubMed/NCBINatale, F. D., Erto, A., Lancia, A., Musmarra, D., 2011. Mercury adsorption on granular activated carbon in aqueous solutions containing nitrates and chlorides. J. Hazard. Mater. 192, 1842-1850. PMid:21803490
View Article PubMed/NCBINtim, S., A., Mitra, S., 2012. Adsorption of arsenic on multiwall carbon nanotube- zirconia nanohybrid for potential drinking water purification. J. Colloid Interf. Sci., 375, 154-159. PMid:22424815
View Article PubMed/NCBIOikari, A., Kukkonen, J., Virtanen, V., 1992. Acute toxicity of chemicals to Daphnia magna in humic waters. Sci. Total Environ. 117-118,367-377. 90103-Y
View ArticleOlivares, H. G., Lagos, N. M., Gutierrez, C. J., Kittelsen, R. C., Valenzuela, G. L., Hidalgo Hillo, M. E., 2016. Assessment oxidative stress biomarkers and metal bioaccumulation in macroalgae from coastal areas with mining activities in Chile. Environ. Monit. Assess. 188, 1-11. PMid:26661961
View Article PubMed/NCBIPan, L., Ren, J., Liu, J., 2005. Effects of benzo
(k) fluoranthene exposure on the biomarkers of scallop Chlamys farreri. Comp. Biochem. Phys. C: Toxicology & Pharmacology 141, 248-256. PMid:16095977
View Article PubMed/NCBIPaul, N., Chakraborty, S., Sengupta, M., 2014. Lead toxicity on non-specific immune mechanisms of freshwater fish Channa punctatus.Aquat. Toxicol,152 (5), 105-112. PMid:24747082
View Article PubMed/NCBIPellegrini, M., Annick. L., Michèle, S., Roger, P., Robert, V., Liliane, P., 1993. Interactions between the toxicity of the heavy metal's cadmium, copper, zinc in combinations and the detoxifying role of calcium in the brown alga Cystoseira barbata. J. Appl. Phycol. 5,351-361.
View ArticlePeterson, H. G., 1991. Toxicity testing using a chemostat-grown green alga, Selenastrum Capricornutum. Plant. Toxic. Assess. 2:107-117.
View ArticleQin, H., Liu, A., Gu, W., Tao, H., Li, J., Li, B., Sun, S., Jiang, H., 2015. Acute toxicity of six heavy metals on three aquaculture organisms.Asian J. Ecotoxicol. 10, 287-296.
Qin, Q., Qin, S., Wang, L., Lei, W., 2012. Immune responses and ultrastructural changes of hemocytes in freshwater crab Sinopotamon henanense exposed to elevated cadmium.Aquat. Toxicol. 106-107, 140-146. PMid:22155426
View Article PubMed/NCBIQu, X., Alvarez, P. J. J., Li, Q., 2013. Applications of nanotechnology in water and wastewater treatment.Wat. Res. 47, 3931-3946. PMid:23571110
View Article PubMed/NCBIRafa, P., Pawe, G., Malgorzata, P., Arkadiusz, D., 2010. Relationship between chronic exposure to lead,cadmium and manganese,blood pressure values and incidence of arterial hypertension.Medycyna Pracy 61, 5-14. https:// doi.org/10.1111/j.1548-1387.2010. 01132.x
Rai, L. C., Singh, A. K., Mallick, N., 1990. Employment of CEPEX enclosures for monitoring toxicity of Hg and Zn on in situ structural and functional characteristics of algal communities of River Ganga in Varanasi, India. Ecotoxic. Enviorn. Saf. 20, 211-221. 90060-I
View ArticleRainbow, P. S., 1992. Accumulation of heavy metals by marine organisms and its significance. Mar. Envir. Sci. 11, 44-52.
Reddad, Z., Gerente, C., Andres, Y., Cloirec, P. L., 2002 Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ. Sci. Technol. 36, 2067-2073. PMid:12026994
View Article PubMed/NCBIRhee, J. S.,Kim, B. M.,Jeong, C. B.,Leung, K. M. Y., Park, G. S., Lee, J. S., 2013. Development of enzyme-linked immunosorbent assay (ELISA)for glutathione S-transferase (GST-S) protein in the intertidal copepod Tigriopus japonicus and its application for environental monitoring.Chemosphere,93(10), 2458-2466. PMid:24112658
View Article PubMed/NCBIRickwood, C. J., Galloway, T. S., 2004. Acetylcholinesterase inhibition as a biomarker of adverse effect: a study of Mytilus edulis exposed to the priority pollutant chlorfenvinphos.Aquat. Toxicol. 67, 45-56. PMid:15019250
View Article PubMed/NCBIRose, M., Fernandes, A., Mortimer, D., Baskaran, C., 2015.Contamination of fish in UK freshwater systems: risk assessment for human consumption. Chemosphere 122,183-189. . 2014.11.046 .2014.11.046 PMid:25532764
View Article PubMed/NCBIRossiello, M. R., Aresta, A. M., Prisco, M., Kanduc, D., 1991. DNA hypomethylation during liver cell proliferation induced by a single dose of lead nitrate. Bollettino Della Societa Italiana Di Biologia Sperimentale 67, 993-997. 90030-M
View ArticleRyvolova, M.,Krizkova, S.,Adam, V., Beklova, M., Trnkova, L., Hubalek, J., Kizek, R., 2011. Analytical methods for metallothionein detection.Current Analytical Chemistry 700(3), 243-261.
View ArticleRzymski, P., Niedzielski, P., Klimaszyk, P., Poniedzialek, B., 2014.Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ. Monit. Assess. 186, 3199-3212. PMid:24407963
View Article PubMed/NCBISalem, A., Sene, R. A., 2011. Removal of lead from solution by combination of natural zeolite-kaolin-bentonite as a new low-cost adsorbent.Chem. Eng. J. 174, 619-628.
View ArticleSfakianakis, D. G., Tsatsakis, A. M., Kentouri, M., Renieri, E.,2015. Effect of heavy metals on fish larvae deformities:A review.Environ. Res.137, 246-255. PMid:25594493
View Article PubMed/NCBIShamsijazeyi, H., Kaghazchi, T., 2010. Investigation of nitric acid treatment of activated carbon for enhanced aqueous mercury removal. J. Ind. Eng. Chem. 16, 852-858.
View ArticleSheng, Z.,Zhu, J. Q.,2014. Research progress on molecular toxicology of meta llothionein in aquatic animals.Chin. J. Biol. 31(2), 77-81 (in Chinese).
View ArticleStackhouse, R. A., Benson, W. H., 1989. The effect of humic acid on the toxicity and bioavailability of trivalent chromium. Ecotox. Environ. Saf. 17,105-111. 90014-6
View ArticleSun, N., Huang, W., Yu, H., 2015. Analysis and evaluation of enrichment characteristics of heavy metals in sediments and marine organisms in Zhanjiang port. Mar. Envir. Sci. 34, 669-672.
Sun, Y. M., Ma, Z. Y., Huang, H. P., 2015. Contamination and assessment of heavy metals shellfish in the intertidal zone of sea islands in China.China Environ.Sci. 35(2), 574-578 (in Chinese).
Tang, J., Tang, Y., Sun, H., Li, J., Wu, Y., Zhao, H., 2013. Damage effects of single factor and combined toxicity of heavy metals Cu2+ and Pb2+ on Loach egg DNA. Acta Hydrobiologica Sinica, 37 (3), 501-506.
Thomas, R. E., Lindeberg, M., Harris, P. M., Rice, S. D., 2007. Induction of DNA strand breaks in the mussel (Mytilus trossulus) and clam (Protothaca staminea) following chronic field exposure to polycyclic aromatic hydrocarbons from the Exxon Valdez spill.Mar. Pollut. Bullet. 54, 726-732. PMid:17328928
View Article PubMed/NCBITolins, M., Ruchirawat, M., Landrigan, P., 2014. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure.Annals Global Health 80, 303-314. PMid:25459332
View Article PubMed/NCBITopcuoglu, S., Kirbasoglu, C., Gungor, N., 2002. Heavy metals in organisms and sediments from turkish coast of the black sea,1997-1998.Envir. Int. 27, 521-526. 00099-X 00099-X
View ArticleTripathi, S.,Mishra, B. B.,Tripathi, S. P., 2012. Effect of heavy metal cadmium sulphate on the toxicity and biochemical parameters of reproductive cycle of Colisa fasciatus.Researcher 4(4), 65-68.
Tubbing,D. M. J., Santhagens, L. R., Admiraal, W., Beelen, P. V., 1993. Biological and chemical aspects of differences in sensitivity of natural populations of aquatic bacterial communities exposed to copper. Envir. Toxical. Wat. Qual. 8, 191-125.
View ArticleVandegehuchte, M. B.,Vandenbrouck, T.,Coninck, D. D.,de coen W.,2010. Gene transcription and higher-level effects of multigenerational Zn exposure in Daphia magna.Chemosphere,2010,80(9), 1014-1020. PMid:20580408
View Article PubMed/NCBIVijayavel, K., Gopalakrishnan, S., Ramsn, T., Thilagam, H., 2009. Immunotoxic effects of nickel in the mud crab Scylla serrata. Fish Shellfish Immunol. 26 (1), 133-139. PMid:19046900
View Article PubMed/NCBIVukovi, G. D., Marinkovi, A. D., oli, M., Risti, M., Aleksi, R., Peri-Gruji, A. A., Uskokovi, P. S., 2010. Removal of cadmium from aqueous solutions by oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes.Chem. Eng. J. 157, 238-248.
View ArticleWaisberg, M., Joseph, P., Hale, B., Beyersmann, D., 2003. Molecular and cellular mechanisms of cadmium carcinogenesis.Toxicology, 192, 95-117. 00305-6
View ArticleWang, T., Wei, R., An, X., Dong, F., Jia, X., Ma, L., 2015. Accumulative effects of heavy metal lead in crucian carp body. J. Hebei United University 37, 122-126.
Xing, W., 2016. Histological damage and genotoxic effects of heavy metals lead and chromium on Loach [D]. Yan'an, Yan'an University.
Yang, S., Huang, Y. J., Zhang, M., Chen, Z., Xie, J. C., 2015. Ecophysiological effects of heavy metals on insects.Acta Entomologica Sinica 58(4), 427-436 (in Chinese).
Yang, X. Q., 2011. Methodology of dissolved trace metals measurements in natural waters and distribution of dissolved Cd in the Jiulong River-Estuary-Coastal seawater [D]. Xiamen: Xiamen University.
Yang, Y., 2015.Combined toxicity of typical synthetic pyrethroids and typical metals to zebrafish [D]. Hangzhou: Zhejiang University.
Yavuz, O., Altunkaynak, Y., Fuat, G., 2003. Removal of copper,nickel, cobalt and manganese from aqueous solution by kaolinite.Water Res. 37, 948-952. 00409-8
View ArticleYohannes, Y. B.,Ikenaka, Y., Nakayama, S. M. M., Saengtienchai A., Watanabe, K., Ishizuka, M., 2013. Organochlorine pesticides and heavy metals in fish from Lake Awassa, Ethiopia: insights from stable isotope analysis. Chemosphere 91, 857-863. PMid:23422170
View Article PubMed/NCBIZhang, C., Song, C., Hu, G., Meng, S., Fan, L., Qiu, L., Liu, Y., 2018. Research progress on pollution status and elimination technology of heavy metals in fishery water in China. China agronomy bulletin 34, 141-145.
Zhang, Y., Liu, Z., Wang, Y., Yan, Z., Wang, H., Yang, N., 2010. Combined toxicity of Cu 2+ and Cd 2+ on early embryonic development of zebrafish. Environ. Sci. Res. 23, 1415- 1420.
Zhao, Y., 2010. Toxic effects of several heavy metals on guppies [D]. Jinan, Shandong, Shandong Normal University.
Zheng, Q., Han, C., Zhong, Y., Wen, R., Zhang, J., 2014. The determination and evaluation of heavy metal content in Polyodon spathula,Aristiehthys nobilis and Hypophthalmichthys molitrix from Meizhou Areas.J. Jiaying University 32, 52-57.
Zhou, J. M., Dang, Z., Cai, M. F., Situ, Y., Liu, C. Q., 2005. Speciation distribution and transfer of heavy metals in contaminated stream waters around dabaoshan mine.Res. Environ. Sci. 18(3), 5-10 (in Chinese).
