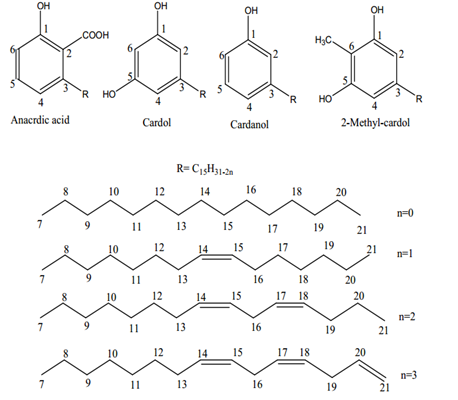
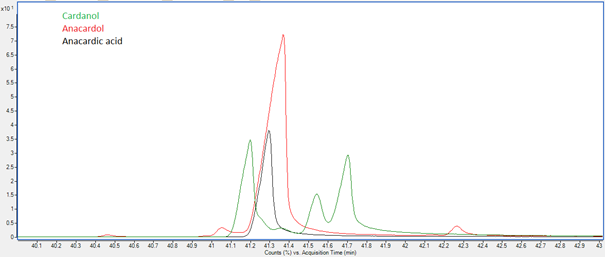
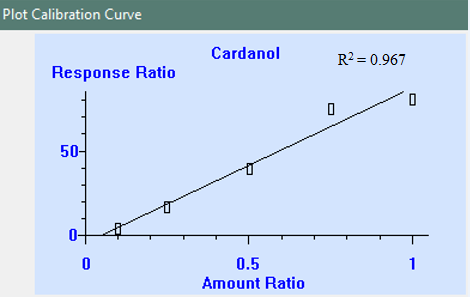
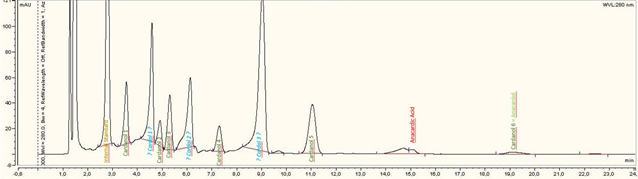
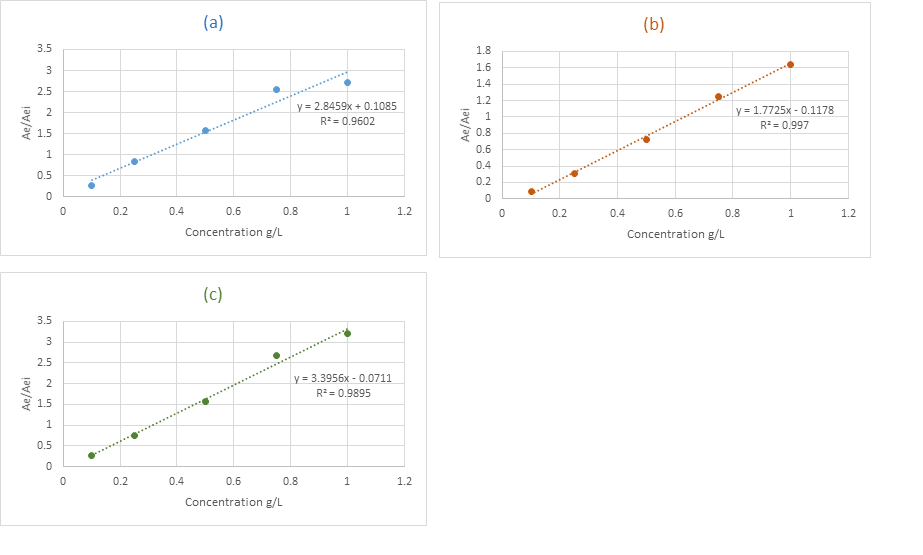
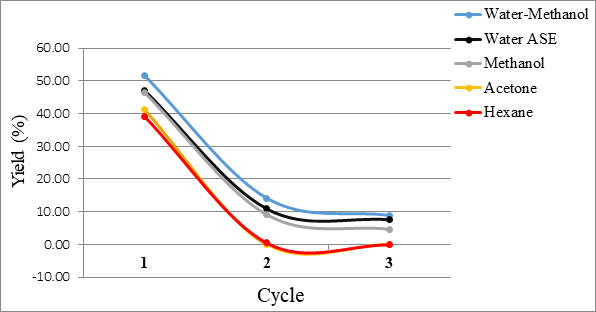
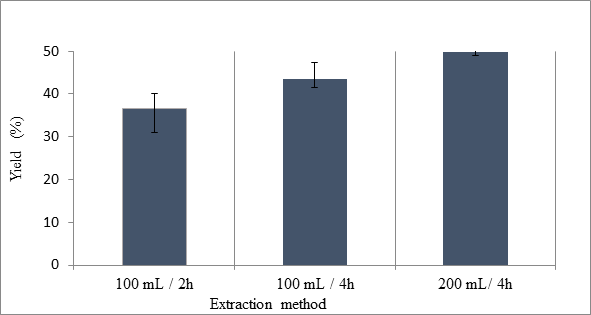
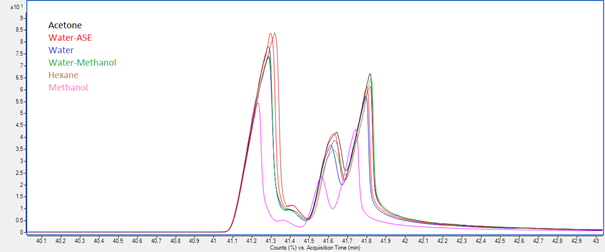
Jeremy Valette b, Alfredo Napoli b, Noel Durand c, Didier Montet c
a. Institut Polytechnique Rural de Formation et Recherche Appliquée (IPR/IFRA) de Katibougou, 22 Route de Koulouba, Dar-salam, Bamako, Mali
b. CIRAD, UR Biowoeb, 73 rue Jean-François Breton, 34398 Montpellier Cedex 5, France
c. Qualisud, Univ Montpellier, Avignon Université, CIRAD, Institut Agro, IRD, Université de La Réunion, Montpellier, France