Pravat Manjari Mishra
Email: pravatmanjari@yahoo.co.in or pravatmanjari@immt.res.in
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 5 ISSUE: 2
Page No: 44-50
Pravat Manjari Mishra
Email: pravatmanjari@yahoo.co.in or pravatmanjari@immt.res.in
Pravat Manjari Mishraa*; Santosh Kumar Sahooa; Deepak Kumar Padhia
a Environment and SustainabilityDepartment, CSIR-Institute of Minerals and Materials Technology, Bhubaneswar-751013, Odisha
Elzbieta Regulska(e.regulska@uwb.edu.pl)
Yuanguo Xu(xuyg@ujs.edu.cn)
Chongfei Yu(chongfei1987@163.com)
Y M Hunge(yuvihunge@gmail.com)
Pravat Manjari Mishra, Biomimetic synthesis of Ag/TiO2 nanocomposites using a natural surfactant for effective degradation of an environmental pollutant(2020)Journal of Earth Sciences & Environmental Studies 5(2) pp:44-50
The present paper involves the synthesis of bio-based Ag/TiO2 nanocomposites using both dry and fresh leaf extracts of Averrhoa carambola L. as reducing, capping and stabilizing agents. The nanocomposites are characterized by UV-vis DRS, XRD, TEM and EDX spectroscopy. TEM analysis shows the formation of somewhat uniformly distributed AgNPs on the surface of TiO2. From the UV-vis DRS studies and band gap energy calculation, it is confirmed that Ag/TiO2 DLE is more active than Ag/TiO2 FLE. The photocatalytic activity of the Ag/TiO2 nanocomposites prepared by both the leaf extracts was studied towards the degradation of methyl orange (MO). It was found that both FLE and DLE induced Ag/TiO2 exhibited higher efficiency towards photocatalytic degradation of MO as compared to chemically prepared Ag/TiO2. It was observed that DLE induced Ag/TiO2 exhibited higher efficiency towards photocatalytic degradation of MO as compared to FLE induced Ag/TiO2.
Keywords: Biomimetic; Nanocomposites; Averrhoa carambola L.; photocatalytic activity .
The unconsumed dyes and toxic chemicals present in the industrial effluents are now the subjects of considerable concern of environmental remediation. Noble metal-doped metal oxide nanocomposites are considered as the areas of great research for the degradation of these environmental pollutants due to their high oxidation activity, good thermal stability and selectivity. Some nanocomposites showed excellent results towards environmental remediation [1-10].
Among them, Ag/TiO2 system is of immense importance and has been a subject of interest in the field of photocatalytic degradation of environmental pollutants. Khan et al. studied the photodecomposition of methylene blue (MB) in an aqueous solution using synthesized Ag-TiO2 nanocomposites under visible light irradiation [11]. The excellent photocatalytic degradation efficiency of Ag–TiO2 microwires were studied by Mandal and Bhattacharyya, 2012 [12]. Nainani et al. studied the photocatalytic efficiency of Ag-TiO2 towards degradation of methyl orange [13]. Enhancement in the photocatalytic oxidation of oxalic acid by silver deposition on a TiO2 surface is studied by Szabó-Bárdos et al. [14].
But the chemical methods which are employed for the synthesis of Ag/TiO2 nanocomposites, involve the use of harmful toxic chemicals. Thus environmental friendly processes need to be adopted for the synthesis of these nanocomposites. Jiang et al., 2013 synthesized two-dimensional TiO2@Ag heterojunction structure using edible corn cripsy, which exhibited efficient photocatalytic activity towards the degradation of methylene blue (MB) [15]. Au/TiO2 and Ag/TiO2 composites synthesized using Citrus limon plant extracts, showed excellent photocatalytic activity towards degradation of organic dye [16]. But very less literature is available on the synthesis of Ag/TiO2 composites by biomimetic approaches. Earlier, we have reported the synthesis of Au/TiO2 using aqueous leaf extract of C. tamala and its remarkable photocatalytic activity towards the degradation of MO [17].
In the present study, we have first time used aqueous leaf extract (both dry and fresh leaf extract) of Averrhoa carambola L. (Order Oxalidales, family Oxalidaceae) for the biomimetic synthesis of Ag/TiO2 nanocomposites. The biomolecules such as reducing sugar, proteins, flavonoids and mainly the carambolaflavone present in the leaf extracts act as reducing, capping and stabilizing agents in the synthesis Ag/TiO2 nanocomposites [18, 19]. The biosynthesized Ag/TiO2 composites showed remarkable photocatalytic activity towards degradation of MO.
2.1 Materials and chemicals used
AgNO3, H2SO4 (0.01 M), NaOH (0.01 N) and C2H5OH (absolute) were purchased from NICE chemicals Pvt. Ltd, Qualigens, Rankem and Merck respectively.
2.1 Preparation of aqueous leaf extract (LE)
FLE (Fresh leaf extract) was prepared by boiling 10 g of thoroughly washed fresh leaves of A. carambola with 100 mL of de-ionised water at 500C for 30 min. and filtered through Whatman 1 filter paper. DLE (Dry leaf extract) was prepared by boiling 2 g of shade-dried leaf powder with 100 mL of ultrapure water at 500C for 15 min and filtered.
2.2 Preparation of 2 wt% Ag/TiO2 nanocomposite
1 gm of Degussa P-25 TiO2 was mixed with 20 mL of distilled water in a beaker and stirred for 30 mins. 187 mL of 1 mM AgNO3 solution (0.02g Ag) was added dropwise for 2 hrs with vigorous stirring. The pH of the resulting suspension was maintained to 5.5 to 5.6 by adding 0.01 N NaOH solution. Then 47 mL of aqueous LE (equivalent to ¼ th of AgNO3 solution used-since this concentration was found to be appropriate from spectroscopic and kinetic studies, Fig. 1) was added at a time to suspension and stirred for 4 hrs vigorously. The colour of the suspension was changed from white to brown and then it was centrifuged. The residue was washed for two times with DI water followed by centrifugation. Finally, the residue was washed with ethanol and centrifuged to collect the residue. The residue was dried overnight in an incubator at 300 C. Then, the residue was subjected to calcination in a muffle furnace for 4 hrs at 4500 C at a rising temperature rate of 50 C/ min. The two catalysts prepared by the above method are labeled as Ag/TiO2-FLE and Ag/TiO2-DLE. Similarly, Ag/TiO2-C is prepared using NaBH4 as reducing agent in place of LE.
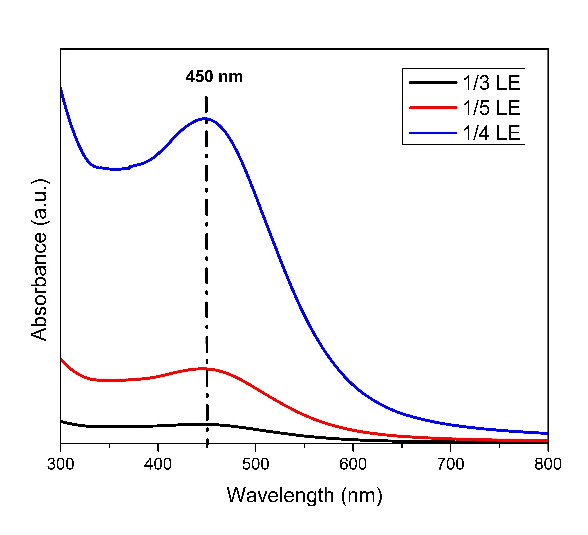
Fig. 1 DRS UV-Visible spectra of AgNPs varying the concentration of leaf extract
2.3 Photocatalytic activity
The photocatalytic activity of the catalysts was evaluated by the photocatalytic degradation of MO in aqueous solution. The photocatalysis was performed in sunlight during 10:00 am to 2:00 pm in sunny days of March.
The photocatalytic degradation of MO was carried out by taking 20 ml of 100 mg/l MO solution in a 100 ml flask, over 1.0 g/l of catalyst. The solution was exposed to sunlight with constant stirring. In all cases, the mixture was kept in the dark for 30 minutes to ensure that the adsorption-desorption equilibrium was reached before irradiation. After visible light irradiation, the sample was withdrawn from the suspension at every 60 minutes during irradiation for the determination of change in absorbance of MO. The catalyst was removed by centrifugation and MO content in the solution was analyzed quantitatively by light absorption at 464 nm.
2.4 Characterization
The nanocomposites were characterized by UV-Vis spectrophotometer (Jasco V-650), XRD (X’Pert Pro P Analytical X-ray diffractometer), TEM technique (Phillips, TECNAI FEI G2 TEM operating at 200Kv) equipped with EDX attachment.
3.1 DRS UV-Visible analysis
From the DRS UV-visible spectra of the samples (Fig. 2 A), it was found that the absorption spectra of Ag/TiO2 nanocomposites synthesized by both DLE and FLE, were shifted towards the visible region and this might be responsible for the enhancement of photocatalytic activity under solar radiation. Significantly enhanced absorption spectra in the visible region of longer wavelength at 500-600 nm are due to SPR effect of metallic AgNPs. The strong absorption at 200-340 nm is characteristic of TiO2. The enhanced absorbance intensity of Ag/TiO2 synthesized by DLE than that of Ag/TiO2 synthesized by FLE confirmed that more no of AgNPs are synthesized by DLE than FLE.
The band gap energy of TiO2, Ag/TiO2 DLE, Ag/TiO2 FLE were calculated by the Tauc approach using the equation 1 and shown in (Fig. 2 B) [20].
ahυ = A(hυ- Eg)n [1]
Where a= absorption coefficient
υ= light frequency
A = proportionality constant
Eg = band gap energy
n= Type of transition in a semiconductor.
It is a direct transition if n = ½ and indirect transition for n = 2. Here the value of n for all the synthesized compounds was taken as 1/2, which confirms that optical transition of nanocomposites are directly allowed [20]. The band gap energy of all the composites are approximated from the plot of (ahυ)n vs hυ by extrapolating the straight line to the X axis intercept. The band gap energies of TiO2, Ag/TiO2 FLE, Ag/TiO2 DLE were found to be 3.12, 2.98, 2.90 eV respectively (Fig. 2 B). The low band gap of Ag/TiO2 DLE and Ag/TiO2 FLE nanocomposites indicates that they have tendency towards visible light motivated photocatalytic ability.
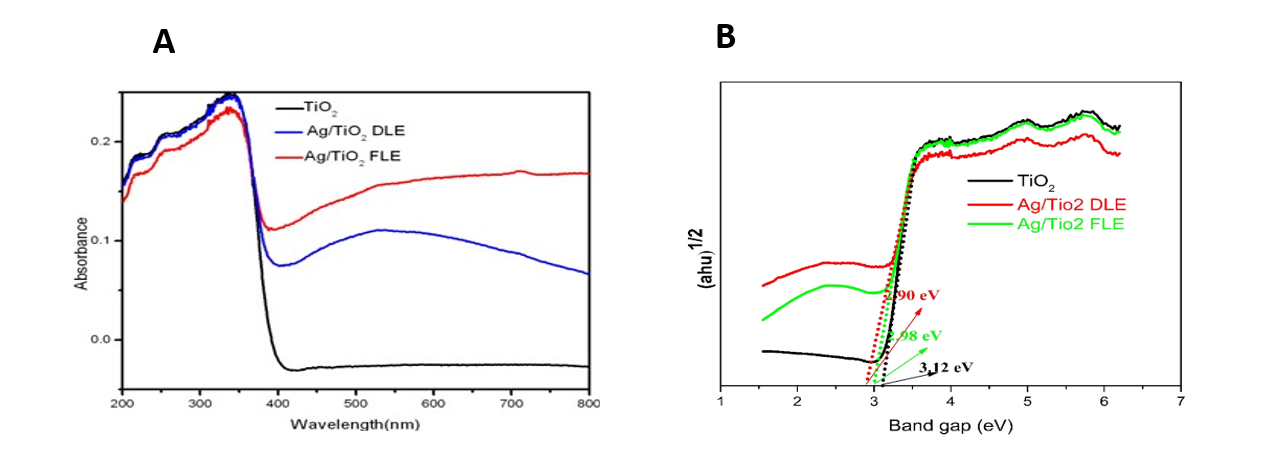
Fig. 2 (A) DRS UV-Visible spectra of TiO2 and Ag/TiO2 nanocomposites; (B) Estimated bandgap energy for TiO2, Ag/TiO2-DLE, Ag/TiO2-FLE nanocomposites.
3.2 XRD Analysis
For pure TiO2 all peaks are well indexed as the anatase phase (JCPDS card No. 83-2243). The XRD patterns of Ag/TiO2 nanocomposites (Fig. 3) almost coincide with that of pure TiO2, and no diffraction peaks is observed for Ag, thus suggesting that the metal particles are well dispersed on the surface of TiO2. Ag/TiO2 nanocomposites did not show any peak shift, indicating that the TiO2 matrix was well maintained as the anatase phase which indicates that the metal dopants are merely placed on the surface of the crystals without being covalently bonded into the crystal lattice. The presence of Ag on TiO2 is also confirmed by EDX spectra which detected Ag element. The average crystalline sizes were calculated by Scherrer equation and found to be 72.29 nm and 76.17 nm for FLE and DLE induced Ag/TiO2 nanocomposites respectively.
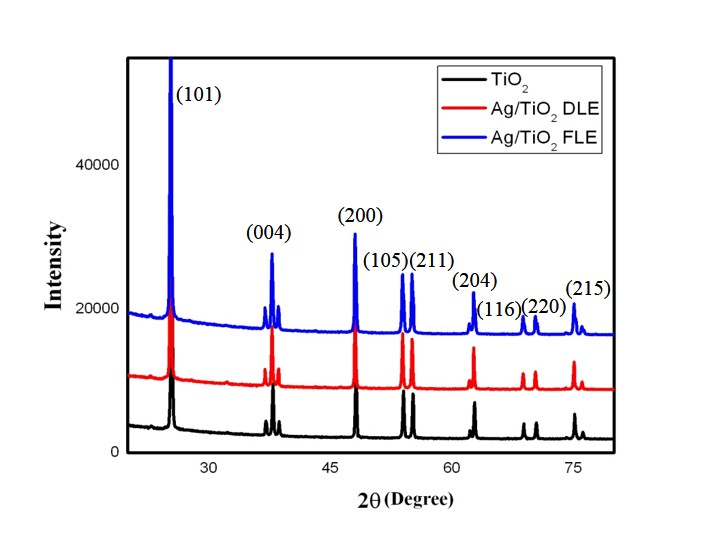
Fig. 3 XRD pattern of TiO2 and Ag/TiO2 nanocomposites
3.3 TEM analysis
The synthesized Ag/TiO2 nanocomposites were characterized by TEM which shows somewhat uniformly distributed AgNPs on titania (Fig. 4). TEM micrographs show a variation of AgNPs size in the range of 1-13 nm. The average particle size of AgNPs was found to be less than 10 nm in the case of both DLE and FLE induced NPs. The presence of silver was confirmed by EDX spectroscopy.
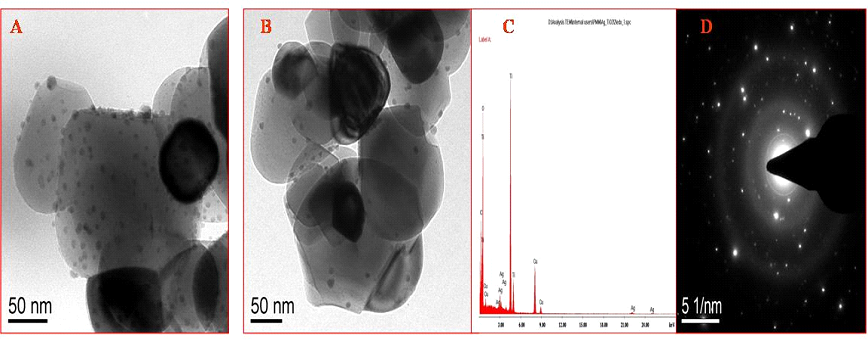
Fig. 4 (A) represents TEM image of Ag/TiO2 nanocomposite synthesized by FLE; (B) represents TEM image of Ag/TiO2 nanocomposite synthesized by DLE; (C) represents EDX of Ag/TiO2 nanocomposite; (D) shows its SAED pattern.
3.4 Photocatalytic activity
The photocatalytic activity of Ag/TiO2-FLE, Ag/TiO2-FLE and Ag/TiO2-C nanocomposites were tested for the degradation of MO in sunlight. The result of the MO degradation by the catalysts was presented in Fig. 5 A. From the study, it was found that Ag/TiO2-DLE exhibited higher efficiency towards photocatalytic degradation of MO than Ag/TiO2-FLE, whereas chemically prepared Ag/TiO2 C exhibited less photocataytic activity in comparison to all. In 30 min. of reaction time, Ag/TiO2-DLE showed 67.2% degradation of MO, whereas Ag/TiO2-FLE showed 53.7% of MO degradation. The percentage of degradation of MO increases with an increase in time in the case of all the catalysts. In 3 hr, the percentages of degradation of MO by Ag/TiO2-DLE, Ag/TiO2-FLE and Ag/TiO2-C were 78.85, 72.9 and 63.2, respectively. The enhanced catalytic activity of Ag/TiO2-DLE than that of Ag/TiO2-FLE confirmed that the more number of AgNPs are synthesized in Ag/TiO2-DLE than in Ag/TiO2-FLE. This is because more number of phytoconstituents of A. carambola leaves are available in DLE than FLE. More the number of biomolecules, stronger the interaction between biomolecules and the nanoparticles. The biomolecules act both as reducing and stabilizing agents for synthesis of more AgNPs. The AgNPs are capped with the biconstituents of A. carambola and the agglomeration between AgNPs is prevented.
With an increase in no. of AgNPs, in the case of Ag/TiO2-DLE, the catalytic activity is enhanced compared to Ag/TiO2-FLE, due to the SPR effect of AgNPs. When the Ag/TiO2 nanocomposites are irradiated (Fig. 5 B) with visible light, the electron from the valance band (VB) of Ag migrates to the conduction band (CB), by absorbing a photon. As a result, the electrons and holes are formed on the surface of the AgNPs. Many researchers reported that due to the SPR effect, the electrons in CB of Ag transferred to the conduction band of TiO2 [21, 22]. Herein, the electrons generated on the surface of the AgNPs due to the SPR effect, migrated to the CB of TiO2 and there they get trapped by the adsorbed oxygen molecules leading to the formation of superoxide radicals. These superoxide radicals and the trapped electrons can combine to produce H2O2, finally forming hydroxyl radicals. The holes formed on the surface of the AgNPs are trapped by water molecules to produce reactive hydroxyl radicals. Those superoxide radicals and hydroxyl radicals anions (strong oxidants) then oxidize the organic pollutant (MO) on the surface of the nanocomposite.
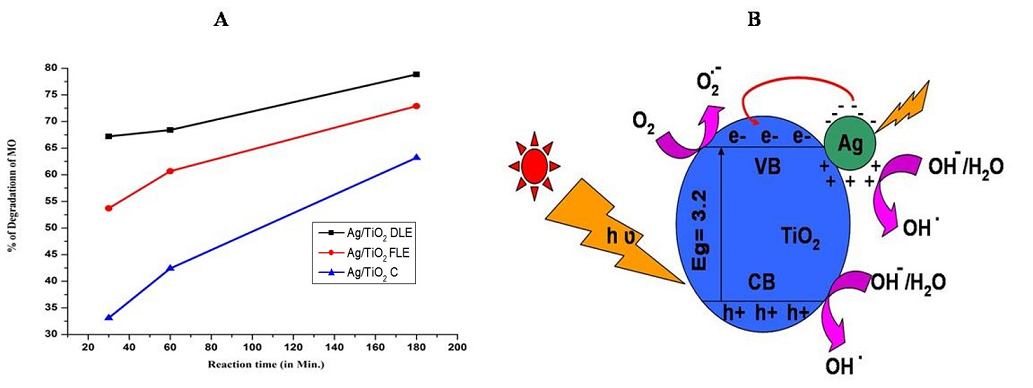
Fig. 5 (A) Degradation of MO using Ag/TiO2-FLE, Ag/TiO2-DLE and Ag/TiO2-C; (B) Schematic diagram of enhanced photocatalytic activity of Ag/TiO2 nanocomposite
This is the first report of the synthesis of Ag/TiO2 nanocomposites using both DLE and FLE of A. carambola. Ag/TiO2-DLE exhibited higher efficiency towards photocatalytic degradation of MO as compared to Ag/TiO2-FLE, due to the synthesis of more number of AgNPs by DLE than FLE, which is also evident from UV-Vis DRS studies of both the nanocomposites as well as from the estimated bandgap energy of Ag/TiO2-DLE, Ag/TiO2-FLE nanocomposites (Fig. 2 A & B). These biosynthesized Ag/TiO2 nanocomposites have shown comparable or higher photocatalytic activities towards degradation of MO in comparison to chemically prepared Ag/TiO2-C. This green, simple and cost-effective method could prove to be a better alternative to chemical synthesis methods and also effective for the large scale preparation of stable Ag/TiO2 nanocomposites.
The authors thank Director, CSIR-IMMT, for providing the facilities to carry out this work and CSIR for funding the project.
Xu YG, Liu J, Xie M, Jing LQ, Xu H, She XJ, Li HM, Xie JM. Construction of novel CNT/LaVO4 nanostructures for efficient antibiotic photodegradation. Chemical Engineering Journal 2019; 357:487-497.
View ArticleXu Y, Liu Q, Liu C, Zhai Y, Xie M, Huang L, Xu H, Li H, Jing J. Visible-light-driven Ag/AgBr/ZnFe2O4 composites with excellent photocatalytic activity for E. coli disinfection and organic pollutant degradation. Journal of Colloid and Interface Science 2017, 512:555-566 PMid:29100160
View Article PubMed/NCBITian Y, Zhou L, Zhu Q, Lei J, Wang L, Zhang J, Liu Y. Hierarchical macro-mesoporous g-C3N4 with an inverse opal structure and vacancies for high-efficiency solar energy conversion and environmental remediation. Nanoscale 2019, 11:20638-20647. PMid:31641721
View Article PubMed/NCBIHunge YM, Yadav AA, Dhodamani AG, Suzuki N, Terashima C, Fujishima A, Mathe VL. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrasonics sonochemistry 2020, 61:104849. PMid:31710997
View Article PubMed/NCBIHunge YM, Yadav AA, Liu S, Mathe VL. Sonochemical synthesis of CZTS photocatalyst for photocatalytic degradation of phthalic acid. Ultrasonics sonochemistry 2019, 56:284-289. PMid:31101264
View Article PubMed/NCBIHunge YM, Yadav AA, Mathe VL. Oxidative degradation of phthalic acid using TiO2 photocatalyst. Journal of Materials Science: Materials in Electronics 2018, 29:6183-6187.
View ArticleAli T, Hunge YM, Venkatraman A. UV assisted photoelectrocatalytic degradation of reactive red 152 dye using spray deposited TiO2 thin films. Journal of Materials Science: Materials in Electronics 2018, 29:1209-1215.
View ArticleHunge YM. Sunlight assisted photoelectrocatalytic degradation of benzoic acid using stratified WO3/TiO2 thin films. Ceramics International 2017, 43:10089-10096.
View ArticleHunge YM, Yadav AA, Mohite BM, Mathe VL. Photoelectrocatalytic degradation of sugarcane factory wastewater using WO3/ZnO thin films. Journal of Materials Science: Materials in Electronics 2018, 29:3808-3816.
View ArticleYadav AA, Hunge YM, Mathe VL, Kulkarni SB. Photocatalytic degradation of salicylic acid using BaTiO3 photocatalyst under ultraviolet light illumination. Journal of Materials Science: Materials in Electronics 2018, 29:5069-15073.
View ArticleKhan MM, Ansari SA, Amal MI, Lee J, Cho MH. Highly visible light active Ag@TiO2 nanocomposites synthesized using an electrochemically active biofilm: a novel biogenic approach. Nanoscale 2013; 5:4427-4435. PMid:23579384
View Article PubMed/NCBIMandal SS, Bhattacharyya AJ. Electrochemical sensing and photocatalysis using Ag-TiO2 microwires. J. Chem. Sci. 2012, 124:969-978.
View ArticleNainani R, Thakur P, Chaskar M. Synthesis of silver doped TiO2 nanoparticles for the improved photocatalytic degradation of methyl orange. Journal of Materials Science and Engineering B 2012; 2:52-58.
Szabó-Bárdos E, Czili H, Horváth A. Photocatalytic oxidation of oxalic acid enhanced by silver deposition on a TiO2 surface. Journal of Photochemistry and Photobiology A: Chemistry 2003, 154:195-201. 00330-1
View ArticleJiang B, Hou Z, Tian C, Zhou W, Zhang X, Wu A, Tian G, Pan K, Ren Z, Fu H (2013) A facile and green synthesis route towards two-dimensional TiO 2@ Ag heterojunction structure with enhanced visible light photocatalytic activity. Cryst. Eng. Comm. 2013; 15(29):5821-5827.
View ArticleLiang W, Church TL, Harris AT (2012) Biogenic synthesis of photocatalytically active Ag/TiO2 and Au/TiO2 composites. Green. Chem. 2012; 14:968-975.
View ArticleNaik GK, Mishra PM, Parida K. Green synthesis of Au/TiO2 for effective dye degradation in aqueous system. Chemical Engg. Journal 2013; 299:492-497.
View ArticleDasgupta P, Chakraborty P, Bala NN. Averrhoa Carambola: An Updated Review. Int. J. Pharm. Res. Rev. 2013; 2(7):54-63.
Mishra PM, Sahoo SK, Naik GK, Parida K. Biomimetic synthesis, characterization and formation mechanism of stable silver nanoparticles using Averrhoa carambola L. leaf extract. Materials Letters 2015, 160:566-571.
View ArticleD. K. Padhi, K. Parida. Facile fabrication of α-FeOOH nanorod/RGO composite: a robust photocatalyst for reduction of Cr(VI) under visible light irradiation. J. Mater. Chem. A. 2014, 2:10300-10312.
View ArticleChen Z, Fang L, Dong W, Zheng F, Shen M, Wang J. Inverse opal structured Ag/TiO2 plasmonic photocatalyst prepared by pulsed current deposition and its enhanced visible light photocatalytic activity. J. Mater. Chem. A 2014; 2:824-832.
View ArticleWang P, Tang Y, Dong Z, Chen Z, Lim TT. Ag-AgBr/TiO2/RGO nanocomposite for visible-light photocatalytic degradation of penicillin G. J. Mater. Chem A 2013; 1:4718-4727.
View Article