-
ANDREW J. GREGORY
PH:419.372.9369; Email: agregor@bgsu.edu
- THOMAS W. LIPP
Email: tomwlipp@gmail.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 1
Page No: 361-377
ANDREW J. GREGORY
PH:419.372.9369; Email: agregor@bgsu.edu
Email: tomwlipp@gmail.com
THOMAS W. LIPP†* AND ANDREW J. GREGORY†*
†School of Earth, Environment and Society, Bowling Green State University, Bowling Green, OH.
Jun Dong(zd_dongjun@zju.edu.cn)
Thomas W. Lipp and Andrew J. Gregory , Environmental Impacts of Energy Development on Prairie-Grouse and Sage-Grouse in the Continental United States(2018)SDRP Journal of Earth Sciences & Environmental Studies 3(1)
Land use for energy development is a necessary component of human land use that is prevalent on many landscapes globally. To date, energy demands have primarily been met through the burning of fossil fuels and biofuels in both first and third world countries. Recently new forms of energy development such as wind, solar and geothermal have increased prevalence to meet the ever growing energy demands. In the U.S., energy demands are still primarily met through coal, oil, and gas extraction as well as wind energy in the Midwest and Intermountain West regions. Oil, gas, and wind energy infrastructure is often built at high densities within the Midwest and Intermountain west; and has been attributed to population declines of local wildlife. Due to the considerable overlap in species distribution with oil, gas, and wind energy development, grouse species of the Tympanuchus and Centrocercus genera have particularly impacted. Impacts include disruption to the acoustic environment that they rely on for communication fidelity, reduced brood, hen and nest survival, and reduced lek attendance. In addition, the vast network of energy infrastructure comprised of roads, power lines, turbines, and pump jacks has increased local fragmentation and habitat loss for all grouse species. As energy demands continue to increase, and with multiple species of grouse nominated for listing under provisions of the Endangered Species Act, negative impacts to grouse attributed to energy development are likely to continue across much of the continental U.S. In this work, we summarize key findings of previous research by; identifying areas of spatial overlap between energy development and grouse habitat, describing the observed direct and indirect impacts that energy development has on grouse and identifying knowledge gaps to be addressed in future research.
Keywords: Avoidance, Energy Development, Fragmentation, Gas, Grasslands, Habitat, Impact, Oil, Prairie-Grouse, Sage-Grouse, Wind
Prairie-grouse and sage-grouse are iconic fauna of grassland and rangeland landscapes, and include Lesser Prairie-Chicken (Tympanuchus pallidicinctus), Greater Prairie-Chicken (Tympanuchus cupido), Sharp-tailed Grouse (Tympanuchus phasianellus), Attwater’s Prairie-Chicken (Tympanuchus cupido attwateri), Gunnison Sage-Grouse (Centrocercus minimus), Greater Sage-Grouse (Centrocercus urophasianus) and the now extinct Heath-hen (Tympanuchus cupido cupido). Currently these species, and several sub-species, occupy ranges which extend from Canadian territories in the north to the Texas Gulf Coast, and from California to the Canadian east coast. (Figure 1) [1; 2]. Prairie-grouse and sage-grouse (collectively referred to hereafter as grouse) have large home range requirements and are sensitive to anthropogenic disturbance, qualities which make them an umbrella and indicator species for rangeland management and conservation [3; 4].
Numerous species of grouse have been petitioned for listing under the Endangered Species Act (ESA) due to range and population declines over the last century. Currently, the Centrocercus minimus is listed as ‘Threatened’ under provisions of the ESA [5]. Similarly, the Tympanuchus cupido attwateri is listed as ‘Endangered’ under provisions of the ESA, and a recovery plan focused on the survival of the T. attwateri and conservation of its habitat is in place [6]. Both sage-grouse and the Lesser Prairie-Chicken are also imperiled, and have each been nominated multiple times for ESA listing. However, listing attempts for these species have not garnered the same level of support; e.g., in July of 2016, the T. pallidicinctus was delisted from ‘Threatened’ by the USFWS [7]. Also, in September 2015, the 8th attempt to list the C. urophasianus under provisions of the ESA resulted in a “Not Warranted” ruling, which was a reversal of the previous “Warranted but Precluded” ruling [8].
A major contributor to the near ubiquitous decline in grouse abundance is anthropogenic land use negatively affecting the availability, quality and distribution of large native rangelands [3; 9; 10]. One particularly intensive human land use that threatens vast tracts of native grassland is high intensity development for oil, gas, and wind energy resources [11; 12]. Petroleum and natural gas extraction are large contributors to U.S. energy consumption, and because of this, there is a constant trade-off between intense land activities such as petroleum extraction (369,000-2,114,000 ha/exajoule/year), natural gas extraction (150-880 ha/exajoule/year) and wildlife conservation [11]. In addition, as renewable energy begins to cement itself as a relevant energy contributor, use of renewable resources, like wind, are expected to increase in capacity by 2025 [13]. With the large amount of development taking place in the coming decade, grouse species are likely to be further impacted by energy development across much of their core distribution. This review aims to address the potential conflicts derived from the spatial overlap between grouse ranges and energy development in the U.S., as well as the relevant knowledge gaps and the need for further scientific investigation.
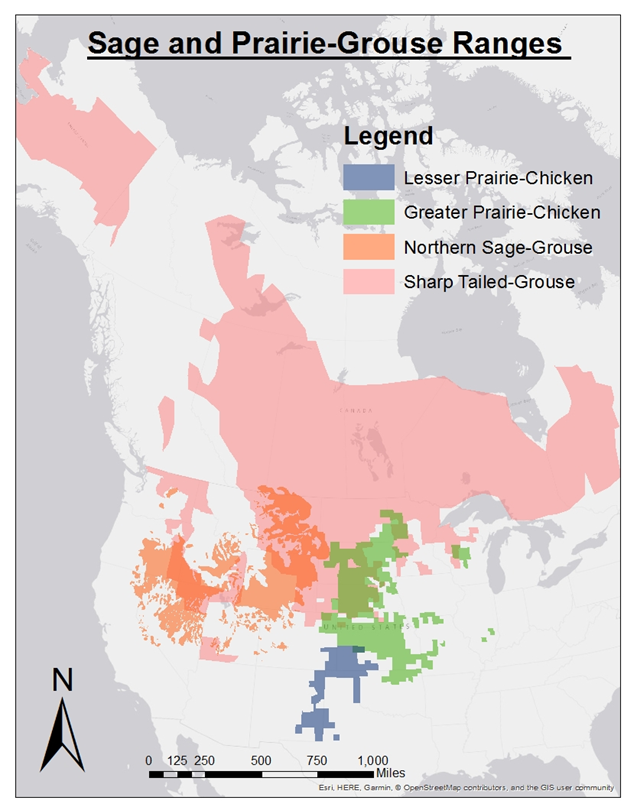
Figure 1: Distribution map for common grouse species in N. America. Species distribution for nearly all grouse in N. America has been reduced in the past century. Land alteration and degradation has reduced the availability of habitat outside of grouse distributions, and reduced the suitability of habitat within distributions.
We searched for literature using online databases including ISI Web of Knowledge and Google Scholar for information relevant to grouse and energy development. All literature searches were conducted from December 2014 - April 2018 using combinations of the key words: Lesser-Prairie Chicken, Prairie Grouse, anthropogenic, sound, acoustic, chronic noise, nest success, energy development, oil and gas, wind energy, fitness, habitat, west, land use, land cover, disease, home range, nest placement, survival, mortality, U.S., energy demand, LPC, energy efficiency and GPC, Sharp tailed grouse, Gunnison Sage Grouse, attenuation, climate change, and climate prediction. Additionally, we searched government databases for unpublished technical reports regarding status and condition of grouse species. We included a total of 106 sources of which 69 (65.1%) were peer-reviewed articles published in journals, 5 (4.7%) were books or book chapters, 15 (14.1%) were technical documents from various organizations including the U.S. Geologic Survey, U.S. Department of the Interior, Western Association of Fish and Wildlife Agencies, U.S. Fish and Wildlife Service and Colorado Division of Wildlife, 3 (2.8%) were meeting proceedings, 9 (8.5%) were theses or dissertations (in situations where a thesis or dissertation preceded the publication of a peer-review paper we censored the thesis or dissertation in favor of using the peer-reviewed paper citation, however we retain the thesis or dissertation in our count statistics), 2 (1.9%) were related news articles and 3 (2.8%) were interactive webpages.
We performed two additional searches using Google Scholar in November of 2015 and April 2018, independent of reviewed literature, to compare the temporal distribution of relevant publications for prairie-grouse and sage-grouse (Figure 2). Search one used the key word “Sage-Grouse” and search two used the key word “Prairie-Grouse”. Each search is a temporal distribution of the 100 most relevant publications provided by Google Scholar. The resulting distributions follow a similar pattern for each search with a gradual build up from 1950 until 2010 where it peaks, followed by a sharp drop off through 2018 (Figure 2).
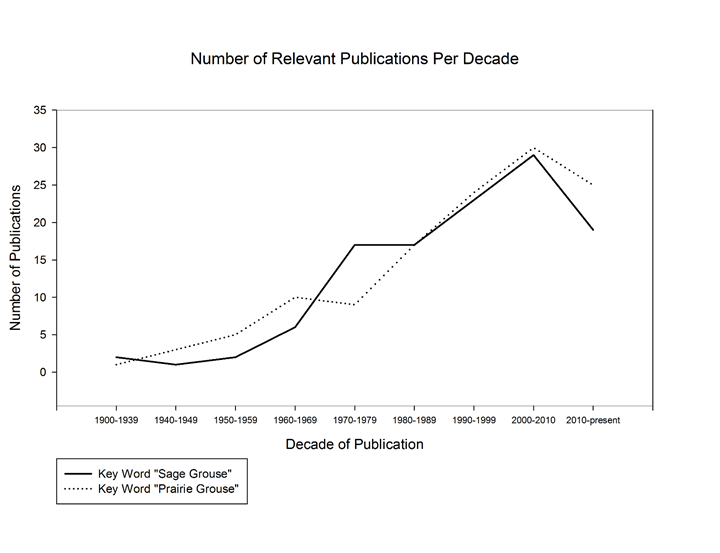
Figure 2: Results from additional search on Google Scholar comparing relevant results from key words “Sage-Grouse” and “Prairie-Grouse”. Both searches returned similar distribution of publications per decade with a gradual increase from 1900 – 2010 where the peak occurred. The sharp drop in publications following the peak in 2010 is likely due to search year (2018) and not a decrease in research effort.
Of the studies included in the review, 85 (80.2%) focused on grouse species. Of the species studied, we are categorizing C. urophasianus ‘sage-grouse’ (genus Centrocercus), and we are categorizing the T. cupido, T. phasianellus and T. pallidicinctus as ‘prairie-grouse’ (genus Tympanuchus). The distribution of species studies included in the review is well dispersed between sage-grouse and prairie-grouse (Figure 3).
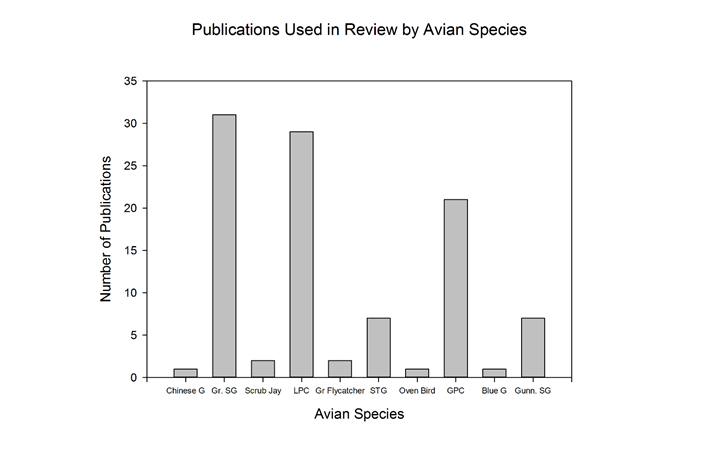
Figure 3: Number of publications used in review; compared by species. Centrocercus urophanasianus and Tymphanuchus pallidicinctus were the focal species of the greatest number of publications used in the review.
Of our reviewed literature, 46 (43.3%) studies observed the relationship between grouse species and energy development. These publications were key in developing our summary of energy development influences on grouse. Since wind, oil and gas resources are the most abundant energy resources in grouse ranges, we focused our literature search on these energy resources. Of our reviewed literature, C. urophanasianus had the most research involving oil and gas (13/27; 48.1%), while T. cupido had the most research involving wind energy (10/19; 52.6%) (Figure 4).
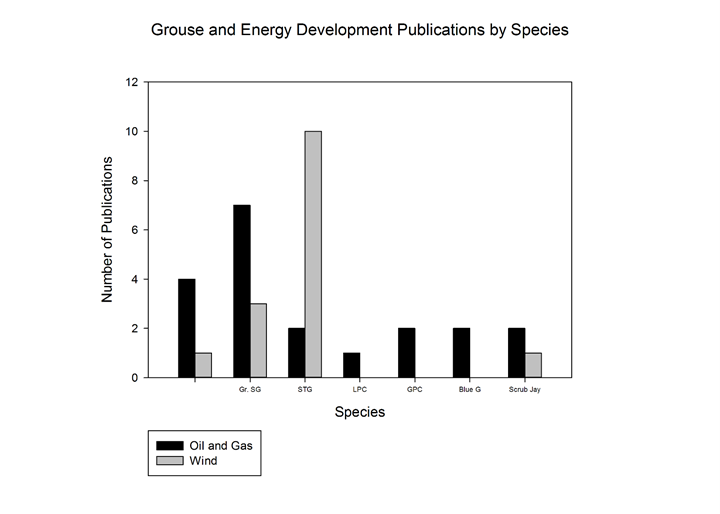
Figure 4: Number of publications used in this review, compared by species and energy resource. Centrocercus urophanasianus had the greatest number of publications involving any type of energy development, as well as the greatest number of publications involving oil and gas development specifically. Tympanuchus cupido had the greatest number of publications involving wind energy development.
From the sources used, we found a distinct increase in the number of studies published concerning energy development and grouse since 2000 (89.1%; 41/46). This bias might be due in part to current socioeconomic issues such as the increasing energy demands in the U.S. [14], the 2008 EPA benchmark to reach 20% wind generated power in the U.S. by 2030 [13; 15] or the attempts to list species such as the T. pallidicinctus and the C. urophanasianus under provisions of the ESA.
GROUSE RANGES AND OVERLAP WITH ENERGY DEVELOPMENT
WHERE ARE GROUSE?
Grouse once inhabited much of central North America and were unable to evade the influences of anthropogenic expansion [16; 17; 18]. Initially, grouse distributions expanded as human settlement broke up the landscape and added a moderate amount of cultivated agriculture—following the plow hypothesis [19]. However, as the ox and horse were replaced by the tractor, and agricultural land use intensified and came to occupy >50% of once rangeland dominated landscapes, rangelands available for grouse declined; furthermore, as rangeland area declined, cattle were grazed at higher densities which further degraded remaining ranges and grouse distributions contracted rapidly [20; 21]. At one point T. cupido inhabited grasslands as far spread as Ohio, North Dakota and Texas [2; 19; 20], but are now limited to the states of Kansas, Nebraska, South Dakota, Wisconsin, Colorado, Oklahoma and Minnesota [2; 20]. Similar stories can be told for other grouse species such as the T. pallidicinctus, which is now confined to portions of New Mexico, Oklahoma, Texas, Colorado and Kansas [22; 23]. Even the enigmatic C. urophanasianus has had its distribution constricted in every state in which it is found [24].
High quality prairie-grouse habitat is characterized by heterogeneous grasslands dominated by warm season grasses with visual obstruction readings between 4-8 [25; 26; 27]. For prairie-grouse, these conditions are most commonly associated with grasslands of the Midwest and Central Plains, which are typified by highly abundant grass and forb species with minimal forest cover [27; 28; 29; 30]. Sage-grouse occupy similar habitat to their prairie-grouse relatives, but vegetative composition within their range is heavily dominated by sagebrush (Artemisia spp.) [3; 18; 31; 32]. It is likely that this vegetative dominance plays an integral role in the habitat selection process for sage-grouse, as they have been observed to have stronger selection toward sagebrush vegetation than other landscape features [3; 31; 32].
In addition to vegetative characteristics, sage-grouse have been observed to select for gentle topography (<10% slope) [31; 32], and prairie-grouse have been observed to utilize areas with low vegetation and high elevation, relative to the surrounding landscape, such as ridgelines [27; 33; 34]. Additionally, patch size and time since disturbance by fire has been observed to play a significant role in grouse population persistence, with research indicating ideal, high-quality grassland patch size to be between 65 -165 ha [10; 29; 35].
WHERE IS ENERGY DEVELOPMENT OCCURING?
Energy development in the U.S. has expanded over the last 20 years. Since 2000, the U.S. has become more energy efficient, created new sources of energy and increased domestic production from already identified sources. As a result of these improvements, domestic energy development infrastructure has expanded by adding roads, transmission lines, pipelines, wind turbines, solar panels, hydro dams, oil and gas pumps and other infrastructure associated with energy development [14]. The increase in energy demand has led to over 76,900,000 ha of land leased for energy development across the U.S. [11]. Of that land, 21.3% (16,389,047 ha) is located within rangeland landscapes of the U.S. [11].
Since 2000, wind energy production has been pushed to become a more significant contributor to U.S. energy production. Estimates of wind energy for the U.S. were around 167 million MWh in 2013, which accounts for nearly 5% of total electricity generation in the U.S. [13], and accounts for over 200,000 ha of energy land leases in rangeland landscapes [11]. As of 2014, mid-western states like Kansas, Colorado, North Dakota, Minnesota and Wyoming have an installed wind capacity of 1,000 - 5,000 MW [36]. Due to the natural abundance of wind, infrastructure can be developed within currently or previously developed or impacted sites such as mines, agricultural fields and cities making it a high potential energy source [14].
Records show that 122,496 federal oil and gas drilling applications were filed from 1929-2004 in the Western U.S [37], with a large portion of U.S. oil and natural gas reserves residing in five geologic basins: Greater Green River, Montana Thrust Belt, Paradox-San Juan, Powder River and Uinta Piceance [38]. Overall, annual oil and gas land use in the U.S. has been found to range between 519 – 2,994 ha/exajoule/yr and accounts for >60% of land leased for energy development in rangeland systems (9,971,273 ha) [11; 17].
OVERLAP
Currently, much of the suitable grouse habitat and developed energy resources are located west of the Mississippi River, and are competing land uses. Unsurprisingly, given the co-location of both land uses, grouse habitat completely overlaps regions with moderate- very high amounts of wind energy development (Figure 5). The overlap between land uses likely comes from the similarities in site selection by both grouse and wind energy developers [15; 33; 39]. Both grouse and wind energy developers search for open grassland or shrub land, as well as high elevations [14; 15; 18; 27; 31; 32]. For grouse, these landscape characteristics make lekking behaviors and vocalizations more effective in attracting females to leks, as they reduce visual and acoustic obstructions [39; 40; 41; 42; 43, 44]. Similarly, wind energy benefits from these characteristics as they are associated with less wind obstruction, promoting maximum energy generation by wind turbines [14; 33].
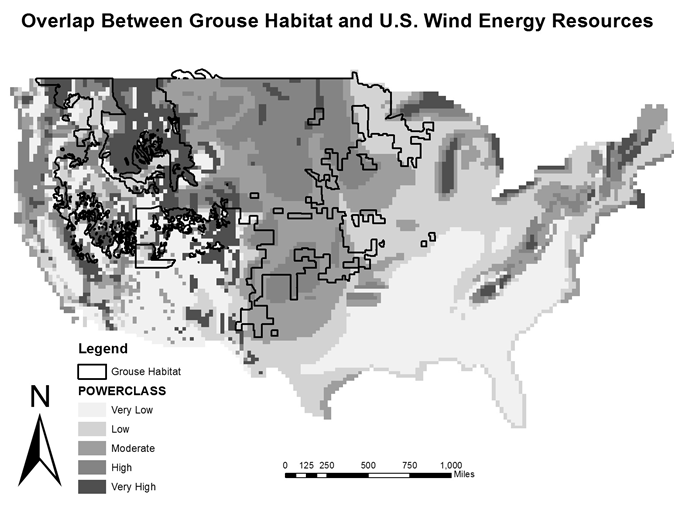
Figure 5: Map of overlap between wind resources and grouse ranges in the continental U.S. Wind resources are most abundant in the New England, Midwest and Rocky Mountain regions. Grouse ranges overlap moderate – very high wind resource areas. GIS data for wind resources obtained from the National Renewable Energy Laboratory operated by the Alliance for Sustainable Energy for the U.S. Department of Energy. GIS data for grouse ranges obtained from the Conservation Biology Institute, Colorado Division of Wildlife.
Similar overlap is observed between grouse ranges and the fossil fuel-rich geologic basins of the Midwest and Intermountain west (Figure 6) [24; 38; 45; 46; 47]. Of particular concern, is the Powder River Basin situated within the Wyoming Basin, which contains about 25% of the sage-grouse within the species range [37]. The Powder River Basin is important to sage-grouse populations due to its high density of sage-grouse leks, and its role as a geographic bridge between populations in Wyoming, South Dakota and Montana [31]. The co-location of grouse habitat and oil and gas rich geologic basins has resulted in thousands of hectares of grouse habitat being overlapped by high density oil and gas development, which typically occurs in valleys or flats at relatively low elevation [46; 47].
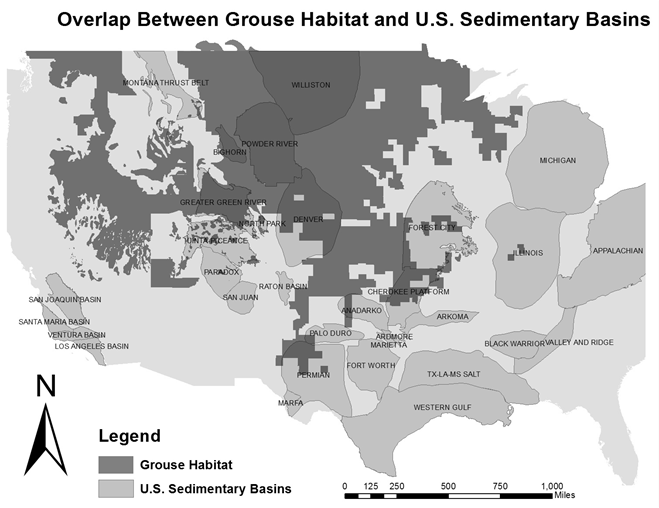
Figure 6: Map of overlap between U.S. geologic basins and grouse ranges in the continental U.S. Grouse ranges overlap several large sedimentary basins including the Powder River, Williston, Greater Green River and Denver basin. Sedimentary basin GIS data obtained from U.S. Geological Survey and state agencies. GIS data for grouse ranges obtained from the Conservation Biology Institute, Colorado Division of Wildlife
Overall, much of the remaining suitable grouse habitat is heavily overlapped by wind, oil and gas development. This overlap has led to landscape scale changes resulting in the degradation of rangeland ecosystems [12; 29; 35; 48]. Competition between these land uses has resulted in grouse species receiving significant direct and indirect impacts to their populations and ranges [18; 44; 47; 49].
DIRECT LOSS OF HABITAT DUE TO OVERLAP
GROUSE HOME RANGE LAND USE
Due to the overlap between grouse habitat and energy development, there has been considerable research investigating how energy development is influencing grouse [31; 33; 50; 51; 52; 53]. Annual grouse activities can be separated into two categories: breeding season and non-breeding season [54]. Previous observations have found grouse species, such as the T. pallidicinctus, have significantly higher mortality during the breeding season months of April-July [49; 55]. This research suggests space use during the breeding season may be more important to grouse populations than space use during the non-breeding season [49]. This information has led to mitigation plans suggesting energy development be limited based on spatial, temporal and habitat needs of the breeding season [17; 56; 57].
Grouse breeding seasons can be broken into three phases: lekking, nesting, and brood rearing [54]. As a lekking species, male grouse congregate in open areas to perform courtship displays involving whooping, booming, cackling, fighting and a multitude of other behaviors in order to draw female attention [33; 44; 58]. Since both suitable lek habitat and maximum wind energy generation are characterized by high elevation and low vegetation areas, there is constant competition between land uses [15; 27; 44]. The competition between the two land uses has fueled multiple investigations into the impact of wind energy infrastructure on lek activities. Results from these studies indicate a negative trend between lek persistence and to distance to wind turbine [4], changes in male vocalization and behavioral tendencies [58], and an increased risk of reduced lek attendance [15]. Similarly, oil and gas development has been observed to have a negative effect on both sage-grouse and prairie-grouse lekking activity by decreasing lek attendance by 29% - 73% [59; 60], decreasing male populations by 61% [48], and increasing lek avoidance [61]. These trends suggest grouse avoid occupying energy development areas and indicate a direct loss of potential lekking habitat for grouse. As most grouse nest within 5km of a lek, proximity to structure also has implications for the availability of nesting habitat [62].
For female grouse, the lekking phase concludes once copulation with a male has occurred, which is followed by transitioning into the nesting phase [54]. Nests are often placed in tall native grasses, or within sagebrush [3; 32; 53; 56; 63; 64]. Vegetation height and visual obstruction (VOR) have been found to be important for nest survival of multiple grouse species, including T. cupido, T. pallidicinctus and C. urophanasianus [3; 26; 56; 63; 65; 66; 67]. Tall vegetation is often selected for at nest sites putatively to conceal the hen and her eggs from predators during this vulnerable reproductive period [3; 26; 67]. While there are a multitude of factors influencing female space use during the nesting phase, studies have generally found that the most consistent indicator of female T. cupido space use to be proximity to lek sites [27; 35; 67]. Following this finding, it is reasonable to suggest nesting habitat suitability can be assessed directly through the condition of nearby leks [4; 62], as nests and leks are generally located within 1km-5km of each other [18; 33; 44].
The close proximity of leks and nests has resulted in nests being subjected to many of the same impacts of energy development that leks are; impacts such as decreased nest success when in close proximity to energy development [3; 26]. With multiple studies finding vegetative characteristics to be the most important factor influencing nest success [3; 26; 56; 67], landscape alteration and surface disturbance caused during construction activities is a primary driver of nesting habitat loss [68]. Both the negative trend between nest success and proximity to energy development as well as the associated surface disturbance from construction activities, suggests energy development has led to a direct loss of potential nesting habitat for grouse [3; 26].
Following a successful nesting phase, females begin brood rearing [54]. While nesting season requires dense, tall, and thick vegetation for visual protection, grouse select for less dense areas of vegetation with a greater forb content when rearing broods [28; 50; 53; 63]. These habitat characteristics provide a greater supply of arthropods for feeding, and easier terrain for the brood to move through [26; 51]. In landscapes with wind energy development, research has found a negative relationship between brood survival and distance to wind turbine [26]. Findings such as this, suggest energy development may be directly reducing suitable brood rearing habitat for grouse [26].
PLACEMENT OF ENERGY INFRASTRUCTURE
With energy demand expected to increase every year, energy developers will be adding additional infrastructure to increase energy production [14]. Production of wind, oil, and gas resources requires the use of wind turbines, pump jacks, access roads, transmission lines, pipeline, and other infrastructure, all of which are likely to be placed where developers can maximize the production of energy resources, regardless of competing land uses or wildlife needs. In addition, to meet energy demands, manufacturing sectors are continually modifying infrastructure components to maximize their potential and efficiency, a trend that can be seen in the continually increasing height of wind turbines; a modification which has been positively correlated with increased fatality for bats, but lower overall avian collision mortality [13].
To make use of the extracted and harnessed energy, developers have to transport the energy from centralized collection locations. Transport of oil and gas is typically done using linear infrastructure components, such as roads and pipelines, which create vast networks of continual resource transport across the landscape [14; 26]. Similarly to get energy to consumers, wind energy is converted to electricity and transported through networks of high voltage transmission and distribution lines.
HOW MUCH DIRECT LOSS OF HABITAT?
In our assessment, direct loss of grouse habitat can be equated to the area of potentially suitable habitat lost due to surface disturbance of energy infrastructure. Studies of the influence of energy development on C. urophanasianus have estimated direct habitat loss to occur within a 62 m radius from affected leks [69]. This estimate coupled with the observed dependency on vegetative composition, density and height [3; 26; 56; 67], and the significant overlap between energy development and grouse ranges [4; 24; 38], allows us to estimate that there have been thousands of hectares of viable grouse habitat directly lost to energy development.
INDIRECT LOSS OF HABITAT DUE TO AVOIDANCE AND FRAGMENTATION
Indirect loss of grouse habitat can be equated to the amount of potentially viable habitat that grouse are unable to use due to the influences of energy development. The most common sources of indirect habitat loss comes through avoidance of landscape features, and subsequent landscape fragmentation. Specifically, research has indicated there are two sensory perceptions that cause avoidance by grouse; visual and acoustic. Studies have shown multiple grouse species, including the T. cupido, T. pallidicinctus and C. urophanasianus, avoid areas visible to vertical landscape features such as trees, powerlines, wind turbines and pump jacks [3; 27; 30; 32; 59; 61; 70; 71]. Commonly, avoidance of these features is attributed to their vertical structure [3; 70; 72], which makes them suitable predator perches for species such as Falco sparverius, Crovus corax, Buteo swainsoni and Buteo jamaicensis [71; 73; 74; 75; 76; 77]. This threat of predation has been seen to cause an increase in physiological stress, which has been observed to lead to reduced fecundity and fitness [4; 78]. Estimates of effect size suggest grouse species avoid visible structures by 100 - 1000 m [70].
In addition to avoiding tall visible structures, grouse have been found to avoid areas louder than surrounding ambient conditions, and areas with variable soundscapes [60; 61; 79]; a finding which is mirrored in other avian species surrounded by energy development [80; 81]. Typical sources of increased and variable noise in grassland landscapes are roads, wind turbines, oil and gas compressors, and oil and gas construction [3; 58; 61; 79; 80; 81; 82]; sources which are abundant across much of the area grouse occupy. Intermittent and chronic noise from wind, oil and gas development have all been found to negatively impact grouse by causing lek avoidance, acoustical masking, decrease in nest success, decrease in clutch size, and increase in brood mortality [4; 27; 58; 61; 79]. Estimates suggest energy development sound sources can reduce the quality of grouse habitat 300 – 500 m away from the sound source; resulting in large portions of developed regions to be impacted by energy development noise [17; 60; 82; 83] .
FRAGMENTATION
Habitat fragmentation is the process of spatially separating a portion of habitat into smaller pieces. Tallgrass prairie and sagebrush have been labeled as some of the most fragmented ecosystems in North America [29; 55; 67]. Fragmentation across these landscapes is an issue for grouse species because of their need for diverse vegetation, increased predation from edge species and limited effective population size [84; 85; 86]. In order to sustain populations, grouse must successfully complete all three reproductive phases: lekking, nesting and brood rearing [54]. Ideal habitat characteristics vary during each of these phases; lekking requires less dense vegetation with minimal acoustic obstruction [33], nesting benefits from increased visual obstruction [3], and brood rearing is most successful in areas abundant with forbs and insects [50; 53]. As patch size decreases, the likelihood of quality habitat for each reproductive phase existing within the patch, is also likely to decrease [87].
Fragmentation also affects predator-prey relationships, as a positive relationship has been observed between fragmentation and the amount of edge habitat [88]. Many grouse predators such as raptors, coyotes, skunks and badgers thrive in fragmented habitats [88], and have a similar avoidance response to anthropogenic disturbance [12]. The increased availability of edge habitat and the avoidance of energy development has been observed to increase grouse mortality, increase nest predation, and increase brood mortality [12; 49; 74].
Additionally, geographic isolation due to habitat fragmentation has resulted in increased genetic drift of C. urophanasianus populations [89], and bottlenecking has resulted in ~25% hatch failure rates for avian species [90]. Both of these population processes are driven by fragmentation of grassland landscapes, and have contributed to significant declines in effective population size among some grouse species [84].
It is difficult to estimate a numeric amount of habitat affected by indirect sources, but we can observe the magnitude of indirect impacts on grouse. For grouse species, indirect loss of habitat is being driven by avoidance behaviors associated with anthropogenic development [57; 91]. Avoidance behaviors coupled with the vast network of anthropogenic features across grouse home ranges, has resulted in fragmentation affecting nearly all grouse species [3; 84; 89]. With research indicating fragmentation is causing severe negative impacts to grouse populations, it can be inferred that nearly all grouse habitat has been and will continue to be indirectly impacted by energy development.
KNOWLEDGE GAPS AND CHALLENGES FOR THE FUTURE
KNOWLEDGE GAPS
Some of the best long-term data existing for grouse, comes in the form of annual management agency lek count datasets. However, lek counts are not collected specifically to evaluate population level impacts of energy development and so are not sufficient to track energy impacts on grouse [57; Personal experience A. Gregory]. Most of the research investigating the influence of energy development on grouse species has come since the early 2000’s. In this short time, much progress has been made toward quantifying the influence of energy development on grouse; however, there is currently a lack of any long-term longitudinal studies of the impacts of energy development on grouse. Given the well-known 4-8 year periodicity associated with some lek count data sets, long-term longitudinal studies assessing the impacts of energy on grouse are much needed [92].
Impacts from climate change are another area with little actual data. Average global temperature is increasing and could lead to negative results for grouse populations due to their thermal sensitivity [93; 94]. Thermal activity in nesting T. pallidicinctus has shown a negative correlation between nest success and nest temperatures [93; 94]. Grouse nests were observed to be 4-6 C° cooler than surrounding landscape temperatures, but maintaining the temperature differential required constant effort by the female [93; 94]. As energy development increases, large densities of energy development may increase landscape temperatures due to the addition of heat sinks, which has been associated with negative impact on nest placement and success for some grouse populations [94].
Climate change in conjunction with human land use is expected to result in a 9.0% - 21.0% decline in habitat abundance and a 3.0% - 30.0% reduction in habitat quality for T. cupido [Gregory et al. unpublished data]. Climate prediction models have been developed to emulate future climate scenarios, with many showing negative impacts to grouse habitat [95; 96]. While not a species found in North America, climate models predicting increased CO2 emissions expect a decrease in habitat quality and quantity for the Chinese Grouse (Tetrastes sewerzoi) [95], an impact that would likely decimate North American grouse species. Another model predicted an increase in bare ground and decrease in shrub and sagebrush litter resulting in an 11.6% loss of sage-grouse habitat by 2050 [96]. While these are predictive models of the future and not current conditions, they provide useful information for developing precautionary conservation and management goals.
CHALLENGES
With energy demand expected to increase annually, increases in energy development are likely to occur [14; 97]. For the past 50 years, increasing energy demands have been primarily met by using fossil fuels, and current information suggests that this trend will continue [14]. From 1929-2004, over 120,000 drilling applications were filed to federal agencies with <2% rejected or withdrawn [37], and natural gas production has risen by over 20% since 1990 [14]. As a supplement to oil and gas, wind energy has experienced a 23-fold increase since 2000 [14], and projections estimate our current wind energy capacity of 62.3 GW will increase to 80-114 GW by 2025 [13]. Additionally, the U.S. Department of Energy has set a goal to satisfy 20% of the energy demand using domestic wind resources by the year 2030 [13; 15]. While wind energy has been labeled as a ‘green energy source’, in 2030, its expected land requirement of 72.1 ha/TW will be 53.5 ha/TW greater than natural gas, and 27.4 ha/TW greater than oil [14].
Challenging tradeoffs arise when trying to balance the increase in energy production with conservation of wildlife habitat. Private and government organizations such as the Western Association of Fish and Wildlife Agencies (WAFWA), Fish and Wildlife Service (FWS) and the state of Wyoming are researching scenarios that result in the highest quality potential habitat for grouse, and recommending research backed management plans based on their findings [57; 71; 79; 98; 99]. Tools developed for habitat conservation like the Crucial Habitat Assessment Tool (CHAT), which uses offset mitigation, are giving state and federal agencies the ability to locate the highest quality, potential habitat based on the defined needs of the species being conserved (WAFWA.org). Tools like CHAT are advantageous for maximizing the quantity of high quality habitat while minimizing the constraints placed on the energy industry (WAFWA.org). Use of before-and-after controlled-impact (BACI) designed studies applied at the appropriate scale will offer insight into the influences brought on by energy development and enhance the ability to extrapolate findings across study systems [17; 88; 100; 101].
An additional method to minimize habitat conservation and energy development conflicts is to develop contemporary regulatory mechanisms. Mitigating the influence of sound through noise muffling components could increase lek attendance, avian communication fidelity, nesting success, and potential habitat for many grouse species including T. pallidicinctuss, T. cupido, C. minimus and C. urophanasianus [18; 102; 103; 104]. Regulations on development proximity and development density could reduce negative population trends in grouse, reduce noise exposure to maintain nesting habitat, decrease nest failure, decrease brood mortality and increase space use [4; 17; 26; 27; 32; 57; 69; 83]. As more knowledge gaps are addressed, regulatory policies should be revised to most effectively mitigate the influence of energy development on wildlife.
RECENT SUCCESS
While the observed impact of energy development on grouse has been primarily negative and there are still knowledge gaps to be addressed, some previously imperiled species of grouse have had recent success. Conservation efforts implemented by groups such as the Western Association of Fish and Wildlife Agencies have aided in a 25% population increase in T. pallidicinctus from 2014-2015 [10]. Additionally, male lek attendance of C. urophanasianus has increased 63% since 2013 [105], and the Wyoming Executive Order 2008-2 C. urophanasianus Core Areas Protection plan has been able to accurately identify suitable habitat for C. urophanasianus within some of the most heavily oil and gas developed areas of the U.S. [57]. With the help of effective management and informed conservation, it has been shown that grouse species can still persist with intense energy development in their native range [10, 105].
Energy development within grassland habitats encompasses 16,389,047 ha of land [11]. With their collective species distributions occurring in the Southwest, Intermountain west, Central Plains and Midwest, grouse home ranges overlap thousands of hectares of U.S. domestic energy development [15; 24; 57; 69]. Conservation of grouse habitat and energy development are both land intensive practices [11]. Supporting both land uses within the same landscape calls for exceptional consideration when developing management strategies [79].
Intense energy development has created an omnipresent anthropogenic influence across rangelands [69; 81]. Direct influences have removed thousands of hectares of potentially viable grouse habitat through large scale surface disturbance caused from the development of energy resources. Indirect influences from energy development have resulted in fragmented and degraded landscapes, resulting in behavioral and reproductive impacts to multiple grouse species [3; 49; 60; 70; 79; 82; 89; 90; 106].
Increased domestic energy production has spurred increased research on the impacts of energy development on grouse over the last few decades. As energy demands and development are expected to increase, it will be important to address knowledge gaps in order to further our understanding of how grouse respond to and utilize their surroundings. Future success of grouse species will likely be dependent on the ability of mitigation and conservation strategies to incorporate both energy development and habitat conservation into their core agenda.
We thank Bowling Green State University for supporting T.W.L. and A.J.G. We also thank our colleagues and journal reviewers, and in particular L. Parsons, who commented on and assisted in revising earlier versions of this paper.
AUTHOR CONTRIBUTION
Thomas Lipp and Andrew Gregory contributed to idea and design of the content, Thomas Lipp contributed to the drafting of the manuscript, Thomas Lipp and Andrew Gregory contributed to the revision of the manuscript and final approval for publishing.
The Cornell Lab of Ornithology at Cornell University (2015) All About Birds. Web. 01 April 2016.
Elmore D, Thacker E, Smith L, Dahlgren D (2014) Distinguishing between greater and lesser prairie-chicken. Oklahoma Cooperative Extension Service Division of Agricultural Sciences and Natural Resources Oklahoma State University.
Pitman J, Hagen C, Robel R, Loughin T, Applegate R (2005) Location and success of lesser prairie-chicken nests in relation to vegetation and human disturbance. Journal of Wildlife Management 69: 1259-1269. 069[1259:LASOLP]2.0.CO;2
View ArticleWinder V, Carrlson K, Gregory A, Hagen C, Haukos D, Kesler D, Larrson L, Matthews T, Mcew L, Patten M, Pitman J, Powell L, Smith J, Thompson T, Wolfe D, Sandercock B (2015a) Factors affecting female space use in ten populations of prairie chickens. Ecosphere 6(9):166.
View ArticleU.S. Fish and Wildlife Service. Species profile for Gunnison sage-grouse (Centrocercus minimus), .
View ArticleU.S. Fish and Wildlife Service (2010) Attwater's prairie-chicken recovery plan, second revision. Albuquerque, New Mexico.
U.S. Fish and Wildlife Service. Species profile for lesser prairie chicken (Tympanuchus pallidicinctus), .
View ArticleU.S. Fish and Wildlife Service, U.S. Department of the Interior (2015) Endangered and threatened wildlife and plants; 12-month finding on a petition to list greater sage-grouse (Centrocercus urophasianus) as an endangered or threatened species.
Braun C (1998) Sage grouse declines in western North America: what are the problems? Proceedings Western Association State Fish and Wildlife Agencies 78:139-156.
Western Association of Fish and Wildlife (2015a) Aerial survey shows lesser prairie chicken population increased 25 percent from 2014 to 2015. Wildlife.state.nm.us
Copeland H, Pocewicz A, Kiesecker J (2011) Geography of energy development in western North America: potential impacts on terrestrial ecosystems in Naugle N (2011) Energy Development and Wildlife Conservation in Western North America (7-22). Washington, DC: Island press.
Burr P, Robinson A, Larsen R, Newman R, Ellis-Felege S (2017) Sharp-tailed grouse nest survival and nest predator habitat use in North Dakota's Bakken oil field. PLoS ONE 12(1).
View ArticleDiffendorfer J, Beston J, Merril M, Stanton J, Corum M, Loss S, Thogmartin W, Johnson D, Erickson R, Heist K (2015) Preliminary methodology to assess the national and regional impact of U.S. wind energy development on birds and bats. U.S. Geologic Survey.
Jones N, Pejchar L, Kiesecker J (2015) The energy footpring: how oil, natural gas, and wind energy affect land for biodiversity and the flow of ecosystem services. BioScience 65(3):290-301.
View ArticleWinder V, Gregory A, McNew L, Sandercock B (2015b) Response of male greater prairie-chickens to wind energy development. The Condor 117: 284-296.
View ArticlePatricelli G, Blickley J (2006) Avian communication in urban noise: causes and consequences of vocal adjustment. The Auk 123(3): 639-649. 123[639:ACIUNC]2.0.CO;2
View ArticleBeck J (2009) Impacts of oil and natural gas on prairie grouse: current knowledge and research needs. National Meeting of the American Society of Mining and Reclamation 66-87.
View ArticleBlickley J, Blackwood D, Patricelli G (2012) Experimental evidence for the effects of chronic anthropogenic noise on abundance of greater sage-grouse at leks. Conservation Biology 26: 461?471. PMid:22594595
View Article PubMed/NCBIRoss J, Arndt A, Smith R, Johnson J, Bouzat J (2006) Re-examination of the historical range of the greater prairie-chicken using provenance data and DNA analysis of museum collections. Conservation Genetics 7(5): 735-751.
View ArticleRoss J (2011) The evolutionary history, demographic independence and conservation status of two North American prairie bird species: the greater prairie-chicken and the lark sparrow. Dissertation. Bowling Green State University, Bowling Green, OH.
Dettenmaier S, Messmer T, Hovick T, Dahlgren D (2016) Effects of livestock grazing on rangeland biodiversity: A meta-analysis of grouse populations. Ecology and Evoloution 7:7620-7627. PMid:29043019
View Article PubMed/NCBIKansas Ornithological Society (2009) Emergency petition species review: petition for species review to list the lesser prairie-chicken (Tymphanuchus pallidicinctus).
Oyler-McCance S, DeYoung R, Fike J, Hagen C, Johnson J, Larsson L, Patten M (2016) Rangewide genetic analysis of lesser prairie-chicken reveals population structure, range expansion, and possible introgression. Conservation genetics 17(3):643.
View ArticleSchroeder M (2004) Distribution of sage grouse in North America. The Condor 106(2): 363-376.
View ArticleJohnson J, Schroeder M, Robb L (2011) Greater prairie-chicken (Tympanuchus cupido), version 2.0. In The Birds of North America (A. F. Poole, Editor). Cornell Lab of Ornithology, Ithaca, NY, USA.
View ArticleLebeau C, Beck J, Johnson G, Holloran M (2014) Short-term impacts of wind energy development on greater sage-grouse fitness. Journal of Wildlife Management 78(3): 522-530.
View ArticleWinder V, Mcnew L, Gregory A, Hunt L, Wisely S, Sandercock B (2014) Space use by female greater prairie-chickens in response to wind energy development. Ecosphere 5(1): 1-17.
View ArticleJones R (1964) Habitat used by lesser prairie chicken for feeding related to seasonal behavior of plants in Beaver County, Oklahoma. Southwestern Naturalist 9: 111-117.
View ArticleRyan M, Loren W, Burger J, Jones D, Wywialowski A (1998) Breeding ecology of greater prairie chickens (Tympanuchus cupido) in relation to prairie landscape configuration. The American Midland Naturalist 140(1): 111-121. 140[0111:BEOGPC]2.0.CO;2
View ArticleLautenbach J, Plumb R, Robinson S, Hagen C, Haukos D, Pitman J (2016) Lesser prairie-chicken avoidance of trees in a grassland landscape. Rangeland Ecology and Management 70(1):78-86.
View ArticleDoherty K, Naugle D, Walker B, Graham J (2006) Greater sage-grouse winter habitat selection and energy development. Journal of Wildlife Management.72(1): 187-195.
View ArticleFedy B, Kirol C, Sutphin A, Maechtle T (2015) The influence of mitigation on sage-grouse habitat selection within an energy development field. PloS ONE 10(4).
View ArticleGregory AJ, McNew L, Prebyl T, Sandercock B, Wisely S (2011) Hierarchical modeling of lek habitats of greater prairie-chickens in Sandercock B, Martin K, Segelbacher G (2011) Ecology, conservation and management of grouse. Studies in Avian Biology (39): 21-32 University of California Press, Berkely, CA.
Hovick T, Dahlgren D, Paper M, Elmore D, Pitman J (2015) Predicting greater prairie-chicken lek site suitability to inform conservation actions. PloS one 10(8).
View ArticleWinder V, Mcnew L, Pitman J, Sandercock B (2017) Space use of female greater prairie-chickens in response to fire and grazing. Rangeland Ecology and Management 70:165-174.
View ArticleAmerican Wind Energy Association. AWEA state wind energy facts.
View ArticleConnelly J, Knick S, Schroeder M, Stiver S (2004) Conservation assessment of greater sage-grouse and sagebrush habitats. Western Association of Fish and Wildlife Agencies. Unpublished report, Wyoming.
U.S. Energy Information Administration. Maps: Exploration, resources, reserves and production.
View ArticleKiesecker J, Evans J, Fargione J, Doherty K, Foresman K, Kunz T, Naugle D, Nibbelink N and Niemuth N (2011) Win-win for wind and wildlife: a vision to facilitate sustainable development. PLoS ONE 6(4).
View ArticleCopelin,F (1963) The lesser prairie-chicken in Oklahoma. Oklahoma Department of Wildlife Technical Bulletin 6, Oklahoma City, Oklahoma, USA.
Giesen K (1991) Population inventory and habitat use by lesser prairie-chickens in southeast Colorado. Federal Aid in Wildlife Restoration Report W-152-R, Colorado Division of Wildlife, Colorado, USA.
Dantzker M, Deane G, Bradbury J (1999) Directional acoustic radiation in the strut display of male sage grouse Centrocercus Urophasianus. Journal of Experimental Biology 202: 2893-2909. PMid:10518472
PubMed/NCBIAspbury A, Gibson R (2004) Long-range visibility of greater sage grouse leks: a GIS-based analysis. Animal Behaviour 67:1127-1132.
View ArticleGregory AJ (2011) Landscape genetics and behavioral ecology of greater prairie-chickens (Typmanuchus cupido). Dissertation. Kansas State University, Manhattan, KS.
Harju, S. M., Dzialak, M. R., Taylor, R. C., Hayden-Wing, L. D., and Winstead, J. B. 2010. Thresholds and Time Lags in Effects of Energy Development on Greater Sage?Grouse Populations. The Journal of Wildlife Management. 74(3): 437-448.
View ArticleNaugle D (2011) Energy development and wildlife conservation in western North America. Island Press, Washington, DC.
View ArticleGregory AJ, Beck J (2014) Spatial heterogeneity in response of male greater sage-grouse lek attendance to energy development. PloS ONE 9(6).
View ArticleTaylor R, Tack J, Naugle D, Mills S (2013) Combined effects of energy development and disease on greater sage grouse. PLoS ONE 8(8).
View ArticleWolfe D, Patten, M, Shochat E, Pruett C, Sherrod S (2007) Causes and patterns of mortality in lesser prairie-chickens Typanuchus pallidicinctus and implications for management. Wildlife Biology 13: 95-104. 13[95:CAPOMI]2.0.CO;2
View ArticleAhlborn G, (1980) Brood-rearing habitat and fall-winter movements of lesser prairie chickens in eastern New Mexico. Thesis. New Mexico State University, Las Cruces, New Mexico, USA
Hagen C, Pitman J, Loughin T, Sandercock B, Robel R, Applegate R (2005a) Impacts of anthropogenic features on habitat use by lesser prairie-chickens. Studies in Avian Biology 39: 63-74.
Matthews T, Tyre A, Taylor J, Lusk J, Powell L (2011) Habitat selection and brood survival of greater prairie-chickens in Sandercock B, Martin K, Segelbacher G (2011) Ecology, conservation and management of grouse. Studies in Avian Biology (39):179-191. University of California Press, Berkely, CA.
Shepherd J, Connelly J, and Reese K (2011) Modeling nest and brood habitats greater sage-grouse in Sandercock B, Martin K, Segelbacher G (2011) Ecology, conservation and management of grouse. Studies in Avian Biology (39) 137-150. University of California Press, Berkely, CA.
Sandercock B, Martin K, Segelbacher G (2011) Ecology, conservation and management of grouse. Studies in Avian Biology (39). University of California Press, Berkely, CA.
Jamison B (2000) Lesser prairie-chicken chick survival, adult survival, and habitat selection and movements of males in fragmented rangelands of Southwestern Kansas. Thesis. Kansas State University, Manhattan, KS.
Kirol C, Sutphin A, Bond L, Fuller M, Maechtle T (2015) Mitigation effectiveness for improving nesting success of greater sage-grouse influenced by energy development. Wildlife Biology 21: 98-108. PMid:26366042
View Article PubMed/NCBISpence E, Beck J, Gregory A (2017) Probability of lek collapse is lower inside sage-grouse core areas: Effectiveness of conservation policy for a landscape species. PLoS ONE 12(11).
View ArticleWhalen C, Bomberger Brown M, McGee J, Powell L, Walsh E (2018) Male greater prairie-chickens adjust their vocalizations in the presence of wind turbine noise. The Condor 120(1):137-148.
View ArticleHovick T, Elmore D, Dahlgren D, Fuhlendorf S, Engle D (2014a) Evidence of negative effects of anthropogenic structures on wildlife: a review of grouse survival and behavior. Journal of Applied Ecology 51: 1680-1689.
View ArticleNichter A, Lipp T, Gregory A (2017) A possible impact of anthropogenic noise on male Lesser Prairie-Chicken lek attendance in Kansas. Grouse. News 54:6-8.
Blickley J, Patricelli G (2010) Impacts of anthropogenic noise on wildlife: Research priorities for the development of standards and mitigation. Journal of International Wildlife Law and Policy 12: 274-292.
View ArticleHolloran M, Anderson S (2005) Spatial distribution of greater sage-grouse nests in relatively contiguous sagebrush habitats. The Condor 107:742-752. doi.org/10.1650/7749.1
View ArticleRiley T (1978) Nesting and rearing habitat of lesser prairie chickens in southeastern New Mexico. Thesis. New Mexico State University, Las Cruces, New Mexico.
Giesen K (1994) Movements and nesting habitat of lesser prairie-chicken hens in Colorado. Southwestern Naturalist 39: 96-98.
View ArticleDavis C, Riley T, Smith R, Suminski H, Wisdom, M (1979) Habitat evaluation of lesser prairie chickens in eastern Chaves County, New Mexico. Department of Fish and Wildlife Science, New Mexico Agriculture Experiment Station, Las Cruces, New Mexico, USA.
Davis D, Horton R, Odell E, Rodgers R, Whitlaw H (2008) Lesser prairie chicken conservation initiative. Lesser Prairie-Chicken Interstate Working Group. Unpublished Report, Colorado Division of Wildlife, Fort Collins, CO.
McNew L, Hunt L, Gregory A, Wisely S, Sandercock B (2013) Effects of wind energy development on nesting ecology of greater prairie-chickens in fragmented grasslands. Conservation Biology 28: 1089-1099. PMid:24628394
View Article PubMed/NCBIBraun C, Oedekoven O, Aldridge C (2002) Oil and gas development in western North America: effects on sagebrush steppe avifauna with particular emphasis on sage-grouse. Transactions of the North American Wildlife Natural Resources Conference.
Manier D, Bowen Z, Brooks M, Casazza M, Coates P, Deibert P, Hanser S, Johnson D (2014) Conservation buffer distance estimates for greater sage-grouse?a review. U.S. Geologic Survey Open-File Report 2014?1239, 14.
View ArticlePruett C, Patten M, and Wolfe D (2008) Avoidance behavior by prairie grouse: implications for development of wind energy. Conservation Biology 23: 1253-1259. PMid:19500121
View Article PubMed/NCBIDinkins D (2013) Common raven density and greater sage-grouse nesting success in southern Wyoming: potential conservation and management implications. Thesis. Utah State Universtity, Logan, Utah.
Walters K, Kosciuch K, Jones J (2014) Can the effect of tall structures on birds be isolated from other aspects of development? Wildlife Society Bulletin 30(2): 250-256.
View ArticleLammers W, Collopy M (2007) Effectiveness of avian predator perch deterrents on electric transmission lines. Journal of Wildlife Management 71: 2752-2758.
View ArticleCoates P, Connelly J, Delehanty D (2008) Predators of greater sage-grouse nests identified by video monitoring. Journal of Field Ornithology 79: 421?428.
View ArticlePrather P, Messmer T (2010) Raptor and corvid response to power distribution line perch deterrents in Utah. Journal of Wildlife management 74: 796-800.
View ArticleSlater S, Smith J (2010) Effectiveness of raptor perch deterrents on an electrical transmission line in southwestern Wyoming. Journal of Wildlife Management 74: 1080-1088.
View ArticleDinkins J, Conover M (2014) Greater sage-grouse (Centrocercus urophasianus) hen survival: effects of raptors, anthropogenic and landscape features, and hen behavior. Canadian Journal of Zoology 92: 319?330.
View ArticleRubolini D, Romano M, Boncoraglio G, Ferrari RP, Martinelli R, Galeotti P, Fasola M, Saino N (2005) Effects of eleveated egg corticosterone levels on behavior, growth and immunity of yellow-legged gull (Larus michahellis) chicks. Hormones and Behavior 47(5): 592-605.
View Article PubMed/NCBIPatricelli G, Blickley J, Hooper S (2013) Recommended management strategies the limit anthropogenic noise impacts on greater sage-grouse in Wyoming. Human-Wildlife Interactions 7(2): 114-133.
Francis C, Ortega C, Cruz A (2009) Noise pollution changes avian communities and species interaction. Current Biology 19: 1415-1419. PMid:19631542
View Article PubMed/NCBIFrancis C, Paritsis J, Ortega C, Cruz A (2011) Landscape patterns of avian habitat use and nest success are affected by chronic gas well compressor noise. Landscape Ecology 26: 1269-1280.
View ArticleLipp T (2015) Geospatial analysis of how oil and gas energy development influences lesser prairie-chicken spatial ecology in Kansas. Thesis. Bowling Green State University, Bowling Green, OH.
Hunt, J (2004) Investigation into the decline of populations of the lesser prairie-chicken (Tympanuchus pallidicinctus Ridgway) in southeastern New Mexico. Dissertation, Auburn University, Auburn, Alabama, USA.
Johnson J, Bellinger R, Toepfer J, Dunn P (2004) Temporal changes in allele frequencies and low effective population size in greater prairie chickens. Molecular Ecology 13: 2617-2630. PMid:15315675
View Article PubMed/NCBIMcNew L, Gregory A, Wisely S, Sandercock B (2012) Demography of greater prairie-chickens: regional variation in vital rates, sensitivity values, and population dynamics. Journal of Wildlife Mangement 76:987-1000.
View ArticleJohnson D, Holloran M, Connelly J, Hanser S, Amundson C, Knick S (2014) Influences of environmental and anthropogenic features on greater sage-grouse populations 1997-2007 in Greater sage-grouse: ecology and conservation of landscape species and its habitats. forest and rangeland ecosystem science center. U.S. Departments of the Interior and Geologic Survey 407-450.
Hanski I (2011) Habitat loss, the dynamics of biodvisersity, and a perspective on conservation. AMBIO 40:248-255. DOI 10.1007/s13280-011-0147-3
View ArticleStephens S, Koons D, Rotella J, Willey D (2003) Effects of habitat fragmentation on avian nesting success: a review of the evidence at multiple spatial scales. Biological Conservation 115: 101-110. 00098-3
View ArticleBreidinger L, Mock K, Messmer T (2013) Greater sage-grouse and natural gas development in Utah: using population genetic data for conservation efforts. Western North American Naturalist 73(2): 177-183.
View ArticleBriskie J, Mackintosh M (2004) Hatching failure increases with severity of population bottlenecks in birds. Proceedings of the National Academy of Sciences of the United States of America 101:558-561. PMid:14699045
View Article PubMed/NCBIPlumb R (2015) Lesser prairie-chicken movement, space use, survival, and response to anthropogenic structures in Kansas and Colorado. Thesis. Kansas State University, Manhattan, KS.
Fedy BC, Aldridge CL (2011) The importance of within-year repeated counts and the influence of scale on long-term monitoring of sage-grouse. Journal of Wildlife Management 75: 1022?1033.
View ArticleBoal C, Haukos D, Grisham B (2010) Understanding the ecology, habitat use, phenology and thermal tolerance of nesting lesser prairie-chickens to predict population level influences of climate change. Great Plains Landscape Conservation Cooperative.
Hovick T, Elmore R, Allred B, Fuhlendorf S, Dahlgren D (2014b) Landscapes as a moderator of thermal extremes: a case study from an imperiled grouse. Ecosphere 5: 1-12.
View ArticleLyu N, Sun Y (2013) Predicting threat of climate change to the chinese grouse on the qinghai-tibet plateau. Wildlife Biology 20: 70-82.
Homer C, Xian G, Alderbridge C, Meyer D, Loveland T, O'Donnell M (2015) Forecasting sagebrush ecosystem components and greater sage-grouse habitat for 2050:lLearning from past climate patterns and Landsat imagery to predict the future. Ecological Indicators 55: 131-145.
View ArticleFillipini M, Hunt C (2012) US residential energy demand and energy efficiency: a stochastic demand frontier approach. Energy Economics 34: 1484-1491.
View ArticleSvedarsky W, Westemeier R, Robel R, Gough S, Toepher J (2000) Status and management of the greater prairie-chicken Tympanuchus cupido pinnatus in North America. Wildlife Biology 6: 277-284.
Jamison B, Dechant J, Johnson D, Igle L, Goldade C, and Eulis B (2002a) Effects of management practices on grassland birds: lesser prairie-chicken. Northern Prairie Wildlife Research Center, Jamestown, North Dakota, USA.
Kemp M, Peterson J, Gardner R (2001) Scale-dependence and the problem of extrapolation: implications for experimental and natural coastal ecosystems in Gardner R, Kemp M, Kennedy V, Peterson J (2001) Scaling Relations in Experimental Ecology (3-47). Chichester, New York: Columbia University Press.
Weins J (2001) Understanding the problem of scale in experimental ecology. Gardner R, Kemp M, Kennedy V, Peterson J, Scaling Relations in Experimental Ecology (61-80). Chichester, New York: Columbia University Press. PMid:11673475
PubMed/NCBISparling D (1983) Quantitative analysis of prairie grouse vocalizations. The Condor 85: 30-42.
View ArticleHabib L, Bayne E, Boutin S (2007) Chronic industrial noise affects pairing success and age structure of ovenbirds Seiurus aurocapilla. Journal of Applied Ecology 44:176-184.
View ArticleSlabbekoorn H, Ripmeester E (2008) Birdsong and anthropogenic noise: implications and applications for conservation. Molecular Ecology 17: 72-83. PMid:17784917
View Article PubMed/NCBIWestern Association of Fish and Wildlife (2015b) WAFWA report documents greater sage-grouse population rebound. WAFWA.org.
Gregory A, Wisely S, McNew L, Sandercock B (in press) A landscape perspective on rates of multiple paternity and brood parasitism among Greater Prairie-Chickens across Kansas. Wilson Journal of Ornithology.
