Wei Weia,b, Zongxin Rana, b, Weidong Penga, b
a College of Architecture and Environment, Sichuan University, Chengdu, 610065 China
b Institute of New Energy and Low Carbon Technology, Sichuan University, Chengdu, 610065 China
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 2
Page No: 600-608
Wei Weia,b, Zongxin Rana, b, Weidong Penga, b
a College of Architecture and Environment, Sichuan University, Chengdu, 610065 China
b Institute of New Energy and Low Carbon Technology, Sichuan University, Chengdu, 610065 China
Pamela J Welz(welzp@cput.ac.za)
Wei Wei, Zongxin Ran, Weidong Peng, Influence of nitrogen control on the coupling system of winery wastewater and microalgae cultivation(2019)SDRP Journal of Earth Sciences & Environmental Studies 4(2)
This study aimed to research the nutrient uptake by two microalgae at different initial total nitrogen concentrations in winery wastewater, thus laying a theoretical foundation for the future breeding of microalgae with winery wastewater and exploring the nitrogen and phosphorous removal efficiency by using microalgae cultivation technology as well. Six concentrations of total nitrogen (TN) based on winery wastewater were designed. The two species cultured in winery wastewater with proper initial TN (40.25~360 mg/L for Chlamydomonas reinhardtii; 40.25~120 mg/L for Scenedesmus dimorphus) not only grew well, but also achieved outstanding removal efficiencies for TN and total phosphorous (TP). After 17 days cultivation, for Chlamydomonas reinhardtii, Scenedesmus dimorphus and the co-cultured species, the lowest TN removal rate was 71.69%, 72.84% and 70.67% as initial TN was 1100 mg/L respectively; however, the highest TN removal rate for Chlamydomonas reinhardtii reached to 95.15% as initial TN was14 mg/L. For Scenedesmus dimorphus and the co-cultured species, the highest TN removal rates were 98.85% and 99% respectively as initial TN was 5 mg/L. In addition, the regular of TP removal by two algae was entirely distinct and Chlamydomonas reinhardtii was more positive in TP uptake. These results also suggest that different ratio of N/P caused by diverse initial TN concentration could influence largely algal growth and further nutrient removal.
Keywords: Winery wastewater; microalgae; Chlamydomonas reinhardtii; Scenedesmus dimorphus; nitrogen
The development of human societies brings about dozens of pressing problems such as wastewater generation. The distillery wastewater derived by winery industries is rich in compounds of nitrogen (N), phosphorus (P), organics and other nutrients, which will be harmful because of the high pollution potential including eutrophication and toxicity-related impacts [1]. To remove the pollutants in winery wastewater, several treatment strategies have been proposed, such as USAB-UBF-SBR, UASB-biological contact oxidation and AFB-CASS processes [2-4]. However, these conventional pollutants removal processes are too costly to provide a satisfactory solution and would be a waste of resources, thus limiting their application. Besides, improper wastewater treatment may lead to more carbon emission relating to greenhouse effect directly. Therefore, an ideal process should be able to achieve comprehensive wastewater treatment with minimum energy and cost input [5].
Microalgae cultured in the wastewater may provide an effective way to solve the problem. Benefits from microalgae included not only being an available resource but also reducing the demand for the fresh water. The attention to microalgae cultivation for a variety of utilize has been noticeably paid over the last decade, owing to their high photosynthetic efficiency in CO2 fixation, high growth rates and high biomass production [6]. In the environmental field, coupling microalgae cultivation with wastewater treatment seems to be a promising solution to overcome the drawbacks of current treatment processes, which saves the high costs of microalgae cultivation and purifies the wastewater at the same time. Microalgae can usually uptake nitrogen and phosphorous efficiently in a wide range of wastewater [7,8]. It’s also valuable to get biomass generated and other products [9]. Several studies have tested green alga Chlorella vulgaris for a good potential for nutrient removal from various types of digestate such as dairy fertilizer [10], cattle slurry and raw cheese whey [11], and urban WWTP [12]. In these studies, the removal rates of phosphates were reported to be 63% ~ 94% and the removal rate of ammonia was nearly 100%.
Studies have confirmed that a variety of different properties of waste water after appropriate treatment can be used to cultivate energy microalgae, such as green algae, Cyanobacteria, Chrysophyte etc. Chlamydomonas is a genus of unicellular algae that has been used for decades as a model organism for molecular biology [13,14], which is also applied to biomedical engineering [15]. However, investigations about Chlamydomonas involved in distillery wastewater treatment or its pollutants tolerance were insufficient. On the contrary, Scenedesmus dimorphus, a kind of simple, easily harvested green microalgae, are more reported to culture combining with wastewater [16,17]. It is very necessary to figure out the growth and nutrient up taking characters of Scenedesmus and Chlamydomonas for resource recycling. Besides, the application of microalgae for comprehensive treatment of wastewater is of great interest as it avoids the need for additional treatment processes [5].
This paper investigated the influence of nitrogen on the growth of two different algae Scenedesmus and Chlamydomonas and showed the characters of the two algae in winery water. The results would contribute to the further research on using algae to treat winery wastewater.
2.1 Materials
Chlamydomonas reinhardtii and Scenedesmus dimorphus were isolated from college of life sciences, Sichuan University, China. Algalpre-culture was prepared by inoculating Water Culture medium with Chlamydomonas reinhardtii and Scenedesmus dimorphus in 250 ml flasks respectively. Cultures were maintained at room temperature (20℃) with 12h: 12h (light : dark) cycle under illumination from lamps at 25±2.5 μmol m−2 s−1. To maintain algae suspended, the culture was shaken manually twice a day. The pH adjustment of the medium to 7.0±0.2 was done using NaOH. The raw wastewater was obtained from a winery industry of Chengdu. Prior to experimentation, the raw wastewater was first stirred 30 min adding bentonite then centrifuged at a low speed (3500 r/min), filtered through Millipore filter (synthetic fabric CN-CA), next adjusted the pH to 7.0±0.2, autoclaved at 121℃ for 30 min at last. Compositions of the distillery wastewater after autoclaving were shown in Table 1, and unless noted otherwise, only the soluble and sterile fraction was used for microalgae growth and biodegradation test.
Table 1: compositions of winery wastewater after autoclaved
|
Parameters |
After autoclaved |
|
pH |
6.87 |
|
COD, mg/L |
74700 |
|
TN, mg/L |
1610 |
|
TP, mg/L |
657.3 |
COD: chemical oxygen demand; TN: total nitrogen; TP: total phosphorus.
2.2 Experimental Design
Biomass generation of Chlamydomonas reinhardtii and Scenedesmus dimorphus at 4 different diluted wastewater (0.83%, 1%, 1.25%, 2%, wastewater : water, v/v) were observed for 7 days to make sure a proper dilution condition [4]. Then, they were separately and together inoculated into winery wastewater at a proper diluted dose at 6 different nitrogen concentrations (5, 14, 40.25, 120, 360, 1100 mg/L) set by NaNO3. All the experimental sets were inoculated with 0.6% of stock culture and were done with three replicate at room temperature (25℃) with 12h: 12h (light : dark) cycle under illumination from lamps at 50±5μmol m−2 s−1. Every other day, 10mL culture was withdrawn from each flask to determine the biomass of microalgae, and the contents of TN, TP, COD (Chemical Oxygen Demand) as well.
2.3 Analysis
The algal dry weight was determined by weighing method. First, put the medium in the centrifuge tube. Then centrifuge and wash for three times. After that, vacuum drying at 80℃ or 40℃, and then weighing.
Specific growth rates (µm) were estimated in relation to the following method [18]:
µm=(lnX2-lnX1)/(t2-t1) (1)
where t1 and t2 were the duration of cultivation, X1 and X2 were the concentrations of biomass at day t1 and t2 respectively.
TN and TP concentrations in the samples were measured by the Chinese National Standard (HJ 636-2012 and GB 11893-89, respectively) [19,20]. The COD was determined by using the standard potassium dichromate method based on the GB 11914-1989 with a Speed Digester (5B-1(F)) [21]. The samples were diluted properly if necessary.
The nutrient removal efficiency (Ei) was calculated according to the following Eq. (2):
Ei = (Si, 0- Si, f)/ Si, 0×100% (2)
Where Si, 0 (mg/L) indicated the initial nutrient concentrations, Si, f (mg/L) indicated the final nutrient concentrations.
3.1 The growth potential of algae cultured in winery wastewater
Each microalgae has its appropriate ecological habitat and could deal well with certain wastewater treatment, cultivation and bio-energy production [22]. Six concentrations (1.1%, 1.4%, 1.7%, 2.7%, 6.8%, 13.5%, wastewater : water, v/v) of winery wastewater were selected according to Yu’s experiment to screen for the suitable condition that Chlamydomonas reinhardtii and Scenedesmus dimorphus are capable of growing well and removing nutrients of TN and TP in winery wastewater [4]. The results showed that Chlamydomonas reinhardtii and Scenedesmus dimorphus could grow well in most of wastewater despite in 6.8% and 13.5% winery wastewater. It indicated the occurrence of an inhibition phenomenon due to high nutrient contents as other previous reports [23,24].
The results presented in Fig.1 illustrated that both Chlamydomonas reinhardtii and Scenedesmus dimorphus were able to sufficiently remove nutrients from wastewater after being cultivated for 6 days in four concentrations of winery wastewater. Obviously, the higher concentration among 1.7% to 2.7% is more appropriate for economy and practical applicability. So, the medium for further research was based on 2.5% winery wastewater in which initial TP kept 16.4 mg/L.
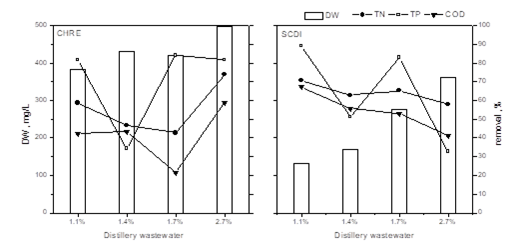
Fig.1: comparison of dry weight (DW) and nutrient removal of Chlamydomonas reinhardtii and Scenedesmus dimorphus at different wastewater concentration (the initial TN/TP/COD was 18.18/ 7.42/ 843.33 mg/L; 21.81/ 8.90/ 1012 mg/L; 27.26/ 11.13/ 1265 mg/L; 43.62/ 17.81/ 2024 mg/L, respectively)
3.2 Nutrients removal characteristics of microalgae
Then presenting Chlamydomonas reinhardtii, Scenedesmus dimorphus and co-cultured species to rapidly grow in winery wastewater by changing the nitrogen concentration were conducted. The results in Fig.2 A showed that both two microalgae and their co-cultured species grew slowly when cultured in the low nitrogen concentration (initial TN concentration < 14 mg/L). For the first 3 days, all tested microalgae were at an adaptive phrase. The next several days within the cell cycle, Chlamydomonas reinhardtii grew much better. Good growth could be observed when cultivated in 40.25 ~ 360 mg/L. However, the TN concentrations for Scenedesmus dimorphus and co-cultured species growing well are relatively narrower (ranging from 40.25 to 120 mg/L). As initial TN concentration was 120 mg/L, Chlamydomonas reinhardtii got the highest biomass (about 1045 mg/L) on the 17th day while Scenedesmus dimorphus got 743.75 mg/L. However, the co-cultured species reached the highest biomass (about 762.5 mg/L) on the 17th day as initial TN was at 40.25 mg/L, which indicated an interspecific competition between these two kinds of microalgae.
The examination for TN and TP removal of the tested microalgae revealed that Chlamydomonas reinhardtii, Scenedesmus dimorphus and the co-cultured species displayed vast nutrients uptake from the winery wastewater (Fig.2 B and C). Results showed that TN removal of all the tested microalgae was over 70% and Scenedesmus dimorphus had an advantage over Chlamydomonas reinhardtii for TN removal. Besides, the concentration of initial TN made different results of nutrients removal. With the increase of algae biomass, TN concentrations were gradually reduced in the six groups and decreased markedly at the first week, which showed the same dynamic regular with Wang’s work [22]. In terms of Chlamydomonas reinhardtii, TN removal rates were higher within lower initial concentrations (TN < 120 mg/L). The lowest TN removal rate was 71.69% when the initial TN was 1100 mg/L while the highest reached to 95.15% under 14 mg/L at the end of experiment. Once the absorption of nutrient by microalgae was constant, the lower dry weight of microalgae would cause a higher rate of absorption of nutrient by microalgae with a unit mass. Therefore, when the concentration of TN was 1100 mg/L, the growth of microalgae was inhibited to variety degrees. The excessive absorption of TN by algal cells was not conducive to the splitting of algal cells and then resulting in a significantly higher nutrient absorption rate of microalgae. Besides, the path of TP removal mainly includes the absorption of algae as well as the adsorption and precipitation of phosphate. From the concentration of TP and the growth curve of microalgae, it showed that TP removal was basically consistent with the growth trend of microalgae. TP removal rate finally got the highest 90.11% as initial TN was 40.25 mg/L. As initial TN was 5 mg/L, the TP removal rate got just 9.81% in the last part. Therefore, the change of initial TN concentration showed a more sensitive removal of TP than TN. Compared with Chlamydomonas reinhardtii, Scenedesmus dimorphus also got the lowest TN removal rate (72.84%) at the same condition and the similar regularity on nutrients removal but less than Chlamydomonas reinhardtii for TP removal. As the initial TN was as lower as 5 mg/L, it showed a -1.73% of TP removal which demonstrated Scenedesmus dimorphus had non absorption of TP under low TN. In addition, the highest TP removal for Scenedesmus dimorphus was 70.80% while initial TN was 40.25 mg/L. As for the co-cultured species, Scenedesmus dimorphus had a larger competitiveness in the biological interaction. The effect of nitrogen on the nutrients removal by co-cultured species kept pace with Scenedesmus dimorphus mostly. However, TP removal got 85.50% with higher than Scenedesmus dimorphus as initial TN was 40.25 mg/L, which also showed synergism by Chlamydomonas reinhardtii. Fig.2 C showed that the TP removal rate went down at later growth state, which is similar to Wang’s study that phosphorus could be released by algae with its death [25].
Amazing reductions in COD concentration were also found for all samples in Fig.3 after 17 days cultivation. It has been reported that several microalgae are mixotrophic species as they can assimilate CO2 and organic carbon simultaneously [26,27]. Results showed the change of initial TN concentration had no significant effect on COD removal. However, as the initial TN concentration reached 1100 mg/L, there were apparently lower COD removal rates. At the end of cultivation, Scenedesmus dimorphus and the co-cultured species showed higher removal capacity of COD. The COD removal rates by the two cultivation systems are over 85%, the final COD concentration decreased to 62 mg/L and 97 mg/L respectively, which met the national discharge standards of COD in most western countries.
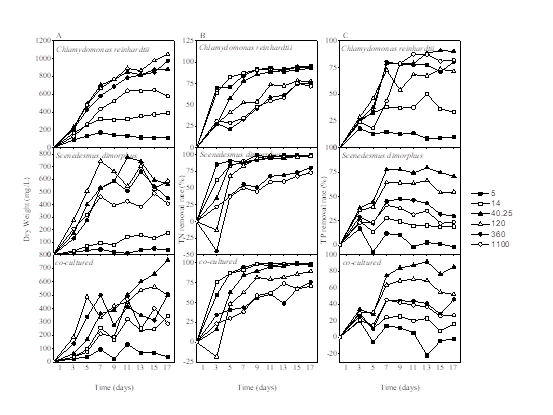
Fig.2: Biomass production (DW, mg/L, A) TN removal rate (B) and TP removal rate (C) of microalgae cultured in winery wastewater with different initial TN concentration.5, 14, 40.25, 120, 360 and 1100 represent six domesticated concentrations of initial TN; the initial TP concentration for all samples was 16.4 mg/L
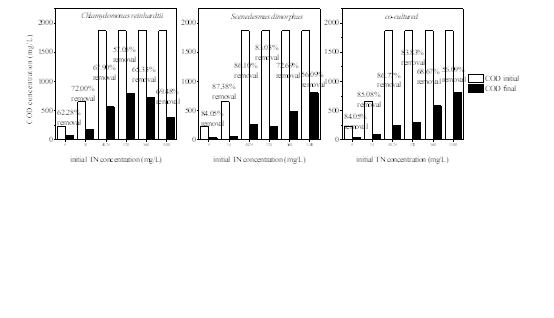
Fig.3: Removal rates of COD when microalgae cultured in winery medium with different initial TN concentrations. 5, 14, 40.25, 120, 360 and 1100 represent six domesticated concentrations of initial TN; the initial TP concentration for all samples was 16.4 mg/L
3.3 Dynamics of Microalgae Growth
The results above have showed that the appropriate initial TN concentration with 40.25 mg/L would promote both microalgae growth and nutrient removal in winery wastewater. Fig.4 and Table 2 illustrated the relationship among remaining nutrient concentration and microalgae biomass when initial TN concentration was 40.25 mg/L. It’s clearly that both the remaining nutrients of TN and TP kept a linearity bond with algae biomass concentration. However, linearity bond of remaining TP with the co-cultured species was not strong, in which R2 was lower than 0.75. This might be due to the complex competition between two species. In a word, all correlation coefficients signified TN and TP are both important nutrients for algae cell grew closely.
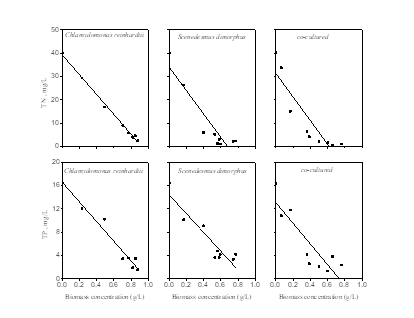
Fig.4: The relationship between remaining nutrient concentration and biomass concentration for different algae
Table 2: The relationship among the remaining TN, TP and biomass concentration for different algae
|
Microalgae |
Remaining nutrient |
Linear regression equation |
R2 |
|
Chlamydomonas reinhardtii |
TN |
Y=39.34328-42.61845X |
0.99414 |
|
TP |
Y=16.59233-16.92862X |
0.96096 |
|
|
Scenedesmus dimorphus |
TN |
Y=34.11879-50.2472X |
0.83322 |
|
TP |
Y=14.4285-16.17035X |
0.84463 |
|
|
co-cultured species |
TN |
Y=31.3941-50.7X |
0.77374 |
|
TP |
Y=13.07001-17.77157X |
0.73622 |
On the other side, algae growth correlated with not only the remaining nutrient, but also the ratio of TN/TP. Sun’s experiment showed when the ratio of N/P was at 80, Karenia mikimotoi displayed a highest growth rate [28]. However, Long pointed out microalgae could not grow well when the ratio of N/P was too low or too high [29]. Hence, this study analyzed the remaining ratio of N/P and SGR (specific growth rate) on Chlamydomonas reinhardtii and Scenedesmus dimorphus. Results in Fig.5 and Table 3 proved the molar ratio of N/P linked with algae growth essentially which were not the same as Sun’s study [28]. This might owe to the different kinds of microalgae and diverse concentration designs. It was found a kind of power function equation, Y=a*X^b (both a and b were constants; X: the ratio of N/P; Y: SGR), to state that dynamic relation more precisely (R2 > 0.75) when ratio of N/P exceeding 20. What’s more, Chlamydomonas reinhardtii showed a faster growth rate and lower TN uptake compared with Scenedesmus dimorphus at the same nutrient level. Generally speaking, the initial TN determined the accumulation of microbial biomass. Besides, the ultimate TP concentration played a more susceptible role for not only growth limit and low SGR but also a low nutrient removal rate eventually. This was in accord with Song’s study [30].
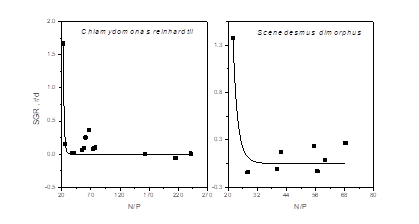
Fig.5: Correlation between the molar ratio of N/P and SGR (specific growth rate)
Table 3: Dynamics of two microalgae between the molar ratio of N/P and SGR (specific growth rate)
|
EQUATION |
Y = a*X^b |
|||
|
ALGAE |
N/P |
A |
b |
R2 |
|
Chlamydomonas reinhardtii |
20~250 |
6.36584E19 |
-14.59687 |
0.89810 |
|
Scenedesmus dimorphus |
20~80 |
2.47057E16 |
-12.13113 |
0.89772 |
Previous studies showed less attention on Chlamydomonas reinhardtii and Scenedesmus dimorphus applied to winery wastewater treatment. In this study, we investigated the nitrogen effect on algae growth and nutrient uptake based on winery wastewater, which is conductive to further research on wastewater treatment using microalgae, and also to lay a theoretical foundation for the future breeding of microalgae with winery wastewater. The two species cultured in winery wastewater with proper initial TN (40.25~360 mg/L for Chlamydomonas reinhardtii; 40.25~120 mg/L for Scenedesmus dimorphus) not only grew well, but also achieved outstanding removal efficiencies for TN and TP. After 17 days cultivation, Chlamydomonas reinhardtii got highest 1045 mg/L biomass as initial TN was 120 mg/L; the removal rate for TN, TP and COD reached to 77.63%, 83.62% and 73.12% respectively. Scenedesmus dimorphus biomass was about to 775 mg/L as initial TN was 40.25 mg/L; the removal rate for TN, TP and COD reached to 96.85%, 70.80% and 82.06% respectively. Besides, characteristics of the co-culture species were closer to Scenedesmus dimorphus in all aspects.
In a word, lower initial TN confined biomass accumulation and sequently influenced nutrient uptake. Higher initial TN would cause a higher ratio of N/P which also is adverse to nutrient uptake. Moreover, the results confirmed that Scenedesmus dimorphus was more positive on nutrient uptake but less on growth.
The work was supported by Nature Science Foundation of Sichuan Province (2017GZ0383, 2017SZ0181), Chengdu Technology Bureau (2015-HM01-00013-SF), U.S department of Energy Award (DE-EE-0003373, Nature), Science Foundation of China (31100374), 985Construction Project.
Krishnamoorthy S, Premalatha M, Vijayasekaran M. Characterization of distillery wastewater – An approach to retrofit existing effluent treatment plant operation with phycoremediation. Journal of Cleaner Production 2017;148:735-50
View ArticleLi K, Wang T, Zhang Z. A reconstruction of the vinasse wastewater treatment process. China water& wastewater 2004;20(5):83-5 (in Chinese)
Guo Q, Huang J, Kou Q, Zeng J. Application of UASB in the large-scale treatment of rice flavor liquor lees. Technology of Water Treatment 2013;39(6):125-8 (in Chinese)
Yu J, Wang P, Ran Z. Study on Purification of Vinasse Wastewater by Chlamydomonas reinhardtii. Journal of Sichuan Normal University (Natural Science) 2016;39:900-4 (in Chinese)
Gupta SK, Ansari FA, Shriwastav A, Sahoo NK, Rawat I, Bux F. Dual Role of Chlorella sorokiniana and Scenedesmus obliquus for Comprehensive Wastewater Treatment and Biomass Production for Bio-fuels. Journal of Cleaner Production 2016;115:255-64
View ArticleŠoštarič M, Golob J, Bricelj M, Klinar D, Pivec A. Studies on the Growth of Chlorella vulgaris in Culture Media with Different Carbon Sources. Chemical & Biochemical Engineering Quarterly 2009;23(4):471-77
Abou-Shanab RAI, Ji MK, Kim HC, Paeng KJ, Jeon BH. Microalgal species growing on piggery wastewater as a valuable candidate for nutrient removal and biodiesel production. Journal of Environmental Management 2013; 115(3):257-64 PMid:23270891
View ArticleShi J, Podola B, Melkonian M. Application of a prototype-scale Twin-Layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresource technology 2014;154:260-6 PMid:24412478
View ArticleChiu SY, Kao CY, Chen TY, Chang YB, Kuo CM, Lin CS. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresource technology 2015;184:179-89 PMid:25499744
View ArticleWang L, Li Y, Chen P, Min M, Chen Y, Zhu J, et al. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresource technology 2010;101(8):2623-8 PMid:19932957
View ArticleFranchino M, Comino E, Bona F, Riggio VA. Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate. Chemosphere 2013;92(6):738-44 PMid:23706373
View ArticleCho S, Lee N, Park S, Yu J, Luong TT, Oh Y-K, et al. Microalgae cultivation for bioenergy production using wastewaters from a municipal WWTP as nutritional sources. Bioresource technology 2013;131:515-20 PMid:23453233
View ArticleGutman BL, Niyogi KK. Chlamydomonas and Arabidopsis. A dynamic duo. Plant Physiology 2004;135(2):607-10 PMid:15208408
View ArticleRochaix JD. Chlamydomonas reinhardtii as the photosynthetic yeast. Annual Review of Genetics 1995;29(1):209-30 PMid:8825474
View ArticleRosalesmendoza S. Future directions for the development of Chlamydomonas-based vaccines. Expert Review of Vaccines 2013;12(9):1011-9 PMid:24053395
View ArticleCheng H. The isolation culture and its application of Scenedesmus in pig wastewater treatment. Dissertation for the Doctoral Degree, Zhejiang:Zhejiang University, 2013:8-9
Yao X. The analysis of chlorella alga and its biomass in domestic wastewater. Dissertation for the Master Degree, Shandong:Shandong Normal University, 2017:16-17
Changling LI, Yang H, Yuji LI, Wang W. Effect of Culture Models on Metabolism and Protein Components of Microalgae Chlorella vulgaris. Journal of Food Science & Biotechnology 2014;33(1):56-62 (in Chinese)
HJ 636-2012. China’s national environmental protection standard “standards for surface water”, Ministry of Environmental Protection of the People’s Republic of China, 2012
GB 11893-89. China’s national environmental protection standard “standards for surface water”, Ministry of Environmental Protection of the People’s Republic of China, 1989
GB 11914-1989. China’s national environmental protection standard “Water quality-Determination of the chemical oxygen demand-Dichromate method”, Ministry of Environmental Protection of the People’s Republic of China, 1989
Wang M, Yang Y, Chen Z, Chen Y, Wen Y, Chen B. Removal of nutrients from undiluted anaerobically treated piggery wastewater by improved microalgae. Bioresource technology 2016;222:130-8 PMid:27718397
View ArticleDe GI, Vargas VA, Blanco S, González MC, Soto R, Garcíaencina PA, et al. A comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresource technology 2010;101(14):5150-8 PMid:20219356
View ArticlePark J, Jin H-F, Lim B-R, Park K-Y, Lee K. Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresource technology 2010;101(22):8649-57 PMid:20663665
View ArticleWang H, Mu X. Cultivation of high oil content chlorella pyrenoidosa on urban sewage. Journal of South-Central University for Nationalities(Natural Science Edition) 2010;30(3):38-42 (in Chinese)
Lee YK, Ding SY, Hoe CH, Low CS. Mixotrophic growth of Chlorella sorokiniana in outdoor enclosed photobioreactor. Journal of Applied Phycology 1996;8(2):163-9
View ArticleYang S, Liu G, Meng Y, Wang P, Zhou S, Shang H. Utilization of xylose as a carbon source for mixotrophic growth of Scenedesmus obliquus. Bioresource technology 2014;172:180-5 PMid:25261865
View ArticleSun J, Liu D, Chan Z, Wei T. Growth of Platymonas helgolandica var. tsingtaoensis, Cylindrotheca closterium and Karenia mikimotoi and their survival strategies under different N/P ratios. Chinese Journal of Applied Ecology 2004;15(11):2122-2126 (in Chinese) PMid:15707326
Long MA, Wang PG, Zhu BH, Pan KH. Inter-specific competition of Karenia mikimotoi and Platymonas subcordiformis under different level of nutrients. Marine Environmental Science 2013;32(2):221-6 (in Chinese)
Song M. Selection of high oil-yielding microalgae and Optimization of its nitrogen and phosphorous removal and lipid accumulation. Dissertation for the Doctoral Degree, Shangdong:Shandong University, 2016:78-79
