Nile Red Staining as a Subsidiary Method for Microplastic Quantification: A Comparison of Three Solvents and Factors Influencing Application Reliability
Corresponding Author
E-mail address: elke.fischer@uni-hamburg.de, phone: +49 40 42838-7419
Affiliation
Matthias Tamminga1, Elena Hengstmann1, Elke Kerstin Fischer1*
1 Center for Earth System Research and Sustainability (CEN), University of Hamburg, Bundesstraße 55, 20146 Hamburg, Germany
Article Reviewed By:
Gopinath Nallani(Gopinath.Nallani@fmc.com)
Tlou Chokwe(tchokwe@randwater.co.za)
Citation
Matthias Tamminga, Nile Red Staining as a Subsidiary Method for Microplastic Quantifica-tion: A Comparison of Three Solvents and Factors Influencing Application Reliability(2017)SDRP Journal of Earth Sciences & Environmental Studies 2(2)
Abstract
The identification of microplastics is a challenging task to the scientific community, especially as ana-lytical methods limit sample numbers due to high expenses and time consuming procedures. Quantifying microplastics by staining with Nile Red can be helpful in distinguishing these particles from other inorganic (e.g. sediment) or organic (e.g. plant material) matter. The benefits of acetone, chloroform and n-hexane as solvents for Nile Red were investigated. Various polymer types, namely high-density and low-density polyethylene (HDPE, LDPE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET), polyamide (PA), polyvinyl chloride (PVC) and cellulose acetate (CA), several post-consumer products (freezing bag, bottle cap, plastic bottle, styrofoam, fishing line, food container, pipe and cigarette butt) as well as biogenic material (algae, hard plant material, soft plant material) were used as reference material. Chloroform has demonstrated to be the most suitable solvent achieving recovery rates of 83.3% for the group of HDPE and LDPE, PP and PVC being the most demanded polymer types in Europe. However, the proposed method does not reach the reliability of Raman-spectroscopy or Fourier transform infrared spectroscopy. Though, it can aid the assessment of microplastic abundances. Staining with Nile Red does not require expensive equipment and allows the quick evaluation of a large number of samples.
Introduction
Microplastics, being defined as plastic particles smaller than 5 mm in their longitudinal orientation, gained rising scientific interest in the last decades.1-3 They can be differentiated into primary (e.g. abrasive scrubbers) and secondary microplastics resulting from the fragmentation of larger particles, i.e. through UVB-degradation.2,4 Microplastic pollution of aquatic ecosystems can have a variety of ecological consequences. Among these the ingestion of plastic particles by aquatic species, including negative implications on their metabolism,5,6 the trophic transfer of plastics1,7 and possible ecotoxicological impacts should be considered.8,9
The quantification of microplastics is based on the distinction of these particles from other inorganic (e.g. sediment) or organic (e.g. plant material) matter. Various methods depending on the difference in density of particles have been applied in order to separate sediment from particularly lighter compartments of the sample.3, 10-12 The identification of microplastics as synthetic polymers poses a major challenge due to interference issues associated 35 with organic particles.13,14 Currently, the assessment of the polymer composition via (micro-)Fourier transform infrared (FTIR) spectroscopy,13,15,16 (micro-)Raman-spectroscopy6,17 and Pyrolysis-gaschromatography with mass spectrometry18-20 are approaches commonly applied allowing consistent predictions on the abundance and composition of microplastics within a sample.21,22 However, these methods rely on expensive equipment and include time-consuming procedures.14,21
Besides spectroscopic techniques a quantitative differential staining approach based on the lipophilic dye Nile Red (9-diethylamino-5H-benzo[α]phenoxazine-5-one) has been applied.23-28 The phenoxazone Nile Red was first used in the field of microbiology for detecting intracellular lipid droplets as well as for flow cytofluorometry29,30 and has firstly been adapted for the purpose of microplastic analysis by Andrady.27 Though this approach does not reveal information on the chemical structure of the particles, it allows a quick and inexpensive estimation of the microplastic load in a sample.26 The wavelength of the maximum emission and the intensity of the fluorescence strongly depend on the specific solvent used for extraction.30 In terms of microplastic analysis acetone and n-hexane were used so far.26-28 In microbiology, use of n-heptane, chloroform, xylene, and ethanol solvents in Nile Red staining were additionally reported.29 Recently, Shim et al. presented a comparison of eight possible solvents for Nile Red to improve the staining approach for the quantification of microplastics.28 Using n-hexane, recovery rates of 98% for polyethylene (100-300µm) within a matrix of natural sand could be achieved.
This study evaluates the comparative benefits and efficiencies of acetone, chloroform and n-hexane as possible solvents for Nile Red concerning their suitability for microplastic quantification.
Materials & Methods
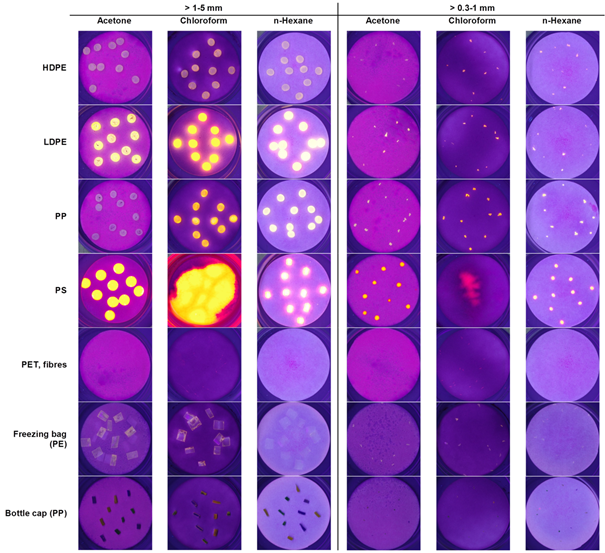
Six polymer-types (Goodfellow Inc.), nine post-consumer products made of or containing artificial polymers and three types of biogenic material were investigated regarding their condition when treated with Nile Red with varying solvents. Major characteristics in terms of size distribution of particles in different test material used for the staining experiments are displayed in table 1. All experiments were carried out for two size fractions (>0.3-1 mm and >1-5 mm). Particles belonging to the small size fraction or post consumer products were produced from larger material by cutting, grinding or carving. In total, four different shapes were considered: granules (approx. spherical, microbeads), fragments (irregular shape), fibres and films. In terms of the standard test material, irregular shapes mainly resulted from the grinding process of larger particles. The artificial polymers were transparent or whitish, except for bottle caps and fishing lines, which were coloured yellow, green or blue. For each sample ten particles were evenly placed on a filter membrane (413, VWR International, particle retention 5-13 µm) to allow the subsequent delimitation of single particles and the detection of potential degradation caused by the solvent treatment. Since the single arrangement of small fibres (>0.3-1 mm) was challenging, small piles of these were placed at fixed spots on the filter. All filters were kept in glass petri dishes.
The Nile Red concentration was set to 1 mg/ml in chloroform and acetone.23 For the third solvent a 100 mg/l stock solution in acetone was diluted ten times with n-hexane to generate a 10 mg/l working solution according to methods described by Song et al. (2014).26 The solutions were thoroughly stirred until no visible particles of Nile Red remained. Each sample was treated with 1 ml of the respective solution and allowed to rest for 48 h covered with a watch glass under a fume cupboard until all moisture evaporated. Subsequently, the dyed membrane filters were photographed (Pentax K-30, exposure time 2’’, ISO 100, resolution 2420x2343) under UV-light (Omnilux UV 18W G13, 365 nm). Special care was given to avoid contamination during the whole analysis as far as possible (e.g. samples were covered whenever possible, humidity within the laboratory was increased to reduce aerial contamination).
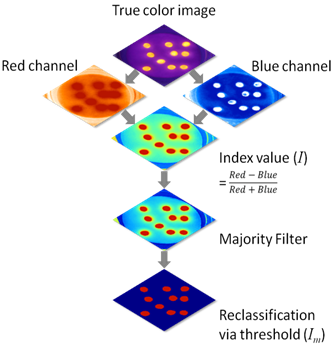
All filters were examined for stained particles. In order to reduce the processor-imposed subjectivity a standard evaluation protocol, which utilizes image analysis techniques was applied. Fig 1 visualizes the workflow in an R environment31 using RSAGA.32 The RSAGA package enables the usage of SAGA (System for Automated Geoscientific Analyses)33 in R. The RGB-composites were separated into single channels. An index (I) based on the normalized difference of the red channel and the blue channel was used in this study for distinguishing microplastics from the background. The fluorescence showed to be the highest in red visible light and lowest in blue for all solvents (for the vast majority of samples). For n-hexane green fluorescence had a similar intensity as red fluorescence, in accordance to findings reported before.26,28 To allow an improved delimitation of stained particles, a majority filter (eight surrounding pixels) was applied. This filter replaces cell/pixel values based on the majority of their adjacent cells/pixels (cells must share an edge) within a raster. As a last step, a reclassification using a threshold (Im) was carried out. Im was determined manually and adapted for each solvent individually to avoid hindering the performance of the specific method (for acetone and chloroform Im = 0.05; for n-hexane Im = 0.03). These results were used for quantification by counting areas, that were classified as fluorescent (I ≥ Im). Particles that were partially stained were also taken into account, when they could be recognised as the original objects by their shape.
Results
Table 2 shows the results of the staining experiment. In general, chloroform achieves the highest recovery rates for plastics (>1-5mm = 58.3% and >0.3-1mm = 47.8%) whilst having the lowest impact on biogenic matter (30.0% and 13.3%). The performance of n-hexane was similar, however fewer plastic (52.8% and 42.8%) and more biogenic particles (43.3% and 13.3%) got stained. The issue of co-staining biogenic matter by n-hexane was reported by Shim et al. as well.28 Compared to the other staining methods, the results achieved by acetone do not match up with respect to the materials recovered. Differences in performance are not only related to material composition, but also highly to the shape of investigated particles, as reported previously by Shim et al.28
Fibres have proven to be especially difficult to stain with recovery rates being well below the mean of granules and fragments for all solvents. Considering the three most demanded plastic polymer types in Europe (PE, PP, PVC: 58.8% of total demand) chloroform performed better than the other solvents in obtaining good recoveries to estimate microplastic numbers.34
Furthermore, the size of each particular particle has a significant influence on recovery rates (Fig 2). While 95% of all particles larger than 1 mm could be stained, only 71.7% of their smaller counterparts were identified. This difference is very likely due to the criteria of recurring shapes. Smaller particles that were stained partially were more difficult to identify than larger ones. Thus, it is not the staining procedure itself, but rather the evaluation method implemented that limits higher recovery rates for small particles.
Cellulose acetate in the form of cigarette butts had the tendency to (partly) melt when exposed to chloroform or acetone. This effect was even more severe for PS treated with chloroform, where the particles were completely dissolved. Hence, the quantification of cigarette butts was still possible in contrast to PS. We suspect the vulnerability of PS to be less significant when it is included in a field sample, as other compartments (e.g. sand, remaining biogenic material) should reduce the exposure intensity. Furthermore, styrofoam did not melt, indicating that surface properties play a major role in this context, as well.
In accordance to the findings of Shim et al.28 PE and PP were effectively stained by Nile Red in solution with n-hexane, whereas PET and PA were not detectable. Concerning EPS, only the large fraction could be quantified in both n-hexane and chloroform. We hypothesize that this is due to the specific surface and density properties of the expanded material.
Compared to spectroscopic analysis methods such as (micro-)FTIR13,15,16 or Raman-(micro)spectroscopy6,17 the proposed approach is less time consuming and costly, but also less accurate and lacks information on the chemical composition of the sample. Nevertheless, staining polymer particles with Nile Red is less time-consuming and less cost intensive than (micro-)FTIR or Raman-(micro)spectroscopy.14,21 All 120 samples in this study could be processed within 48 hours, excluding 48 hours of drying.
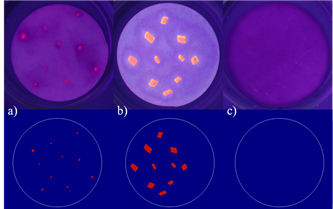
The evaluation method with R based on image analysis techniques produced results being in good accordance with the visual impression of the true colour images in general (Fig 3). Additionally, edges were well displayed, hence allowing the assessments of shapes and sizes in future investigations. Though chloroform was the only solvent showing the ability to stain fibres (Fig 4, c), these results could not be considered, as they did not match the criteria of the evaluation protocol.
Conclusion
In general, chloroform has demonstrated to be the most suitable solvent in quantifying micro-plastics by achieving recovery rates of 83.3% for the most demanded polymer types in Europe. Nevertheless, this method does not reach the reliability of spectroscopic approaches like (micro-)FTIR or Raman-(micro)spectroscopy. Still, quantifying microplastics by differential staining with Nile Red is relatively simple, economical and fast. In comparison to visual examination a misinterpretation of mineral and calcareous biogenic particles (e.g. shells) can be ruled out. Therefore, it can represent a subsidiary method to quantify microplastic contamination, especial-ly when a high number of samples cannot be examined completely by analytical approaches. In such cases, the proposed method can support the investigation of the remaining sample volume to allow an ensured quantitative extrapolation of the findings. Moreover, it can be particularly useful when recovery rates of test material, blank samples and spiked reference samples are to be assessed in terms of quality assurance for laboratory processing and protocols applied. All solvents showed the tendency to at least partly stain biogenic matter, which emphasizes the ne-cessity to embed a pre-treatment for the destruction of biogenic matter into the operational pro-tocol for microplastic identification. The transferability of the proposed method towards the analysis of field samples needs further assessment.
Acknowledgement
The authors would like to thank Constantin vom Bruch for kindly revising the language of this article.
Images and Tables
|
Origin |
Material/polymer type |
Shape |
|
|
> 0.3-1 mm |
> 1-5 mm |
||
|
Standard test material |
HDPE |
fragment |
granule |
|
LDPE |
fragment |
granule |
|
|
PP |
fragment |
granule |
|
|
PS |
fragment |
granule |
|
|
PET |
fragment/fibre |
granule/fibre |
|
|
Nylon (PA) |
fibre |
fibre |
|
|
Kevlar (PA) |
fibre |
fibre |
|
|
Post consumer products |
Freezing bag (PE) |
film |
film |
|
Bottle cap (PP) |
fragment |
fragment |
|
|
Plastic bottle (PET) |
fragment |
fragment |
|
|
Styrofoam (EPS) |
fragment |
granule |
|
|
Fishing line (PA) |
fibre |
fibre |
|
|
Food container (PS) |
fragment |
fragment |
|
|
Pipe (PVC) |
fragment |
fragment |
|
|
Cigarette filter (CA) |
fibre |
fibre |
|
|
Cigarette filter used (CA) |
fibre |
fibre |
|
|
Biogenic material |
Algae |
fragment |
fragment |
|
Hard plant material (wood) |
fragment |
fragment |
|
|
Soft plant material (leafs) |
fragment |
fragment |
|
|
|
|
Solvent |
|||||
|
|
|
Aceton |
Chloroform |
n-Hexan |
|||
|
|
Fraction (mm) |
>1 |
>0.3 |
>1 |
>0.3 |
>1 |
>0.3 |
|
Standard test material |
HDPE |
0 |
10 |
100 |
100 |
90 |
70 |
|
LDPE |
100 |
100 |
100 |
90 |
100 |
90 |
|
|
PP |
0 |
100 |
100 |
100 |
100 |
100 |
|
|
PS |
100 |
100 |
- |
- |
100 |
100 |
|
|
PET, granules |
0 |
100 |
20 |
100 |
0 |
0 |
|
|
PET, fibres |
0 |
0 |
0 |
30 |
0 |
0 |
|
|
Nylon (PA) |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Kevlar (PA) |
0 |
0 |
0 |
0 |
0 |
10 |
|
|
Post consumer products |
Freezing bag (PE) |
20 |
0 |
70 |
30 |
0 |
0 |
|
Bottle cap (PP) |
30 |
0 |
100 |
10 |
40 |
0 |
|
|
Plastic bottle (PET) |
100 |
100 |
100 |
100 |
0 |
0 |
|
|
Styrofoam (EPS) |
0 |
0 |
100 |
40 |
100 |
0 |
|
|
Fishing line (PA) |
50 |
40 |
60 |
40 |
20 |
0 |
|
|
Food container (PS) |
100 |
100 |
- |
- |
100 |
100 |
|
|
Pipe (PVC) |
100 |
100 |
100 |
100 |
100 |
100 |
|
|
Cigarette filter (CA) |
60 |
0 |
90 |
50 |
100 |
100 |
|
|
Cigarette filter used (CA) |
40 |
0 |
100 |
70 |
100 |
100 |
|
|
Biogenic material |
Algae |
10 |
0 |
10 |
0 |
0 |
0 |
|
Hard plant material (wood, bark) |
60 |
100 |
20 |
10 |
30 |
10 |
|
|
Soft plant material (leafs) |
80 |
70 |
60 |
30 |
100 |
30 |
|
|
Recovery rates |
Plastics |
39,4 |
41,7 |
58,3 |
47,8 |
52,8 |
42,8 |
|
Biogenic material |
50,0 |
56,7 |
30,0 |
13,3 |
43,3 |
13,3 |
|
|
Granules |
33,3 |
- |
70,0 |
- |
81,7 |
- |
|
|
Fragments |
82,5 |
78,9 |
75,0 |
66,7 |
60,0 |
62,2 |
|
|
Fibres |
25,0 |
6,7 |
41,7 |
31,7 |
36,7 |
35,0 |
|
|
Films |
20,0 |
0,0 |
70,0 |
30,0 |
0,0 |
0,0 |
|
|
PE, PP, PVC |
41,7 |
51,7 |
95,0 |
71,7 |
71,7 |
60,0 |
|
|
PE, PP, PVC both fractions |
46,7 |
83,3 |
65,8 |
||||
References
Galgani F. Marine litter, future prospects for research. Frontiers in Marine Science;2: 1-5. Available from: .
View ArticleAndrady AL. Microplastics in the marine environment. Marine Pollution Bulletin. 2011; 62:1596-1605. Available from: .
View ArticleThompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, et al. Lost at sea: where is all the plastic? Science [Internet]. 2004;304(5672):838?838. Available from: .
View ArticleMoore CJ, Lattin GL, Zellers AF. Quantity and type of plastic debris flowing from two urban rivers to coastal waters and beaches of Southern California. Journal of Integrated Coastal Zone Management. 2011;11(1):65-73.
Lusher AL, Hernandez-Milian G, O'Brien J, Berrow S, O'Connor I, Officer R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: the True's beaked whale Mesoplodon mirus. Environmental Pollution. 2015;199: 185-191.
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, et al. Microplastic Ingestion by Zooplankton. Environmental Science & Technology [Internet]. 2013 Jun 6 [cited 2015 Mar 21]. Available from: .
View ArticleCamedda A, Marra S, Matiddi M, Massaro G, Coppa S, Perilli A, Ruiu A, Briguglio P, de Lucia GA. Interaction between loggerhead sea turtles (Caretta caretta) and marine litter in Sardinia (Western Mediterranean Sea). Marine Environmental Research. 2014;100: 25-32.
Galloway TS. Micro- and nano-plastics and human health. In: Marine Anthropogenic Litter. 2015: 343-366.
Fossi MC, Coppola D, Baini M, Giannetti M, Guerranti C, Marsili L, Panti C, de Sabata E, Cl? S. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: the case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus). Mar. Environ. Res. 2014;100: 17-24. Available from: .
View ArticleKedzierski M, Le Tilly V, Bourseau P, Bellegou H, C?sar G, Sire O, et al. Microplastics elutriation from sandy sediments: A granulometric approach. Marine Pollution Bulletin [Internet]. 2016 Apr [cited 2016 Apr 15]; Available from: .
View ArticleClaessens M, Van Cauwenberghe L, Vandegehuchte MB, Janssen CR. New techniques for the detection of microplastics in sediments and field collected organisms. Marine Pollution Bulletin [Internet]. 2013 May [cited 2015 Mar 21];70(1-2):227?33. Available from: .
View ArticleImhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnology and Oceanography: Methods. 2012;10(7):524?37.
Song YK, Hong SH, Jang M, Han GM, Rani M, Lee J, et al. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Marine Pollution Bulletin [Internet]. Forthcoming 2015 [citied 2016 Aug 15]. Available from: .
View ArticleHidalgo-Ruz V, Gutow L, Thompson RC, Thiel M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environmental Science & Technology [Internet]. 2012 Mar 20 [cited 2015 Mar 21];46(6):3060?75. Available from: .
View ArticleTagg AS, Sapp M, Harrison JP, Ojeda JJ. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Analytical Chemistry [Internet]. 2015 Jun 16 [cited 2016 Mar 28];87(12):6032?40. Available from: .
View ArticleCole M, Webb H, Lindeque PK, Fileman ES, Halsband C, Galloway TS. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci Rep [Internet]. 2014 31;4. Available from: .
View ArticleVan Cauwenberghe L, Vanreusel A, Mees J, Janssen CR. Microplastic pollution in deep-sea sediments. Environmental Pollution [Internet]. 2013;182:495?9. Available from: .
View ArticleDekiff JH, Remy D, Klasmeier J, Fries E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environmental Pollution [Internet]. 2014a Mar [cited 2015 Mar 21];186:248?56. Available from: .
View ArticleNuelle M-T, Dekiff JH, Remy D, Fries E. A new analytical approach for monitoring microplastics in marine sediments. Environmental Pollution [Internet]. 2014 Jan [cited 2015 Mar 21];184:161?9. Available from: .
View ArticleFries E, Dekiff JH, Willmeyer J, Nuelle M-T, Ebert M, Remy D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environmental Science: Processes & Impacts [Internet]. 2013 [cited 2015 Mar 21];15(10):1949. Available from: .
View ArticleIvleva NP, Wiesheu AC, Niessner, R. Microplastic in Aquatic Ecosystems. Angewandte Chemie International Edition [Internet]. 2016 Sep [cited 2016 Sep 20]: 1-50. Available from: .
View ArticleL?der MG, Gerdts G. Methodology used for the detection and identification of microplastics?A critical appraisal. In: Marine anthropogenic litter. Springer; 2015: 27-201.
Fischer EK, Paglialonga L, Czech E, Tamminga M. Microplastic pollution in lakes and lake shoreline sediments ? A case study on Lake Bolsena and Lake Chiusi (central Italy). Environmental Pollution. 2016 Mar 4;213:648-57.
Desforges J-PW, Galbraith M, Dangerfield N, Ross PS. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Marine Pollution Bulletin [Internet]. 2014 Feb [cited 2015 Mar 20];79(1-2):94?9. Available from: .
View ArticleShim WJ, Song YK, Hong SH, Jang M, Han GM, Rani M. Microplastic as an emerging contaminant in marine environment. ???????? 2014 ???????? ???. 2014;288?303.
Song YK, Shim WJ, Jang M, Hong SH, Han GM. A Nile Red Staining Method for Microplastic Identification and Quantification. ? 52 ? ??????? ??????. 2014;143?143.
Andrady AL. Measurement and Occurrence of Microplastics in the Environment. In: Presentation at the 2nd Research Workshop on Microplastic Debris Tacoma, WA. 2010.
Shim WJ, Song YK, Hong SH, Jang M. Identification and quantification of microplastics using Nile Red statining. 2016. Marine Pollution Bulletin.
Greenspan P, Mayer EP, Fowler SD. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. The Journal of Cell Biology. 1985 Mar 1;100:965-73.
Greenspan P, Fowler SD. Spectrofluorometric studies of the lipid probe, Nile Red. Journal of Lipid Research. 1985;26:781-89.
R Core Team. R: A language and environment for statistical computing. Version R-portable 2.11.1 [software]. 2016 [cited 2016 Sep 10]. Available from: .
View ArticleBrenning, A. Statistical geocomputing combining R and SAGA: The example of landslide susceptibility analysis with generalized additive models. In: Boehner J, Blaschke T and Montanarella L, editors. SAGA - Seconds Out (= Hamburger Beitraege zur Physischen Geographie und Landschaftsoekologie). 2008;19:23-32.
Conrad O, Bechtel B, Dietrich H, Fischer E, Gerlitz L, Wehberg J, et al. System for Automated Geoscientific Analyses (SAGA) v. 2.1.4. Geoscientific Model Development. 2015 Jul;8(7): 1991-2007.
Plastics Europe. Plastics ? The facts 2015 [Internet]. 2015 Nov 9 [cited 2016 Sep 15]. Available from: .
View Article
