Jihoon Kang
E-MAIL: jihoon.kang@utrgv.edu
Adsorptive and kinetic characterization of aqueous zinc removal by biochars
Corresponding Author
Affiliation
Sergio Mireles1, Yongsik Ok2, Chu-Lin Cheng1,3, Jihoon Kang1*
1 School of Earth, Environmental and Marine Sciences, University of Texas Rio Grande Valley, Edinburg, Texas, USA.
2 School of Natural Resources and Environmental Science, Kangwon National University, Korea.
3 Department of Civil Engineering, University of Texas Rio Grande Valley, Edinburg, Texas, USA.
Article Reviewed By:
Kazuhiro Akutsu(k_akutsu@cross.or.jp)
Tevfik Aysu(tevfikaysu@yyu.edu.tr)
Zhirong Geng(gengzr@nju.edu.cn)
Citation
Jihoon Kang, Adsorptive and kinetic characterization of aqueous zinc removal by biochars(2016)SDRP Journal of Earth Sciences & Environmental Studies 1(3)
Abstract
In many urban areas, stormwater runoff is often contaminated with zinc (Zn) as it washes through galvanized surfaces in buildings and tire dusts from pavements. Biochars have shown a great potential to treat stormwater runoff contaminated with heavy metals due to their favorable physical and chemical characteristics. Biochar materials produced from pyrolysis of oak tree and wood at 400°C and 450 °C, respectively, were studied to characterize adsorption behaviors of Zn from aqueous solution to assess their applicability as a filter media for stormwater treatment. Two adsorption isotherm models, Freundlich and Langmuir, were fitted to the batch-scale experimental data. The kinetics of Zn adsorption was investigated under two contrasting physical condition (stagnant vs. agitated). The adsorption isotherm was better fitted with the Langmuir model (R2 = 0.99) than the Freundlich model (R2 = 0.62-0.72). Oak tree biochar (~ 21,400 mg kg-1) outperformed wood biochar (~ 6,100 mg kg-1) in the Zn adsorption due to higher molar ratio of oxygen to carbon in the oak tree biochar. The Zn adsorption by the biochars were less effective under stagnant condition, suggesting that external energy for agitation is needed when considering biochar as a stormwater filter media. Overall the kinetics data of Zn adsorption fitted well with the pseudo-second order model (R2 = 0.99), indicating that chemisorption was dominant mechanism for the Zn adsorption onto the biochars. This study highlights a potential for biochar to be an effective adsorbent to remove Zn with relatively short contact time for stormwater and industrial applications.
Introduction
Urban stormwater runoff often can contain a broad range of pollutants such as excessive amounts of nutrients, heavy metals, and petroleum hydrocarbons washed off from impervious surfaces (Aryal et al., 2010; Reddy et al., 2014). Discharge of these pollutants into local waterways can disrupt aquatic ecosystems and cause harmful effects to public health and drinking water supplies (Gaffield et al., 2013). Mitigation of non-point source (NPS) pollution has been challenging in the United States (US) and other countries. According to the US Environmental Protection Agency, the NPS pollution has been a major source of water quality impairments in US (USEPA, 2002).
Zinc (Zn) concentrations in urban runoff are often found to be high due to its vast usage. Major sources of Zn loss into stormwater include the heavy use of galvanized materials for many outdoor uses such as gutter systems, drainage pipes, and plating systems (Van der Perk, 2013). Worn automobile tires can also contribute to Zn release into stormwater while the tires begins to wear off. (Golding, 2008). Various treatment methods such as chemical precipitation, ion exchange, membrane separation, flotation, evaporation removal, and adsorption on activated carbon (AC) have been used to remove heavy metals from wastewater (Barakat, 2011). Most of these methods are often expensive and ineffective in low concentration range (< 100 mg L-1) which might occur in stormwater (Kurniawan et al., 2006; Perez-Martin et al., 2008). The most cost-effective method for removing heavy metals out of solution is considered to be adsorption by agro-based waste materials (Demirbas, 2008). Biochar is a carbon-rich product produced by the combustion of biomass such as wood, manure, or crop residue in an oxygen-limited environment (pyrolysis). By the charring the biomass, much of the carbon becomes “fixed” into a more stable form and therefore can minimize carbon dioxide emission to the atmosphere from decaying organic matter. When the biochar is applied to soils, the carbon can be effectively sequestered while improving soil structure and fertility (Liang et al., 2008). In addition, biochar has been viewed as a low-cost filter media to remove inorganic and organic pollutants in water due to its highly porous structure and reactive surfaces (Ahmad et al., 2013; Mohan et al., 2014).
This study evaluated two biochar materials derived from oak tree and wood in removing Zn from aqueous solution as a stormwater filter media. Study objectives were to 1) determine physicochemical and morphological characteristics of the biochar materials, 2) determine the effectiveness of Zn adsorption onto the biochar materials through batch isotherm, and 3) determine parameters for adsorption kinetics of Zn onto the biochar materials under stagnant and agitated conditions. Our results discusses the potential applications of the biochars toward aqueous Zn removal in stormwater based on our batch adsorption and kinetics experiments.
Materials & Methods
2.1 Biochar materials
Two commercially available biochars produced from oak tree and wood pyrolyzed at 400°C and 450 °C, respectively, were obtained for this study (Gangwon charmsoot company, Hoengseoug-gun, Korea). For each of the biochar materials, the elemental composition (C, H, N, S, and O) of biochars was determined on the dry basis by an elemental analyzer (Flash EA 1112 series, CE instruments, UK). The pH of biochars was measured using a pH meter in a 1:10 (w/v) suspension in deionized (DI) water. Specific surface area of the biochars were measured via N2 adsorption multilayer theory using an ASAP-2020M analyzer (Micromeritics Instrument Corp., USA). The data were fitted to the BET (Brunauer–Emmett–Teller) equation in order to calculate the surface area (Park et al., 2015) (Table 1). Pore volume was estimated from N2 adsorption at P/Po ~ 0.5.
2.2 Adsorption isotherm
Batch Zn adsorption isotherms were determined similar to the method of Kang et al. (2009). Briefly, 0.45 g of biochar material was equilibrated in test tubes with 36 mL (1:80 solid-to-solution ratio) of zinc sulfate heptahydrate (ZnSO4×7H2O) containing 0, 1, 2.5, 5, 10, 25, 50, and 100 mg Zn L-1 in DI water with two replications. During preliminary test, oak tree biochar was found to be very effective in removing Zn out of solution at 100 mg L-1. For the oak tree biochar, higher doses were added at 250 and 500 mg L-1. The samples were shaken for 48 h on an end-to-end shaker at 90 oscillations min-1 and supernatants were filtered through 0.2-µm membrane filters. The filtrates were analyzed according to USEPA Zincon Method (Cleceri et al., 2012). Zincon is dry powder form of 2-carboxy-2’hydroxy-5’sulfoformazyl benzene indicator. In the analysis, Zn in the sample is complexed with cyanide. Adding cyclohexanone causes a selective release of Zn. The Zn reacts with zincon indicator to form a blue-colored complex directly proportion to the amount of Zn in the sample. Results were measured at a wavelength of 620 nm through a benchtop spectrophotometer (HACH DR3900, Loveland, CO, USA).
The amount of Zn adsorbed by the biochar materials (q, mg kg-1) was determined by:
q = (C0V – CV)/M [1]
where C0 is the concentration of Zn in input solution (mg L-1), V is the volume of liquid (L), C is the concentration of Zn in solution after the 24-h equilibration time, and M is the mass of biochar (kg). The mean Zn adsorption data from two replicated samples were fitted to the Langmuir model because this macroscopic model fits the L-type curve, generating quantitative parameter related to the maximum Zn sorption capacity (McBride, 1994).
q = (Smax KLC)/(1 + KLC) [2]
where Smax is the maximum Zn sorption capacity (mg kg-1) and KL is an affinity constant related to bonding energy (L mg-1). These parameters were determined with a linearized form of the Langmuir equation:
C/q = 1/(KLSmax) + C/Smax [3]
A linear regression of C/q against C yields that the slope equals 1/Smax and the intercept equals 1/(KLSmax). The coefficient of determination (R2) was calculated from the linearized form.
The Freundlich model was also used to fit our experiment data (Spark, 2003):
q = KF (C)1/n [4]
where KF is the adsorption constant (mg kg-1) and n is an empirical constant related to the intensity of adsorption (L kg-1). These parameters were determined with a linearized form of the Freundlich equation with R2.
log q = log KF + 1/n log C [5]
A linear regression of log q against log C yields that the slope equals 1/n and the intercept equals log KF.
2.3 Adsorption Kinetics
The adsorption kinetics of aqueous Zn by the biochars was studied based on the contact time under two contrasting physical conditions: agitated condition vs. stagnant condition. These conditions were tested to compare Zn adsorption onto the biochars with (agitated) and without (stagnant) external energy input. Samples in duplicates were prepared by adding 10 g of each biochar in 800 mL of 50 mg Zn L-1 solution (1:80 solid-to-solution ratio) at room temperature. The stagnant condition was achieved by leaving the samples with no agitation while the agitated samples were prepared on an end-to-end shaker at 90 oscillations min-1. A small aliquot of samples (~ 30 mL) was withdrawn at 1, 4, 24, and 48 h under each of the conditions. The collected samples were filtered through 0.2-µm membrane filters and analyzed for Zn. The collected kinetics data were fitted with a linearized form of pseudo-second order reaction (Plazinski et al., 2009):
t/q = 1/(k2qe2) + t/qe [7]
where q and qe are the amounts of Zn adsorbed (mg kg-1) onto the biochar at various time, t, and at equilibrium, respectively, and k2 is the rate constant of the pseudo second-order model for Zn adsorption (kg mg-1 h-1). A linear regression of t/q against t yields that the slope equals 1/qe and the intercept equals 1/(k2qe2). Initial sorption rate (qi in mg kg-1 h-1) was calculated from the intercept of eq. [7] when t approaches 0 (Ho and Ofomaja, 2006).
qi = k2qe2 [8]
Results
3.1 Properties of biochar materials
The properties of tested biochar materials are presented in Table 1. Wood biochar (476 m2 g-1) showed higher surface area than oak tree biochar (271 m2 g-1) probably due to higher pyrolysis temperature effect while both has similar C content (Mukome and Parikh, 2015). Pore volume in wood biochar was about two-fold greater than that in oak tree biochar reflecting higher pyrolysis temperature with wood biochar. However, pore diameter was similar between the biochar materials. The pH values (9.9 to 10.2) of the biochars were alkaline probably due to the release of alkali salts from feedstock during the pyrolysis process (Ahmad et al., 2012, Kim et al., 2013). The molar ratio of hydrogen to carbon (H/C) often used as a proxy for aromaticity was two-fold higher in wood biochar. This result indicated that a greater degree of carbonization in wood biochar occurred as H is primarily associated with the organic matter in biomass (Uchimiya et al., 2010; Tan et al., 2015). The lower molar ratio of oxygen to carbon (O/C) in wood biochar suggested that the surfaces of wood biochar were more aromatic and less hydrophilic due to higher extent of carbonization and loss of polar functional group at higher pyrolysis temperature (Chen et al., 2012a, b; Ahmad et al., 2012; Kim et al., 2013).
3.2 Adsorption isotherms
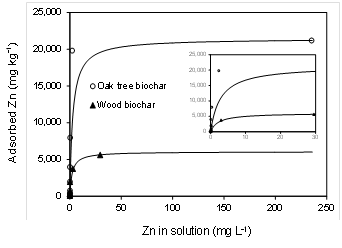
The Langmuir and Freundlich equations are classical models for adsorption isotherm. Both of these equations were implemented to graph the adsorption data for the wood and oak tree biochars. The corresponding R2 were 0.99 for Langmuir model but much lower for Freundlich model (R2 = 0.62 - 0.72). Langmuir model was chosen due to its best fit to experimental data (Fig.1). The Langmuir isotherm assumes monolayer adsorption of adsorbate on a homogenous surface of adsorbent (McBride, 1994). Our result is in agreement with Chen et al. (2011) who reported that the Langmuir model (R2 ~ 0.99) for Zn adsorption data fitted better than the Freundlich model (R2 = 0.86 - 0.94).
The value of Smax for oak tree biochar (21,440 mg kg-1) was 3.5-times greater than that for wood biochar (6,069 mg kg-1) (Table 2, Fig. 1). The adsorption mechanisms of heavy metals onto biochars include ion exchange, electrostatic attraction, chemical precipitation, and complexation with functional groups on biochar surfaces (Ahmad et al., 2013; Tan et al., 2015). In particular, oxygen-containing functional groups in biochars such as carboxyl, hydroxyl, and phonologic surfaces are critical in binding metal ions through electrostatic attraction, complexation, and/or co-precipitation. In this study, higher O content and molar O/C ratio in the oak tree biochar compared to wood biochar (Table 1) was likely the key factor resulting in greater adsorption of Zn.
3.3 Adsorption Kinetics
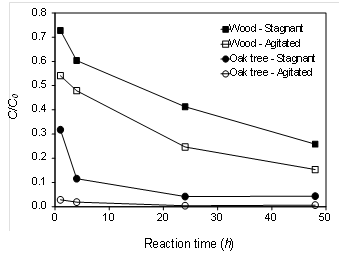
Rapid Zn adsorption to the oak tree biochar was observed within 1-h of contact time, reducing its initial concentration (C0 = 50 mg L-1) up to 97 % under agitated condition (Fig. 2). Oak tree biochar under stagnant condition also reduced Zn concentration by 96 % after 48 h of contact time. Wood biochar was relatively ineffective in reducing Zn concentration even under agitated condition. Agitation is an important parameter in adsorption phenomena, influencing the distribution of the solute in the bulk solution and the formation of the external boundary film (Kyzas, 2012). The rate of aqueous Zn removal in this study was clearly improved by agitation in both biochar materials, indicating that the agitation was likely to reduce the boundary-layer resistance. Therefore, proper mixing energy input is likely to be required to maximize adsorption of Zn onto the biochars.
The sorption rate of Zn to the biochars was initially rapid and slowed down as equilibrium was approached (Fig. 2). In general, sorption process is mainly controlled by the sorbate to sorbent transport, film diffusion through the boundary layer, intraparticle diffusion of ions or molecules, and chemisorption (Alberti et al., 2012). Our kinetics data fitted well with the pseudo-second order chemical reaction (R2 = 0.99), suggesting that chemical reaction and/or chemisorption was a significant rate-limiting step (Table 3). The initially rapid sorption of Zn in the oak tree biochar may be attributed to the interaction with the O-containing surface functional groups and then slow diffusion of Zn into the inner surfaces of biochar was likely to occur. For instance, the value of qi was highest in the oak tree biochar under agitated condition (2,916 mg g-1 h-1) while the wood biochar under stagnant condition yielded the lowest qi (14 mg g-1 h-1). This result suggested that contact time for Zn removal in filter media containing oak tree biochar could be much shortened.
Conclusion
Adsorption of heavy metals from aqueous solution plays an important role in designing stormwater treatment systems. Two biochars materials tested in this study showed contrasting Zn adsorption capacity with the oak tree biochar being much more effective than wood biochar. Oak tree biochar has significantly higher O/C molar ratio and this was likely to contribute to the greater adsorption of Zn. Oak tree biochar showed immediate sorption of Zn at initial sorption stage within 1-h reaction time, which makes this biochar more favorable as a filter media. To improve the adsorption of Zn onto the biochars, proper agitation is recommended for its best performance. Our study highlights a potential for biochar to be an effective adsorbent to treat Zn-contaminated stormwater with relatively short residence time.
Acknowledgement
This research was supported by faculty startup fund, Undergraduate Research Initiative fund, and graduate assistantship from the University of Texas Rio Grande Valley.
Images and Tables
|
Properties |
Oak Tree biochar |
Wood biochar |
|
Physicochemical |
|
|
|
C % |
88.71 |
89.84 |
|
H % |
1.21 |
2.42 |
|
O % |
9.72 |
7.52 |
|
N % |
0.36 |
0.23 |
|
S % |
0.00 |
0.00 |
|
Molar H/C |
0.16 |
0.32 |
|
Molar O/C |
0.08 |
0.06 |
|
pH |
10.2 |
9.9 |
|
Structural |
|
|
|
Surface area (m2 g-1) |
271 |
476 |
|
Pore volume (cm3 g-1) |
0.12 |
0.21 |
|
Adsorption model |
Parameter |
Oak tree biochar |
Wood biochar |
|
Langmuir |
Equation |
y = 0.00005x + 0.00013 (R2 = 0.99) |
y = 0.00016x + 0.00036 (R2 = 0.99) |
|
|
Smax (mg L-1) |
21440.41 |
6069.64 |
|
|
KL (L mg-1) |
0.35 |
0.45 |
|
Freundlich |
Equation |
y = 0.56391x + 3.51207 (R2 = 0.62) |
y = 0.59942x + 3.11592 (R2 = 0.72) |
|
|
KF (mg kg-1) |
3251.41 |
1305.92 |
|
|
n (L kg-1) |
1.77 |
1.67 |
|
Physical condition |
Parameter |
Oak tree biochar |
Wood biochar |
|
Stagnant |
Equation |
y = 0.0155x + 0.0054 (R2 = 0.99) |
y = 0.0194x + 0.0733 (R2 = 0.99) |
|
|
qe (mg kg-1) |
64.33 |
51.44 |
|
|
k2 (kg mg-1 h-1) |
0.0449 |
0.0052 |
|
|
qi (mg kg-1 h-1) |
185.66 |
13.65 |
|
Agitated |
Equation |
y = 0.0155x + 0.0054 (R2 = 0.99) |
y = 0.0151x + 0.0003 (R2 = 0.99) |
|
|
qe (mg kg-1) |
66.28 |
58.04 |
|
|
k2 (kg mg-1 h-1) |
0.6638 |
0.0079 |
|
|
qi (mg kg-1 h-1) |
2916.40 |
26.72 |
References
Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N., Mohan, D., Ok, Y. S. 2013. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99, 19-33.
View ArticleAlberti, G., Amendola, V., Pesavento, M., Biesuz, R. 2012. Beyond the synthesis of novel solid phases: review on modelling of sorption phenomena. Coord. Chem. Rev. 256(1), 28-45.
View ArticleAryal, R., Vigneswaran, S., Kandasamy, J., Naidu, R. 2010. Urban stormwater quality and treatment. Korean J. Chem. Eng. 27(5), 1343-1359.
View ArticleBarakat, M. A. 2011. New trends in removing heavy metals from industrial wastewater. Arabian J. Chem. 4(4), 361-377.
View ArticleChen, X., Chen, G., Chen, L., Chen, Y., Lehmann, J., McBride, M. B., Hay, A. G. 2011. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 102(19), 8877-8884.
View ArticleChen, Z., Chen, B., Chiou, C. T. 2012a. Fast and slow rates of naphthalene sorption to biochars produced at different temperatures. Environ. Sci. Technol. 46(20), 11104-11111.
View ArticleChen, Z., Chen, B., Zhou, D., Chen, W. 2012b. Bisolute sorption and thermodynamic behavior of organic pollutants to biomass-derived biochars at two pyrolytic temperatures. Environ. Sci. Technol. 46(22), 12476-12483.
View ArticleCleceri, L. S., Greenberg, A. E., Eaton, A. D. 2012. Standard methods for the examination of water and wastewater. 22nd edition. American Public Health Association, American Water Works Association, and Water Environment Association, Washington, DC, USA.
View ArticleDemirbas, A. 2008. Heavy metal adsorption onto agro-based waste materials: a review. J. Hazard. Mater. 157(2), 220-229.
View ArticleGaffield, S. J., Goo, R. L., Richards, L. A., Jackson, R. J. 2003. Public health effects of inadequately managed stormwater runoff. Am. J. Public Health. 93(9), 1527-1533.
View ArticleGolding, S. 2008. Suggested practices to reduce zinc concentrations in industrial stormwater discharges. Washington State Department of Ecology, Water Quality Program. (accessed on Feb 25, 2015).
View ArticleHo, Y. S., Ofomaja, A. E. 2006. Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J. Hazard. Mater. 129(1), 137-142. ,%20137.pdf
View ArticleKang, J., Hesterberg, D., Osmond, D. L. 2009. Soil organic matter effects on phosphorus sorption: a path analysis. Soil Sci. Soc. of Am. J. 73(2), 360-366.
View ArticleKim, W. K., Shim, T., Kim, Y. S., Hyun, S., Ryu, C., Park, Y. K., Jung, J. 2013. Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresour. Technol. 138, 266-270.
View ArticleKurniawan, T. A., Chan, G. Y., Lo, W. H., Babel, S. 2006. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 366(2), 409-426.
View ArticleKyzas, G. Z. 2012. Commercial coffee wastes as materials for adsorption of heavy metals from aqueous solutions. Materials 5(10), 1826-1840.
View ArticleLiang, B., Lehmann, J., Solomon, D., Sohi, S., Thies, J. E., Skjemstad, J. O., Wirick, S. 2008. Stability of biomass-derived black carbon in soils. Geochim. Cosmochim. Acta. 72, 6069-6078. ,%206096-6078,%202008%20Liang.pdf
View ArticleMcBride, M. B. 1994. Environmental chemistry of soils. Oxford university press.
View ArticleMohan, D., Sarswat, A., Ok, Y. S., Pittman Jr, C. U. 2014. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent?a critical review. Bioresource Technol. 160, 191-202.
View ArticleMukome, F.N.D. and Parikh, S.J. 2015. Chemical, Physical, and Surface Characterization of Biochar. In Y.S. Ok, S.M. Uchimiya, S.X. Chang, and N. Bolan (Eds), Biochar: Production Characterization, and Applications. CRC Pres, Boca Raton, FL.
Park, J. H., Cho, J. S., Ok, Y. S., Kim, S. H., Kang, S. W., Choi, I. W., ... & Seo, D. C. 2015. Competitive adsorption and selectivity sequence of heavy metals by chicken bone-derived biochar: Batch and column experiment. J. Environ. Sci. Health Part A, 50(11), 1194-1204.
P?rez-Mar?n, A. B., Ballester, A., Gonz?lez, F., Bl?zquez, M. L., Mu?oz, J. A., S?ez, J., & Zapata, V. M. 2008. Study of cadmium, zinc and lead biosorption by orange wastes using the subsequent addition method. Bioresource Technol. 99(17), 8101-8106.
Plazinski, W., Rudzinski, W., Plazinska, A. 2009. Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv. Colloid Interface Sci. 152(1), 2-13.
Reddy, K. R., Xie, T., Dastgheibi, S. 2014. Removal of heavy metals from urban stormwater runoff using different filter materials. J. Environ. Chem. Eng. 2(1), 282-292.
Sparks, D. L. 2003. Environmental soil chemistry. Academic press.
Tan, X., Liu, Y., Zeng, G., Wang, X., Hu, X., Gu, Y., Yang, Z. 2015. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125, 70-85.
Uchimiya, M., Lima, I.M., Klasson, K.T., Wartelle, L.H., 2010. Contaminant immobilization and nutrient release by biochar soil amendment: roles of natural organic matter. Chemosphere 80, 935-940.
USEPA. 2002. National water quality inventory, 2000 report. United States Environmental Protection Agency. Document Number EPA-841-R-02-001.
Van der Perk, M. 2013. Soil and water contamination. CRC Press.
