H.R. Banafshe
Email: banafsheh_h@kaums.ac.ir
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 1
Page No: 203-210
H.R. Banafshe
Email: banafsheh_h@kaums.ac.ir
Mona Masoomi1, Mohammad Karimian2, Abolfazl Azami Tameh1, Negar Khassafi1, Hamid Reza Banafshe3*
1Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran
2Department of Molecular and Cell Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran
3 Department of Pharmacology, School of Medicine, Kashan University of Medical Sciences, Kashan, Iran
Mona Masoomi, Mohammad Karimian, Abolfazl Azami Tameh, Negar Khassafi, Hamid Reza Banafshe, Behavioral changes and expression level of NMDA receptor subunits in a rat model of neuropathic pain induced by chronic constriction injury(2022)Journal of Cellular and Molecular Physiology 4(1) p:203-210
Background: Neuropathic pain is caused by damage to the peripheral and central nervous system. Many molecular mechanisms engage in the development of neuropathic pain, among which spinal cord NMDA receptors play a significant role in neuropathic sensitivity. This study examined the behavioral changes and the expression level of NMDA receptor subunits in a rat model of Chronic Constriction Injury (CCI).
Methods: Experiments were performed on male Wistar rats weighing 230-280 g. in two groups of control and CCI. Thermal allodynia, mechanical allodynia, and thermal hyperalgesia were evaluated on one day before and days 3, 7, 14 and 21 after surgery. The expression pattern of the glutamate receptor subunits of NR2A, NR2B, and NR3B was assessed in spinal cord tissue in lumbar segments using Real-Time PCR technique.
Results: The behavioral response of animals in the CCI group versus the control group showed a significant increase in non-painful mechanical and thermal stimuli (allodynia) and painful thermal stimuli (hyperalgesia). Moreover, the expression of NR2A and NR2B genes was increased in the CCI group compared to the control group, which was significant only for the NR2B gene.
Conclusion: This study shows that the CCI pain model effectively increases the response to both thermal and mechanical stimuli. In addition, the results of the glutamate receptor expression suggest that increasing the expression of the NR2B subunit may be considered as a mechanism for the development of neuropathic pain.
Keywords: Neuropathic pain, Allodynia, Hyperalgesia, Glutamate
The neuropathic pain is one of the most common types of chronic pain, whose treatment has many problems despite increasing advancement of medical science. The neuropathic pain is caused by damaged and impaired central, peripheral or autonomic nervous systems [1]. The neuropathic pain is associated with an unpleasant sensation of irritation and dysesthesia, increased sensitivity to hyperalgesia and feeling of pain with allodynia [2]. Some of the most common types of neuropathic pains are diabetic neuropathy-induced pain, post-herpetic neuralgia pain, phantom limb pain and spinal cord injury (SCI) neuropathy [3]. Despite numerous laboratory and clinical efforts, there is still no effective medication or strategy for the definitive treatment of neuropathic pain.
Pathophysiology of neuropathic pain is extremely complicated and there are many peripheral and central mechanisms involved in this pain. The pain can be caused by damage to afferent nerve fibers and sensitization of afferent terminal due to the release of neuropeptides, or an increase in the number of sodium and calcium channels in the site of damage and changes in neurotransmitter and receptors, especially the increase in alpha-adrenergic receptors [4, 5]. At the brain level, the formation of anatomical changes and the establishment of connections between the nerve fiber types C and A beta, the reduction in the activity of the inhibitory pathways of pain sense and the excessive release of neurotransmitters, such as glutamate in the spinal dorsal horn, contribute to neuropathic pain [6]. A great deal of effort has been made to identify the mechanisms involved in developing neuropathic pains. Recent findings have suggested neurons and glial cells in the development of neuropathic pains. Neuronal mechanisms state that neuropathic pain results from long-term plasticity changes in the pain transmission pathways in the spinal cord and brain [7]. Theories suggest that neurological damages and diseases lead to the release of many mediators such as ATP, prostaglandins, nitric oxide, and glutamate in the environment surrounding the neurons in the brain and the spinal cord, and make stable changes in synaptic activities of pain pathway neurons to induce symptoms of neuropathic pain [8]. These theories have shown that the activation of glial cells in the spinal cord, followed by the production of various cytokines and chemokines, can change the synaptic activity steadily and cause neuropathic pains [9]. Glutamate is the major excitatory neurotransmitter in CNS which effects are exerted through membrane receptors, called ionotropic and metabotropic receptors. The ionotropic receptors are ligand-gated ion channel, while the metabotropic receptors lack the ion channel and are similar to muscarinic acetylcholine receptors by releasing secondary neurotransmitters [10, 11]. The ionotropic receptors are divided into three types according to the compound to which they are attached: α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), kainate and N-methyl-D- aspartate (NMDA). All three types of channels are also opened by glutamate, and a quick synaptic transmission is performed by AMPA and kainate receptors [12]. Glutamate can play an important role in the pathophysiology of pain through binding to glutamate receptors in afferent nerve fibers for pain, especially in the spinal dorsal horn [13].
Due to peripheral neurological damage, intracellular calcium content is increased by stimulating glutamate receptors in the spinal dorsal horn, which can increase the incidence of neuropathic pains [14]. Multiple studies have been recently conducted to detect molecular mechanisms involved in neuropathic pains in altering gene expressions in animal models of CCI, with contradictory results. The purpose of this study was to investigate behavioral changes and changes in NMDA receptor subunit expression in rat CCI model.
2.1 Induction of animal model
Experiments were performed on male Wistar rats weighing 230-280 g. Three to four rats were kept in each cage under 12:12 h light/dark cycle with freely access to water and food. The CCI model was applied on the common sciatic nerve as previously described [15]. Animals underwent general anesthesia with intraperitoneal ketamine/xylazine injection. After shaving and sterilization of the dorsal surface of left thigh, the skin of was incised and the muscles were bluntly dissected to expose the sciatic nerve. Then, four partial stitches were made on the nerve prior to its, branching by 4-0 chromic gut suture. The stitches around the nerve were created at 1-1.5 mm in such a way that no disturbances were developed in the blood supply of nerves. In sham control group the sciatic nerve was exposed, but it was not ligated and muscles were returned. The rats were housed in separate cages after surgery. All experiments were conducted in accordance with ethical principles and considering animal rights and the Ethics Committee of Kashan University of Medical Sciences approved all experimental procedures.
2.2 Behavioral tests
The behavioral tests included hyperalgesia and allodynia. The allodynia testing was divided into cold allodynia and mechano-allodynia subgroups. Thermal stimulation was performed for thermal hyperalgesia using a plantar test. The cold allodynia and the mechano-allodynia were made by Von Frey filaments and Acetone, respectively. Using Plantar test and infrared radiation to the hind planta, the behavioral tests for animals were performed on the day before the surgery (day zero) and on days 7, 14 and 21 after surgery. Animals were acclimatized with the test conditions for at least 30 minutes prior to the start of the experiment [16]. The Hargreaves' method was used to measure thermal hyperalgesia [17]. In this method, the rats were placed on the upper surface of the Plexiglas cage and were allowed to adapt to the surrounding area for a period of 15-25 minutes above the glass platform. The thermal stimulation in the foot was controlled by infrared radiation using a radiant heat source equipped with a timer. The thermal stimulation was repeated three times with 10-minute intervals for the CCI and the Sham groups. The mean latency of withdrawal responses for each foot was calculated [17]. The cut-off point of the test was 22 seconds to prevent damage to the injured tissue.
The cold allodynia was measured by Acetone spray. According to Choi et al. (1994), the rats were positioned on the upper surface of the Plexiglas cage, and 250 μl of acetone was sprayed to their planta by a thin polypropylene tube connected to the insulin syringe without touching the skin. This experiment was performed 5 times at three-minute intervals for each foot [18]. The frequency of foot withdrawal reflex was expressed as follows: (number of trials accompanied by brisk foot withdrawal/total number of trials) ×100. The tactile hypersensitivity was evaluated by the method described by Kingery et al (2000) [19]. The rats were placed on a wired network (0.8 × 0.8 cm cell) and in a transparent reversed Plexiglas chamber (18 × 18 × 25 cm). After accustoming the animals to the pristine environment and stopping exploratory behaviors (about 15 minutes), a series of von Frey filaments were used to measure mechano-allodynia. In this experiment, 2 to 60-gram filaments (Stolting Co., Wood Dale, IL, the USA) were used, respectively, to increase the force applied to the plantar area of the hind foot. The stimulation was conducted three consecutive times to the plantar area until the rats withdrawn their leg or the fiber bends. The lifting of the foot that occurred during normal motion behavior was ignored. The withdrawal threshold was for the smallest filament size which evoked at least two withdrawal responses during three consecutive applications with the same filament. Each filament was applied for approximately 1 S with an inter-stimulus interval of about 5 S.
2.3 Analysis of the expression level of NMDAR subunits in the spinal cord
For gene expression analysis, the samples were taken from spinal cord tissue of lumbar segments. RNA extraction buffer (CinnaGen, Iran) was used to isolate the total RNA from tissue. Using extracted RNA, the complementary DNA (cDNA) was synthesized by cDNA synthesis kits (Invitrogen, USA). The synthesized cDNA was diluted to 1:10. Polymerase chain reaction (PCR) was performed in 96 × 0.2 ml plates (Bioplastics, USA) containing 5 μl of BioFACT 2X Real-Time PCR Master Mix (for SYBR Green I; BIOFACT, South Korea), 1 μl of primers, 2 μl of double-distilled water and 2 μl of diluted cDNA. RT-PCR technology (BioRad, Germany) was used with a standardized protocol to measure the gene expression [11]. The relative standard curve method was applied by housekeeping gene of hypoxanthine-guanine phosphoribosyl transferase (HPRT) as reference gene. The sequence and characteristics of primers used in this study are summarized in Table 1.
Table 1. Primer sequence, PCR product size and annealing temperature in real-time PCR
|
Gene |
Primer name |
Primer sequence (5'-3') |
Product size |
Annealing temperature |
|
NR2A |
NR2A F1 |
GAGCCAGATGACAACCACCT |
200-bp |
58ºC |
|
NR2A R1 |
TCTTGAGGATGTCGATGCAG |
|||
|
NR2B |
NR2B F1 |
CCAAGAGGAGGAAACAGCAG |
184-bp |
56ºC |
|
NR2B R1 |
TGAGGCGAGTTCTCCTTTGT |
|||
|
NR3B |
NR3B F |
ACCGTGGCACTGTCTTCTCT |
177-bp |
60ºC |
|
NR3B R |
TCAAAGGTTTTGTCCCCAAC |
|||
|
HPRT |
HPRT F |
GGTCCATTCCTATGACTGTAGATTTT |
125-bp |
60ºC |
|
HPRT R |
CAATCAAGACGTTCTTTCCAGTT |
2.4 Statistical analysis
Repeated measures t-test and subsequently Tukey's post hoc test were used to compare the experimental and control groups in each stage. The results are expressed as mean ± standard deviation. All statistical analyzes were performed by SPSS ver. 19 software at a significance level of p< 0.05.
3.1 Behavioral responses from neuropathic pain in the CCI model
Most of the rats with ligated nerve appeared healthy, and none of them showed a sign of autotomy after partial ligation of the sciatic nerve. Unilateral paw movements were slightly changed, but this did not interfere with the normal activity of the rats. Partial ligation of the sciatic nerve significantly reduced paw withdrawal latency to thermal stimulation (p< 0.001) but withdrawal latency of control group did not change significantly in radiant heat plantar test. The use of acetone at the midpoint of the left hind foot resulted in a significant increase in the foot withdrawal rate in the CCI group compared to the Sham group. As shown in Figure 1B, the difference between the behavioral scores of the two groups was significant at all days of the test (p< 0.001). The results of behavioral tests for the mechanical allodynia are shown in Figure 1C. After ligation of the sciatic nerve, the hind foot was sensitive to mechanical stimulation even with weak Von-Frey filaments and a significant increase was seen in CCI in comparison to the Sham group (p< 0.001). There was no evidence of hyperalgesia and allodynia on the opposite side in all the experimental groups.
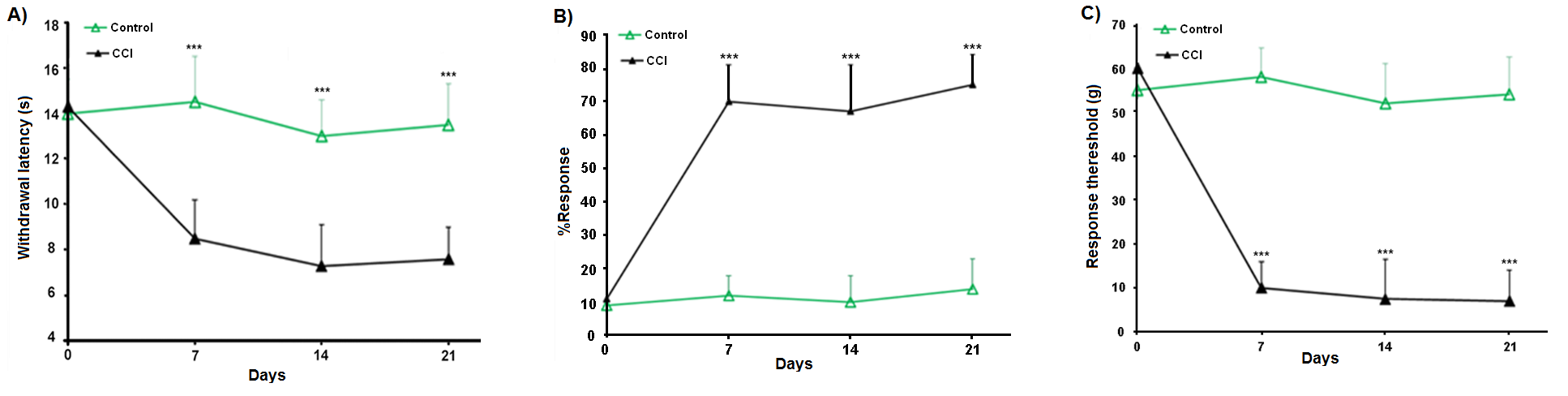
Figure 1. Behavioral responses from neuropathic pain in the CCI model; A) Comparison of the mean response of the two control and CCI groups to the Radiant Heat-induced (thermal hyperalgesia) stimulation. B) Comparison of the mean response of both control and CCI groups to acetone spraying (thermal allodynia). C) Comparison of the mean response of both control and CCI groups to Von-frey (mechanical allodynia) stimulation. *: Significant difference between control and CCI groups (***: p< 0.001).
3.2 The gene expression pattern of the glutamate receptor subunits
Real Time PCR results are summarized in Figure 2. As shown in Figure 2, the expression of NR2A and NR2B genes was increased in the CCI group compared to the control group, which was only significant for the NR2B gene (p< 0.001). However, there was no increase in expression of NR3B gene in the CCI group compared to control (p> 0.05).
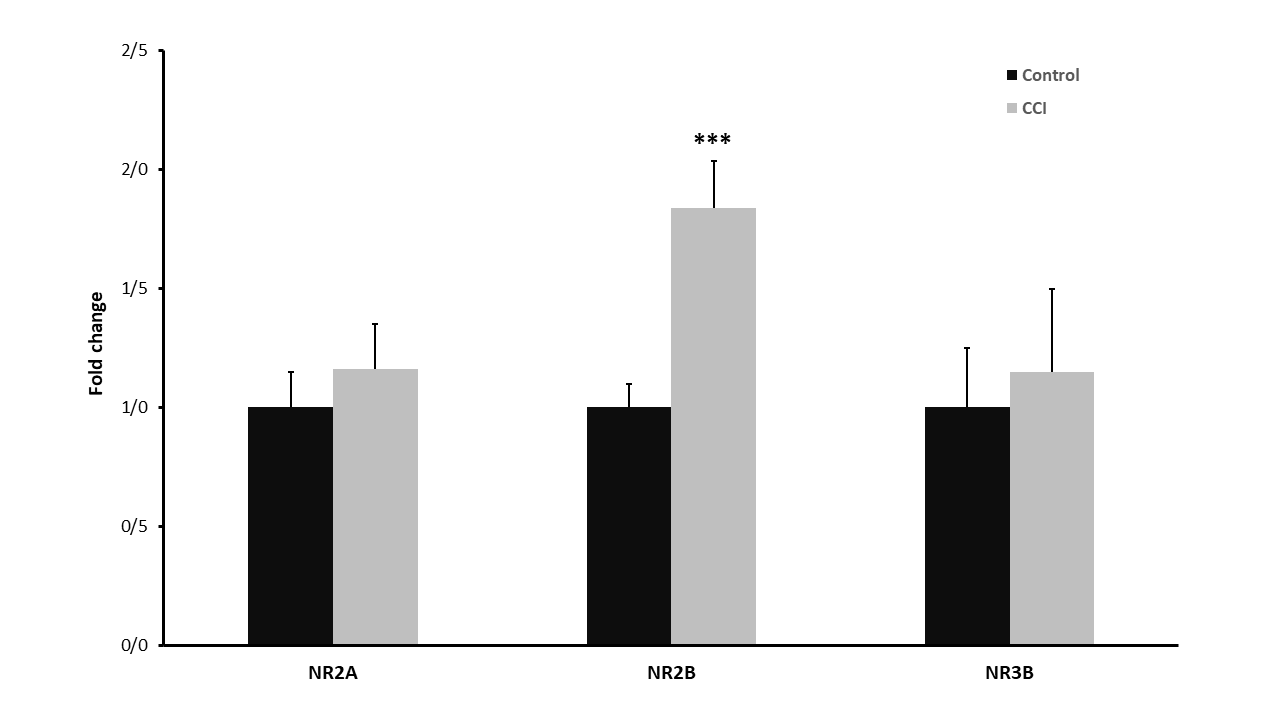
Figure 2. Results of gene expression of NMDAR subunits in the two groups of control and CCI; In the NR2A gene expression the CCI group shows an increase in expression, but not statistically significant, increase of NR2B gene expression in the CCI group is statistically significant and the expression for NR3B gene expression in the two groups showed no change in expression of this gene after CCI (***: p< 0.001).
The neuropathic pain is associated with an unpleasant sensation of irritation and dysesthesia, increased sensitivity to hyperalgesia and feeling of pain with allodynia [20]. Some of the most common types of neuropathic pains are diabetic neuropathy-induced pain, pain following herpes zoster infections, phantom limb pain and spinal cord injury neuropathy [21]. The neuropathic pain can lead to impaired mood and sleep disorder of people due to unpleasantness and high intensity, which affects the secondary daily activities [22]. The neuropathic pains caused by the phantom limb, which have high intensity and feeling of unpleasant conditions, impair the lives of many people who have lost one of their organs due to occupational accidents or during an accident or wars. These people suffer from severe fulminant pain after a long time after amputation, called phantom limb pain, and no effective treatment is yet known [23]. Post-herpetic neuralgia pain is another type of neuropathic pain that is less prevalent but more intense. The severity and importance of this type of pain is one of the most important causes of suicide in European societies [22]. One of the most commonly used models of neuropathic pain is the CCI model, introduced by [15].
In this study, behavioral changes and changes in the expression level of NMDA receptor subunits were investigated in the rat model of CCI. The effect of partial ligation of the sciatic nerve significantly reduced the unilateral foot withdrawal time in thermal stimulation. Moreover, the use of acetone at the midpoint of the left hind foot resulted in a significant increase in the foot withdrawal frequency in the CCI group. In addition, after ligation of the sciatic nerve, the hind foot was sensitive to mechanical stimulation. These results appear to be consistent with a number of human neuropathic pain syndromes, which have been reported previously [15]. It has been suggested that hyperalgesia expresses receptor sensitivity, while allodynia is a central phenomenon facilitated by large myelinated fibers [24]. The other part of the study focused on changes in the expression of glutamate receptor subunits after the development of neuropathic pain as the CCI model. The results of our study showed that the expression of NR2A and NR2B genes was increased in the CCI group, which was only significant for the NR2B gene, but there was no increase in the expression of NR3B gene in the CCI group. A previous on the rat CCI model showed that expression of NR1 and NR2B subunits was increased on the third, seventh and fourteenth days after sciatic injury at the mRNA level [25]. Although in 2005, Wilson et al. reported that the protein levels of NR1 and NR2B subunits were decreased in a sciatic injury animal model and the level of the NR2A subunit was not changed [26]. Recent immunohistochemical evidence suggests that NR2B protein shows different expression pattern in spinal cord gray matter laminas of the gray matter spinal cord, which are postulated to be of primary afferent origin [27, 28] . NR2B-selective antagonists showed beneficial effects in neuropathic animal models of hyperalgesia [27, 29]. In the above studies, mechanical allodynia and mechanical hyperalgesia were also sensitive to NR2B-selective antagonists.
The neuropathic pain due to diabetic neuropathy and nerve damage is associated with increased glutamate release from primary afferent terminals and the stimulation of AMPA receptors and metabotropic glutamate receptors [30, 31]. Although glutamate mainly acts on post-synaptic receptors for mediating excitatory neurotransmitters, pre-synaptic NMDARs can increase the release of some neurotransmitters such as substance P from primary afferent terminals in the spinal dorsal horn [32]. In addition, pre-synaptic NMDAR mediates the release of increased glutamate from the primary afferent terminals [33]. The pre-synaptic stimulation of NMDARs may directly reduce neurotransmitter release by inhibiting the voltage-gated calcium channel activity induced by calcium influx and calcineurin stimulation [34]. The pathological role of NMDARs and the therapeutic effects of NMDAR antagonists on neuropathic pain were initially investigated in an animal model of peripheral nerve injury. The use of NMDA could increase the number of whole-cell currents and calcium influx in spinal lamina II neurons in sciatic nerve-ligated rats [35]. In the model of partial ligation of the sciatic nerve, the NR1 phosphorylation was increased in the dorsal horn [36]. However, after nerve injury, the overall level of NR1 and NR2A-2D subunits in the spinal cord remained unchanged [36, 37]. Increasing NMDAR phosphorylation can play a significant role in inducing sensitivity by nerve injury. The partial ligation of the sciatic nerve can increase the NR2B tyrosine phosphorylation in the spinal cord without affecting the protein level [38]. In addition to changes in the expression level, the posttranslational modification of the glutamate receptor subunits may also be another mechanism for inducing neuropathic pain.
In conclusion, the Chronic Constriction Injury (CCI) model of neuropathic pain effectively increases the behavioral response to thermal and mechanical stimuli. In addition, increasing the expression of NR2B subunit may be considered as a mechanism for the development of neuropathic pain. However, it is suggested that the expression of other NMDA subunits should be investigated at the RNA and protein levels in the neuropathic pain models.
Author’s contributions
HRB supervised this study; MM, MK, and AAT processed the experimental tests and data analysis; MM and NK wrote the initial draft of the manuscript.
Funding
This work was supported by the [Kashan University of Medical Sciences] under Grant [92120].
Disclosure statement
The authors report there are no competing interests to declare.
Wang, S., et al., Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury. Journal of Neuroscience, 2005. 25(2): p. 488-495. PMid:15647493
View Article PubMed/NCBIHansson, P. and U. Lindblom, Hyperalgesia assessed with quantitative sensory testing in patients with neurogenic pain, in Hyperalgesia and allodynia. 1992, Raven Press New York. p. 335-343.
Attal, N., et al., Behavioural evidence that systemic morphine may modulate a phasic pain-related behaviour in a rat model of peripheral mononeuropathy. Pain, 1991. 47(1): p. 65-70. 90012-M
View ArticleMc Mahan, S., Neuropathic pain mechanism. Pain, 2002: p. 155-156.
Stucky, C.L., M.S. Gold, and X. Zhang, Mechanisms of pain. Proceedings of the National Academy of Sciences, 2001. 98(21): p. 11845-11846. PMid:11562504
View Article PubMed/NCBIHtut, M., et al., Pain phenomena and sensory recovery following brachial plexus avulsion injury and surgical repairs. Journal of hand surgery, 2006. 31(6): p. 596-605. PMid:16822598
View Article PubMed/NCBIKingery, W.S., et al., The α2A adrenoceptor and the sympathetic postganglionic neuron contribute to the development of neuropathic heat hyperalgesia in mice. Pain, 2000. 85(3): p. 345-358. 00286-9
View ArticleHargreaves, K., et al., A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain, 1988. 32(1): p. 77-88. 90026-7
View ArticleChoi, Y., et al., Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain, 1994. 59(3): p. 369-76. 90023-X
View ArticleBanafshe, H.R., et al., Lithium attenuates pain-related behavior in a rat model of neuropathic pain: possible involvement of opioid system. Pharmacology Biochemistry and Behavior, 2012. 100(3): p. 425-430. PMid:22009032
View Article PubMed/NCBIBennett, G.J. and Y.-K. Xie, A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 1988. 33(1): p. 87-107. 90209-6
View ArticleCarozzi, V., A. Canta, and A. Chiorazzi, Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neuroscience letters, 2015. 596: p. 90-107. PMid:25459280
View Article PubMed/NCBIPetrenko, A.B., et al., The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesthesia & Analgesia, 2003. 97(4): p. 1108-1116. PMid:14500166
View Article PubMed/NCBIJiang, S., D.-F. Fang, and Y. Chen, Involvement of N-Methyl-D-Aspartic Acid Receptor and DL-α-Amino-3-Hydroxy-5-Methyl-4-Isoxazole Propionic Acid Receptor in Ginsenosides Rb1 and Rb3 against Oxygen-Glucose Deprivation-Induced Injury in Hippocampal Slices from Rat. Pharmacology, 2018. 101(3-4): p. 133-139. PMid:29207398
View Article PubMed/NCBITameh, A.A., et al., Role of steroid therapy after ischemic stroke by N-methyl-d-aspartate receptor gene regulation. Journal of Stroke and Cerebrovascular Diseases, 2018. 27(11): p. 3066-3075. PMid:30072177
View Article PubMed/NCBIGoodwani, S., et al., Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neuroscience & Biobehavioral Reviews, 2017. 77: p. 14-31. PMid:28242339
View Article PubMed/NCBIInoue, K. and M. Tsuda, Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nature Reviews Neuroscience, 2018. PMid:29416128
View Article PubMed/NCBIHussaini, S.M. and M.H. Jang, New roles for old glue: astrocyte function in synaptic plasticity and neurological disorders. International neurourology journal, 2018. 22(Suppl 3): p. S106. PMid:30396259
View Article PubMed/NCBIShiao, R. and C.A. Lee-Kubli, Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics, 2018: p. 1-19. PMid:29736857
View Article PubMed/NCBIKumar, A., et al., Pharmacological management of neuropathic pain: Current trends and possible approaches. Archives of Neuroscience, 2017. 4(1).
View ArticleMuthuraman, A., et al., Drug therapy of neuropathic pain: current developments and future perspectives. Current drug targets, 2014. 15(2): p. 210-253.
Cohen, S.P. and J. Mao, Neuropathic pain: mechanisms and their clinical implications. Bmj, 2014. 348: p. f7656. PMid:24500412
View Article PubMed/NCBIXie, X., et al., Analgesic microneedle patch for neuropathic pain therapy. ACS nano, 2016. 11(1): p. 395-406. PMid:28001346
View Article PubMed/NCBIAndersen, H.H., G. Yosipovitch, and A. Galor, Neuropathic symptoms of the ocular surface: dryness, pain, and itch. Current opinion in allergy and clinical immunology, 2017. 17(5): p. 373-381. PMid:28858914
View Article PubMed/NCBIDeer, T.R., et al., The Appropriate Use of Neurostimulation of the Spinal Cord and Peripheral Nervous System for the Treatment of Chronic Pain and Ischemic Diseases: The N euromodulation A ppropriateness C onsensus C ommittee. Neuromodulation: Technology at the Neural Interface, 2014. 17(6): p. 515-550. PMid:25112889
View Article PubMed/NCBIWilson, J.A., et al., NMDA receptor antagonist treatment at the time of nerve injury prevents injury-induced changes in spinal NR1 and NR2B subunit expression and increases the sensitivity of residual pain behaviours to subsequently administered NMDA receptor antagonists. Pain, 2005. 117(3): p. 421-32. PMid:16150544
View Article PubMed/NCBIBoyce, S., et al., Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology, 1999. 38(5): p. 611-23. 00218-4
View ArticleMa, Q.P. and R.J. Hargreaves, Localization of N-methyl-D-aspartate NR2B subunits on primary sensory neurons that give rise to small-caliber sciatic nerve fibers in rats. Neuroscience, 2000. 101(3): p. 699-707. 00419-X
View ArticleTaniguchi, K., et al., Antinociceptive activity of CP-101,606, an NMDA receptor NR2B subunit antagonist. Br J Pharmacol, 1997. 122(5): p. 809-12. PMid:9384494
View Article PubMed/NCBIZhang, H.M., S.R. Chen, and H.L. Pan, Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain. Neuroscience, 2009. 158(2): p. 875-84. PMid:19017536
View Article PubMed/NCBILi, J.Q., et al., Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem, 2010. 112(1): p. 162-72. PMid:19840219
View Article PubMed/NCBILiu, H., P.W. Mantyh, and A.I. Basbaum, NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature, 1997. 386(6626): p. 721-4. PMid:9109489
View Article PubMed/NCBIZhou, H.Y., et al., Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci, 2010. 30(12): p. 4460-6. PMid:20335482
View Article PubMed/NCBIWu, Z.Z., S.R. Chen, and H.L. Pan, Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J Biol Chem, 2005. 280(18): p. 18142-51. PMid:15746091
View Article PubMed/NCBIIsaev, D., et al., Facilitation of NMDA-induced currents and Ca2+ transients in the rat substantia gelatinosa neurons after ligation of L5-L6 spinal nerves. Neuroreport, 2000. 11(18): p. 4055-61. PMid:11192628
View Article PubMed/NCBIUltenius, C., et al., Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett, 2006. 399(1-2): p. 85-90. PMid:16469445
View Article PubMed/NCBIGao, X., et al., Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain, 2005. 116(1-2): p. 62-72. PMid:15936881
View Article PubMed/NCBILiu, X.J., et al., Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med, 2008. 14(12): p. 1325-32. PMid:19011637
View Article PubMed/NCBI