LIU Sha-sha
Email: cyyliushasha@163.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 1
LIU Sha-sha
Email: cyyliushasha@163.com
LIU Sha-sha ,YAN, SHI Ya-nan
The Affiliated Hospital of Chengde Medical University, Hebei Chengde 067000, China
LIU Sha-sha, YAN, SHI Ya-nan, Effects of ATRA on proliferation, migration and cell cycle of gastric cancer MGC-803 cells (2022) Journal of Cellular and Molecular Physiology 4(1) p:211-217
Objective:To study the effects of all-trans-retinoic acid (ATRA) on proliferation, migration and cycle of human gastric cancer MGC-803 cells.
Methods:The proliferation activity of gastric cancer MGC-803 cells treated with different concentrations of ATRA was detected by CCK-8 assay. The morphological changes of MGC-803 cells in each group were observed under inverted microscope. The migration ability of cells in each group was detected by scratch test. Flow cytometry was used to detect the change of MGC-803 cell cycle.
Results:CCK-8 results showed that ATRA could significantly inhibit the proliferation of MGC-803 cells, and the inhibition rate of cell proliferation increased gradually with the increasing of ATRA concentrations and action time, in a dose-time dependent manner(P<0.05). Under inverted microscope, the morphology of MGC-803 cells changed and the number of viable cells decreased significantly after ATRA treatment. The results of scratch assay showed that ATRA could significantly inhibit the migration of gastric cancer MGC-803 cells, and the repair rate of scratch area decreased significantly with the increasing of ATRA concentration(P<0.05).Flow cytometry showed that the proportion of G0/G1 phase cells increased significantly after ATRA treatment, and the proportion of S phase cells decreased (P<0.05).And there was no significant change in G2/M phase cell proportion (P>0.05).
Conclusion:ATRA could significantly inhibit the proliferation and migration of human gastric cancer MGC-803 cells, suggesting that the mechanism might be to block the cells in G0/G1 phase by affecting the cell cycle process.
Key words: gastric cancer; ATRA; proliferation; migration; cell cycle
Gastric cancer, which occurs primarily in the gastric mucosa, is a typical digestive system tumor with high morbidity and mortality and has attracted extensive attention. Although the incidence rate of gastric cancer has decreased in recent years, the early diagnosis of gastric cancer is difficult, and most patients are not found until the late stage [1],so the control and treatment of gastric cancer is still not ideal. Therefore, exploring more effective treatment methods for gastric cancer has become a research hotspot. All-trans-retinoic acid (ATRA) is a natural derivative of vitamin A, which plays a significant role in inhibiting tumor proliferation and inducing its differentiation and apoptosis. It is the main clinical therapeutic drug for acute promyelocytic leukemia [2].In addition, studies have confirmed that ATRA has certain anti-tumor activity in colorectal cancer, liver cancer, lung cancer, breast cancer and other tumors. Vitamin A mainly functions through two nuclear receptors, RAR and RXR. Increasing evidence suggests that ATRA may play an anti-proliferation role in gastric cancer by upregulating RARα expression. In this study, the effects of ATRA on the proliferation, migration and cell cycle of human gastric cancer MGC-803 cells were observed by using CCK-8 method, cell scratch test and flow cytometry, to provide experimental basis for the clinical treatment of gastric cancer.
2.1 Materials and instruments
Human GC cell lines MGC-803 was purchased from the Biotechnology Development Co. Ltd (Shanghai, China), and was maintained in a 37˚C incubator with 5% CO2. RPMI 1640 and PBS were purchased from Biological Industries(Israel); Fetal bovine serum was purchased from cegrogen company(Germany); ATRA was purchased from csnpharm company(USA); CCK-8 was purchased from ruipat Biotechnology Co., Ltd (Hebei, China); CO2 incubator and multiskan FC microplate reader were purchased from thermo(USA);FACScalibur was purchased from BD Company(USA).
2.2 Experimental method
2.2.1 Cell culture
Gastric cancer MGC-803 cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum, placed in 37 ° C, 5% CO2 incubator, and passaged 1:3 when the density reached about 70%, and the logarithmic MGC-803 cells with vigorous growth were used for subsequent experiments.
2.2.2 Cell proliferation was detected by CCK-8 method
The control group and different concentrations (10, 20, 40, 60, 80 μ mol/L) of ATRA drug group, and blank wells (only culture medium was added, no cells). After the cells were passaged according to 1.2.1, the cell count was adjusted to 4×104 / ml, evenly inoculated in 96 well plates at 100 µ L / well. Each group was provided with 6 multiple wells. After 24 hours of incubation, the prepared solution was changed according to groups, and the incubation continued for 24, 48 and 72 hours. Mix the basic culture medium and CCK-8 reagent in the EP tube according to 9 volumes: 1 volume to prepare CCK-8 reaction solution, take out 96 well plates under each action time, and replace each well with CCK-8 reaction solution 100 μL. After incubation for 0.5h, the absorbance value (OD value) of each well at the wavelength of 450nm was detected with a microplate reader. The experiment was repeated three times to calculate the growth inhibition rate of MGC-803 cells with different concentrations of ATRA. Inhibition rate (%) = (control group OD value - experimental group OD value) / (control group OD value - blank group OD value) × 100%, and IC50 was calculated. According to the results of CCK-8, the concentration of 20, 40, 60μmol / L ATRA for 48h as the following experimental conditions.
2.2.3 Observation on the morphological structure of MGC-803 cells
The control group and different concentrations (20, 40, 60 μmol/L) of ATRA drug group, and the logarithmic long-term MGC-803 cells were collected at 2×105 / well were inoculated in 6-well plates for culture. After 24h, when the cell fusion degree was about 60%, the original culture medium was discarded, and the culture medium was changed according to groups. After 48h, the morphological changes of MGC-803 cells in each group were observed under an inverted microscope and photographed.
2.2.4 Scratch test to detect cell migration
The control group (RPMI 1640 medium containing 1%FBS) and different concentrations (5, 10, 20 μmol/L) of ATRA drug group containing 1% FBS, make 5 parallel lines at equal distance with marker pen on the back of 6-well plate, and count again after cell passage according to 1.2.1, and adjust the density to 1.5 × 105 cells / ml were inoculated in 6-well plates at 2ml per well for culture. After 24 hours, when the confluence of the cell monolayer reached 90%, the 200 µL pipette head was used to scratch the parallel line perpendicular to the marker pen. After discarding the old culture medium, PBS was added to gently wash it for 3 times. After washing off the suspended cells, the prepared solution was added in groups, observed under the microscope, and photographed (0h). After incubation in the incubator for 12, 24 and 48h, take photos again and record. The experiment was repeated three times. The scratch area was analyzed and calculated under the image J interface, and statistical analysis was performed. Repair rate of scratch area (%) = [(0h) scratch wound area - (12h / 24h / 48h) scratch wound area] / (0h) scratch wound area × 100%。
2.2.5 Flow cytometry detection of cell cycle
Group according to 1.2.3. When the cell density at the bottom of the culture flask reaches about 60%,wash the cells twice with PBS, and replace them with RPMI-1640 complete medium and ATRA solution of different concentrations according to the group, and culture for 48h. After 48h, it was washed twice with PBS. After trypsin digestion, the medium was discarded by centrifugation at 1500r / min for 5min. After being rinsed and mixed with precooled PBS, the cells were counted, and the medium was discarded by centrifugation again × 106 cells were mixed with 1ml PBS, and 1ml cell suspension was slowly dropped into an EP tube containing 3ml of 70% ice ethanol and fixed at - 20℃ overnight. The next day, after mixing the cells, centrifuge and discard the supernatant, add 3ml PBS for washing, blowing and mixing, and stand at room temperature for 15min. After centrifuging again according to the above centrifugation conditions, discard the PBS, add 1ml PI/RNase reagent into each tube, incubate for 30min at room temperature in the dark, and then filter. Finally, the computer is used to detect the percentage of G0 / G1, S, G2 / M in each phase. The experiment was repeated three times.
2.3 Statistical Analysis
SPSS 26.0 statistical software was used to for data analysis. Data are presented as the mean ± SEM. One way ANOVA was used for statistical analysis. And a value of P < 0.05 was considered statistically significant .
3.1 Effect of ATRA on proliferation of gastric cancer MGC-803 cells
The results showed that ATRA could inhibit the proliferation of gastric cancer MGC-803 cells( Table 1), and with the increase of ATRA concentration and the extension of action time, its inhibitory effect gradually increased in a dose-time dependence (P < 0.05, Fig. 1). The IC50 values of MGC-803 cells treated with ATRA at 24h, 48h and 72h were 78.51 μmol/L, 44.58 μmol/L,25.98 μmol/L.
Table 1. The proliferation inhibition rate of MGC-803 cells of each group(± s,%)
|
ATRA (μmol/L) |
Inhibition rate (%) |
||
|
24h |
48h |
72h |
|
|
10 |
4.47±2.39 |
14.74±5.57# |
27.44±5.80# |
|
20 |
12.33±3.15* |
27.11±4.82*# |
41.72±3.67*# |
|
40 |
27.15±1.83* |
43.51±8.16*# |
63.56±5.80*# |
|
60 |
41.55±6.08* |
65.17±4.01*# |
82.68±1.17*# |
|
80 |
63.25±4.43* |
83.59±4.81*# |
96.34±2.48*# |
Compared with 24h # P < 0.05 ,compared with 10 μmol/L * P < 0.05
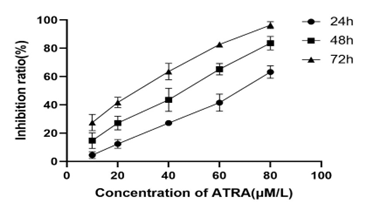
Fig 1. The inhibitory effect of different concentrations of ATRA on proliferation of MGC-803 cells at different times
3.2 Morphological changes of gastric cancer MGC-803 cells
Under the inverted microscope, it was found that MGC-803 cells in the control group adhered to the wall and grew well, and the intercellular connections were tight. After ATRA acts on MGC-803 cells, the cells lose their original state and become elongated, with abnormal morphology and different sizes. The cells are fragmented and the spacing becomes larger, and the number of adherent cells decreases. The above performance is more obvious with the increase of ATRA concentration (Fig. 2).
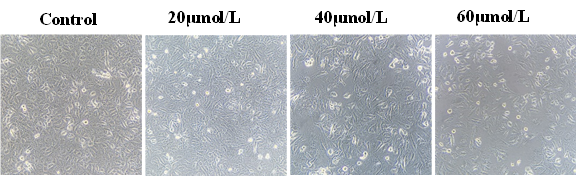
Fig 2. Morphological changes of MGC-803 cells treated with different concentrations of ATRA for 48h
3.3 Effect of ATRA on the migration of gastric cancer MGC-803 cells
After ATRA treated gastric cancer MGC-803 cells for 12h, 24h and 48h, compared with the control group, the healing speed of cell scratch was significantly slowed (Table 2), and the repair rate of scratch area gradually decreased with the increase of drug concentration (P < 0.05, Fig. 3).
Table 2. The repair rate of scratch area of MGC-803 cells of each group(± s,%)
|
|
ATRA concentration (μmol/L) |
Scratch area repair rate(%) |
||
|
12h |
24h |
48h |
||
|
|
0 |
29.74±2.11 |
39.75±0.88 |
51.31±0.96 |
|
|
5 |
25.43±1.35* |
32.88±1.33* |
43.60±0.38* |
|
|
10 |
20.90±0.78* |
26.56±1.25* |
36.58±1.45* |
|
|
20 |
15.70±2.25* |
22.25±2.79* |
30.37±1.91* |
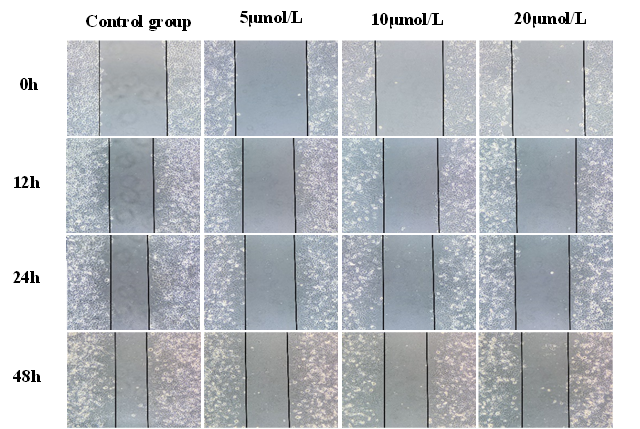
Fig 3. The migration ability of each group cells was detected by scratch assay Effect of ATRA on cell cycle of gastric cancer MGC-803 cells
3.4 Effect of ATRA on the cell cycle of gastric cancer MGC-803
The results showed that ATRA could significantly change the cell cycle of gastric cancer MGC-803 (Fig. 4). ATRA increased the proportion of cells in G0 / G1 phase and decreased the proportion of cells in S phase, and this change became more and more significant with the increase of ATRA concentration (P < 0.05, table 3), while the proportion of cells in G2 / M phase did not change significantly (P > 0.05, table 3), indicating that ATRA can block MGC-803 cells in G0 / G1 phase.
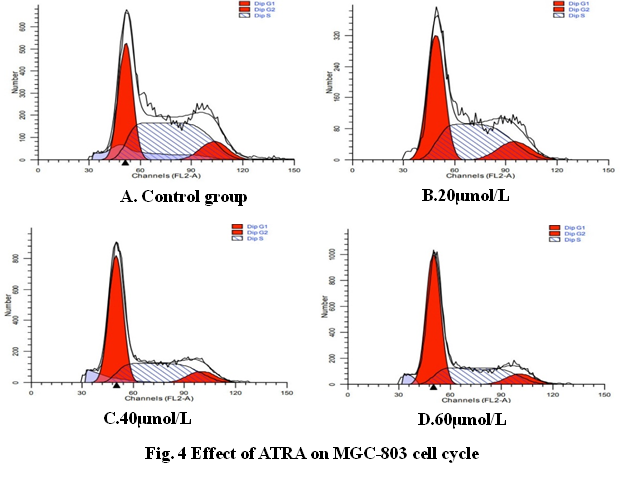
Fig 4. Effect of ATRA on MGC-803 cell cycle
Table 3. The cell cycle progression of MGC-803 cells in each group(± s,%)
|
ATRA (μmol/L) |
Cell cycle (%) |
||
|
G0/G1 |
S |
G2/M |
|
|
0 |
33.08±0.80 |
56.09±0.77 |
10.84±0.97 |
|
20 |
43.19±1.18* |
46.05±0.28* |
10.76±1.42 |
|
40 |
52.39±0.44* |
38.05±0.80* |
9.56±0.68 |
|
60 |
54.48±0.95* |
35.43±0.47* |
10.09±1.09 |
Compared with the control group , *P<0.05
Gastric cancer (GC) is the most common malignant tumor of digestive tract in the world, especially in Asia. It is the second largest cancer causing morbidity and mortality in China, which poses great threat to the national health [3]. Therefore, finding new drug treatment is the focus of current research. Studies have found that ATRA plays a significant role in tumor suppression. ATRA has two kinds of nuclear retinoic acid receptors: retinoic acid receptor (RAR) and retinoid X receptor (RXR), both of which are composed of α, β and γ subtypes, and each subtype has multiple splicing variants [4]. ATRA is metabolized in two ways after it enters cells in a simple dispersion mode. One is that it binds to RA binding protein I (CRABP I) and is transported to the endoplasmic reticulum to be decomposed and digested by cytochrome P450 isoform 26 (CYP26) into inactive products. Second, it binds to cellular retinoic acid binding protein II (CRABP II) and continues to bind to nuclear receptors such as rar after being transported to the nucleus, thus affecting mRNA transcription, protein synthesis and other processes and playing a wide range of biological functions [5].
In recent years, studies have found that ATRA can regulate the expression of a variety of cell transforming growth factors, change cell ultrastructure, promote tumor cell apoptosis and improve the killing efficiency of immune cells, to play a role in inhibiting proliferation and inducing differentiation of tumor cells, but its mechanism of action is not completely the same. Li et al. [6] found that ATRA can significantly inhibit the proliferation of pancreatic cancer, and the mechanism is related to the upregulation of Chmo1A, CRBP-1,etc. In addition, the anti-proliferation and pro apoptosis effects of ATRA on colorectal cancer cells may be related to caspase activation. At the same time, many scholars have found that the regulatory effect of ATRA on a variety of tumors is closely related to its ability to regulate the cell cycle. Interphase (G1,S , G2) and mitosis (M) constitute the whole cell cycle. Interphase is mainly responsible for DNA replication and synthesis of related proteins, ensuring smooth alternation of the whole process. The abnormal cell cycle stimulated by external factors, which leads to uncontrolled cell growth, is an important cause of tumorigenesis. Luo et al. [7] found that the G0 / G1 cycle was blocked after ATRA was applied to osteosarcoma U2OS and MG63 cells, while the expression of cell cycle regulatory gene p21 and cyclin dependent kinase 7 (CDK7) decreased and the expression of cyclin C increased, which significantly inhibited the proliferation ability of the two cells. In addition, marzinke and Clagett Dame et al. [8] found that ATRA can change the cell cycle process of neuroblastoma through clmn gene, induce G1 phase to S phase arrest, and thus inhibit cell proliferation. Zhang et al. [9] confirmed that ATRA also has a significant inhibitory effect on the proliferation of ovarian cancer cells. The reason is that ovarian cancer cells are blocked in the middle and late stages of G1, which greatly reduces the number of cells entering S phase. ATRA mainly plays its role by triggering G1 arrest, affecting DNA synthesis and enhancing the killing function of immune cells [10-11]. Many scholars have found that the antiproliferative effect of ATRA on gastric cancer cells is also related to the regulation of cell cycle. Studies have confirmed that ATRA can block gastric cancer BGC-823 cells in G1 phase and affect DNA synthesis, inhibit cell proliferation and promote apoptosis, and inhibit the migration of BGC-823 cells [12]. In addition, some in vitro experiments have shown that ATRA also inhibits cell proliferation in gastric cancer SGC-7901 cells [13], and the mechanism may be that the expression of p-GSK-3β was decreased by reducing the expression of p-Akt protein, and finally the expression of Cyclin D1 was affected to trigger G1 phase arrest and inhibited proliferation. In this study, we found that ATRA significantly inhibited the proliferation of human gastric cancer MGC-803 cells in a dose-time dependent manner, in the scratch assay, serum-free or low serum medium need to be used to avoid the effect of cell proliferation, since ATRA has a significant Pro apoptotic effect on tumor cells such as liver cancer, gastric cancer and breast cancer [14], large cell sheets die at higher ATRA concentrations, which may be related to the pro apoptotic effects of ATRA and altered growth status of cells in low serum environments. Therefore, to ensure the scientific validity and accuracy of the experiments, ATRA concentrations were reduced by scratch assays, which showed that ATRA significantly inhibited migration of MGC-803 cells, it was also observed under an inverted microscope that the morphology and state of the cells changed significantly after 48 h of ATRA treatment, and the higher the concentration, the more significant the change. To further explore whether the inhibitory effect of ATRA on gastric cancer MGC-803 cells is cell cycle dependent, we further tested the effect of ATRA on the cell cycle of MGC-803 cells by flow cytometry assay, and found that ATRA induced G0 / G1 phase accumulation and arrested cell cycle progression in MGC-803 cells, which was consistent with the above results of ATRA on other cancer cells, suggesting that ATRA may act by blocking G1 to S phase progression. Thus, achieving the effect of inhibiting the proliferation of gastric cancer MGC-803 cells, which provides a new idea for the study of anti-tumor drugs.
In summary, in this experiment, we confirmed that ATRA significantly inhibits the proliferative activity and migration ability of gastric cancer MGC-803 cells, which may be related to its ability to induce cell cycle arrest. This study provides an experimental basis for the application of ATRA in the clinical treatment of gastric cancer, but the specific mechanism needs to be further explored and investigated.
Funding
CHENGDE Science and Technology Research and Development Program. NO. 202109A055.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Patrad E, Niapour A, Farassati F, et al. Combination treatment of all-trans retinoic acid (ATRA) and γ-secretase inhibitor (DAPT) cause growth inhibition and apoptosis induction in the human gastric cancer cell line[J]. Cytotechnology, 2018, 70(2): 865-877. PMid:29417442
View Article PubMed/NCBILiu Y, Wen Q, Chen XL, et al. All-trans retinoic acid arrests cell cycle in leukemic bone marrow stromal cells by increasing intercellular communication through connexin 43-mediated gap junction[J]. J Hematol Oncol, 2015, 8(1): 110. PMid:26446715
View Article PubMed/NCBIZhou JC, Zheng RS, Zhuang GH, et al. Analysis on the trend of gastric cancer incidence and age change in cancer registration regions of China,2000 to 2015[J]. J Pract Oncol, 2020, 34(1): 1-5.
Ni X, Hu G, Cai X. The success and the challenge of all- trans retinoic acid in the treatment of cancer[J]. Crit Rev Food Sci Nutr, 2019, 59(sup1): s71-s80. PMid:30277803
View Article PubMed/NCBILiang XD, Deng XH, Li CH, et al. Update of the effect of all-trans retinoic acid on gap junctional intercellular communication of keratinocytes in psoriasis [J]. China Journal of Leprosy and Skin Diseases, 2020, 36(5): 316-320.
Li J, Orr B, White K, et al. Chmp 1A is a mediator of the anti-proliferative effects of all-trans retinoic acid in human pancreatic cancer cells[J]. Mol Cancer, 2009, 8(1): 1-13. PMid:19216755
View Article PubMed/NCBILuo P, Yang X, Ying M, et al. Retinoid-suppressed phosphorylation of RARα mediates the differentiation pathway of osteosarcoma cells[J]. Oncogene, 2010, 29(19): 2772-2783. PMid:20190807
View Article PubMed/NCBIMarzinke MA, Clagett-Dame M. The all-trans retinoic acid (atRA)-regulated gene Calmin (Clmn) regulates cell cycle exit and neurite outgrowth in murine neuroblastoma (Neuro2a) cells[J]. Exp Cell Res, 2012, 318(1): 85-93. PMid:22001116
View Article PubMed/NCBIZhang D, Holmes WF, Wu S, et al. Retinoids and ovarian cancer[J]. J Cell Physiol, 2000, 185 (1):1-20. 185:1<1::AID-JCP1>3.0.CO;2-O
View ArticleSchenk T, Stengel S, Zelent A. Unlocking the potential of retinoic acid in anticancer therapy[J]. Br J Cancer, 2014, 111(11): 2039-2045. PMid:25412233
View Article PubMed/NCBIHe CD, Wei J, Li LJ. All-trans retinoic acid induces cell-cycle arrest in human cutaneous squamous carcinoma cells by inhibiting the mitogen-activated protein kinase-activated protein 1 pathway[J]. Clin Exp Dermatol, 2014, 39(3): 354-360. PMid:24635079
View Article PubMed/NCBIYang YY, Hu AL, Zhang SM, et al. Effect of all-trans retinoic acid on the migration of human gastric cancer BGC-823 cells[J]. Acta Univ Med Anhui, 2014, 49(7): 867-871.
Zhang S, Shi R, Chen S, et al. All-trans retinoic acid inhibits the prolife-ration of SGC7901 cells by regulating caveolin-1 localization via the ERK/MAPK signaling pathway[J]. Oncol Lett, 2018, 15(2): 1523-1528.
View ArticleWei X, Chen SL, Jia N, et al. Effect of all-retinoic acid on the apoptosis of human gastric cancer SGC-7901 cells [J]. Acta Univ Med Anhui, 2017, 52(8): 1119-1122.
