Ji Eun Park
Phone: +82.2.539.5408
E-mail: n2616@naver.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 2
Page No: 184-192
Ji Eun Park
Phone: +82.2.539.5408
E-mail: n2616@naver.com
Ji Eun Park, Young Mi Kim
Theraderm Co., Ltd. R&D Department, 10-6, Yeondong-daero 86-gil, Gangnam-gu, Seoul 135-502, South Korea
Kim M, Park J, et al. Protective effect of kudzu root vinegar and adenosine against UVB-induced oxidative stress in human keratinocytes. Journal of Cellular and Molecular Physiology (2020); 3(2) pp: 184-192
Ultraviolet (UV) irradiation generates reactive oxygen species (ROS) in cells, which induces sunburn cell formation, melanoma, photoaging, and skin cancer. This study examines the anti-photodamage effects of kudzu root vinegar and adenosine in UVB-exposed human keratinocytes (HaCaT cells).
UVB significantly decreased HaCaT cell viability, whereas kudzu root vinegar and adenosine did not exhibit cytotoxic effects and increased the viability of HaCaT cells.
To investigate the protective effects of kudzu root vinegar and adenosine on UVB-induced oxidative stress in HaCaT cells, ROS, matrix metalloproteinases (MMPs), and mitogen-activated protein kinase (MAPK) were analyzed.
UVB-induced treatment reduced the activity of antioxidant enzymes; however, kudzu root vinegar and adenosine increased their activity. These results indicated that kudzu root vinegar and adenosine exert cytoprotective activity against UVB-induced oxidative stress in HaCaT cells. Moreover, they suppressed the UVB-induced downregulation of MMPs and inhibited the phosphorylation of MAPK induced by UVB-irradiation. Therefore, kudzu root vinegar and adenosine offer anti-oxidative effects, via lowering ROS production, suppressing JNK activation, and downregulating expression of MMPs.
Our findings suggest that kudzu root vinegar and adenosine have potential application in preventing skin damage owing to UVB exposure.
Keywords: reactive oxygen species (ROS), HaCaT cell, UVB, skin damage, anti-aging
The skin, the largest organ in the body, can be divided into the epidermis, dermis, and hypodermis. The epidermis is made of keratinocytes, which are consistently affected by various harmful environmental factors such as ultraviolet (UV) irradiation from the sun.[1] There are three kinds of UV irradiation: UVA (~320–540 nm), UVB (~280–320nm), and UVC (~100–320 nm) light. Among them, UVB exposure to the skin results in considerable mitochondrial malfunction, oxidative stress, inflammation, and activation of apoptosis leading to skin aging.[2, 3] We may reasonably conclude that UVB exposure is a serious risk factor for skin cancer. Accumulated data indicate that UVB has a primarily deleterious effect on the induction of direct DNA damage or the production of reactive oxygen species (ROS).[4-7]
ROS are known to be involved in a number of skin diseases, including tumor promotion and cancer, autoimmune skin diseases, phototoxicity, photosensitivity, and aging.[8] The skin contains keratin, which increases the horny skin supplement that is dissipated by ROS. The dermis consists of elastin and collagen, which are responsible for the strength and elasticity of the skin and are also reduced by ROS.[9]
Direct ROS and DNA damage alter signaling pathways such as mitogen-activated protein kinase (MAPK) that are involved in cell proliferation and survival.[10, 11] MAPK is composed of extracellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK), and p38 kinase. The MAPK subfamily is highly relevant to a variety of transcription factors, in particular the activated protein-1 (AP-1), as well as complexes containing c-Fos and c-Jun, which are known to be activated by JNK, p38 MAPK, and matrix metalloprotease (MMP)-9 expression, and are highly dependent on AP-1.[12, 13] Accumulation of ROS induces changes in the MAPK signaling pathway and weakens the activity of antioxidant enzymes. UVB promotes ERK and p38 MAPK signaling in keratinocytes by increasing ROS generation.[14]
ROS caused from UV radiation can induce activation of MMPs, by altering cellular responses. Namely, MMPs produced from keratinocytes, fibroblasts, and inflammatory cells can cause photoaging.[15] Together with MMP-1, MMP-3 and MMP-9 can completely degrade mature fibrillar collagen of the skin.[16, 17] Therefore, UV irradiation of human skin induces extracellular matrix degradation via induction of transcription factors, which in turn increase MMP production.[18, 19]
Kudzu root (KR, Pueraria lobate) is a perennial herb commonly known as “vidarikanda” and is distributed in the tropical parts of India.[20, 21] The plant’s tuber is widely used in ethnomedicine as well as in traditional systems of medicine, particularly in Ayurveda. It has been used in a variety of Ayurvedic formulations as a restorative tonic that has antiaging, spermatogenic, and immune boosting properties; it has been suggested for the treatment of cardiovascular diseases, hepatosplenomegaly, fertility disorders, menopausal syndrome, sexual debility, and spermatorrhoea.[22] Therefore, we have studied its antiaging effects by developing KR vinegar (KRV) (Fig.1).
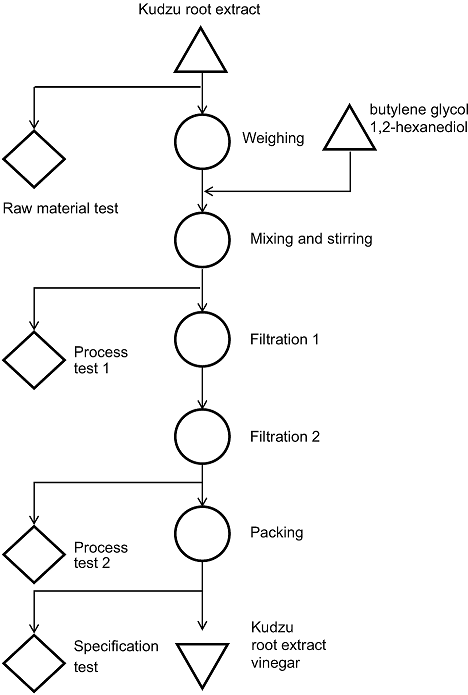
Fig. 1. Sampling in manufacturing process.
In addition, adenosine (AD) is a well-known purine nucleoside that consists of an adenine molecule attached to a ribose sugar molecule moiety.[23] It is present in all biological organs, tissues, and cells. Adenosine has been constituted that regulates cell proliferation, apoptosis, differentiation, angiogenesis, and metastasis in many tumors such as breast, colon, leukemia, liver, and melanoma.[24]
Here, on the basis of the reported effects of KRV and adenosine (AD), the purpose of this study was to examine potential UVB-induced skin aging effects of KRV and AD in vitro by elucidating the underlying mechanism involved and develop a potential new antiaging therapy.
2.1. Materials
Antibodies for JNK and p-JNK were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). Adenosine, gelatin, and GAPDH antibody were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The 2′-7′ dichlorofluorescin diacetate (DCF-DA) was procured from Invitrogen (Carlsbad, CA, USA). Hank’s balanced salt solution (HBSS) was purchased from the Meditech (Herndon, VA, USA).
2.2. Cell culture and treatment
Human keratinocytes (HaCaT cells) were obtained from American Type Culture Collection (ATCC) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin G and 100 mg/ml streptomycin) at 37°C in a humidified incubator containing 5% CO₂ and 95% air. In all experiments, cells were seeded at 1×106 cells/well and incubated with KRV and AD at 50–60% confluence. All chemicals were dissolved in ethanol and the final ethanol concentration was less than 0.1%.
2.3. KRV and AD treatment and UVB irradiation
HaCaT cells were seeded in 96-well plates at densities of 2 × 103 cells/100 µl. Twenty-four hours later, the cells were pretreated with KRV at 37°C and then with AD 3 h later. The cells were washed twice with phosphate-buffered saline (PBS), and the cells were next exposed to UVB radiation (30 mJ/cm2) in PBS by using a Bio-link BLX (Vilber Lourmat, France). Control cells were subjected to the same treatment schedule without UVB irradiation.
2.4. Cell viability
The cell growth effect was measured by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (CellTiter 96® aqueous non-radioactive cell proliferation kit; Promega, Wisconsin, USA). HaCaT cells (2 × 103) were incubated in triplicate in a 96-well plate in the presence or absence of KRV and AD in a final volume of 100 µl at different time intervals for 24 h at 37°C under 5% CO2. MTS solutions (5 mg/ml) were added to each well, and the cells were cultured for another 1 h, after which, the optical density was read at 492 nm (Tecan Trading AG, Switzerland). Cell viability is presented as the relative percentage compared to the control.
2.5. Annexin V staining
Annexin V staining was performed using a fluorescein isothiocyanate (FITC)-Annexin V staining kit (BD Biosciences, San Jose, CA, USA) following the manufacturer’s instructions. Briefly, PA and adenosine-treated cells were washed with PBS and resuspended in binding buffer containing Annexin V and propidium iodide (PI). Fluorescence intensity was measured by flow cytometry (BD Biosciences).
2.6. Measurement of reactive oxygen species (ROS) accumulation
Cells were treated with KRV and AD, and then the cells were exposed to UVB radiation (30 mJ/ cm2) 3 h later and incubated for another 24 h; after this, cells were treated with 25 μM DCF-DA. After incubation for 30 min at 37°C in a 5% CO2 incubator, cells were washed twice with PBS solution, suspended in complete media, and examined under a fluorescence microscope to detect intracellular ROS accumulation.
2.7. Western blot analysis
Cells were harvested and lysed in RIPA buffer, and the resulting protein samples were quantified by using the bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Equal amounts of protein extracts were denatured by boiling at 100°C for 5 min in sample buffer. The proteins were separated by 8–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween 20 buffer (TBS-T; 10 mM Tris, 150 mM NaCl, pH 7.5, and 0.1% Tween 20) for 1 h. The membranes were washed 3 times for 10 min each with TBS-T buffer and incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies. The membranes were washed 3 times for 10 min each with TBS-T buffer. Immunoblot membranes were incubated with Super-signal pico-chemiluminescent substrate or dura-luminol substrate (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instruction and visualized with ImageQuantTM LAS 4000 (Fujifilm Life Science, Japan).
2.8. Gelatin zymography
Gelatin zymography was accomplished in 10% polyacrylamide gel in the presence of gelatin (0.1% w/v) as a substrate for MMP-9. The protein samples were mixed with 5× loading buffer and separated on a 10% SDS-PAGE gel without denaturation. Later, the gel was washed with renaturing buffer (2.5% Triton X-100) for 10 min, twice at room temperature, and incubated for 48 h at 37°C in incubation buffer. After the enzyme reaction, the gel was stained with Coomassie blue R-250 buffer for 1 h and destained with 7.5% acetic acid solution containing 10% methanol. Areas of gelatinase activity were detected as clear bands against the blue-stained gelatin background.
2.9. Senescence-associated β-galactosidase (SA-β-gal) histochemical staining
Cells were cultured in 6-well plates (2 × 105 cells/well), pretreated with KRV and AD, and exposed to UVB. Cells were washed with PBS and fixed for 5 min in 3% formaldehyde. SA-β-gal staining was determined using a senescence β-galactosidase staining kit (Cell Signaling Technology, Danvers, MA, USA). The percentage of blue-stained senescent cells was determined using a light microscope.
2.10. Statistical analysis
Where appropriate, data are expressed as the mean ± SD of at least three independent experiments, and statistical analysis for single comparison was performed using the Student’s t-test; p values less than 0.05 were considered statistically significant.
3.1. Effect of KRV and AD on HaCaT cell viability
The cytotoxic effect of PA and AD on HaCaT cells was determined by the MTS assay (Fig. 2A, B). The cells were treated with various concentration of KRV (20, 50, and 100 µg/ml) and AD (20, 50, and 100 µl) for 24 h. As cell viability was within 80%, it was considered that there was no effect on viability. In addition, the protective effect of KRV and AD against UVB-induced cell toxicity was determined using the MTS assay. UV irradiation (30 mJ/cm2) induced cell death in HaCaT cells. Exposure of UVB (30 mJ/cm2) for 24 h reduced cell viability to approximately 60%, as compared to the non-UV-irradiated group. However, following pretreatment with KRV and AD, the cell viability was increased. Based on these results, a KRV concentration of 100 µg/ml and 100 µl of AD were used in further studies (Fig. 2C).
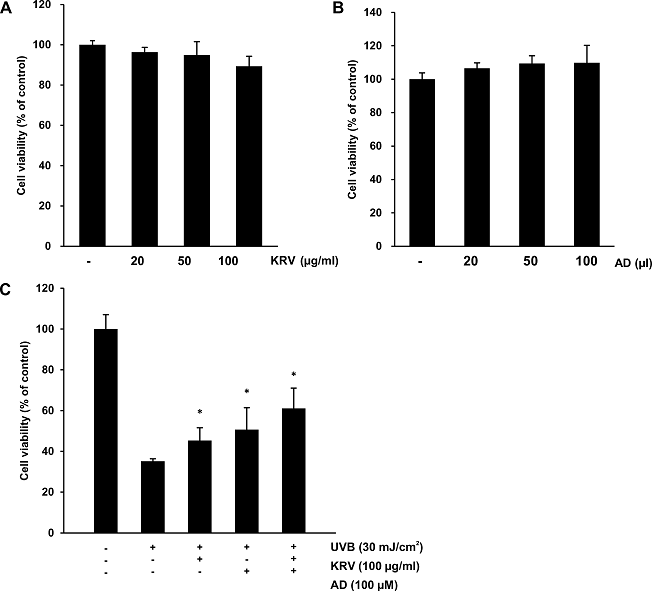
Fig. 2. Cytotoxic effect of KRV and AD in HaCaT cells. (A) HaCaT cells were treated with the indicated concentrations of KRV for 24 h. Cell viability was determined by the MTS assay. (B) HaCaT cells were treated with the indicated concentrations of AD for 24 h. Cell viability was determined by the MTS assay. (C) HaCaT cells were pretreated with KRV and AD, exposed to UVB radiation (30 mJ/cm2) 3 h later, and incubated for another 24 h. Cell viability was determined by the MTS assay. Values are expressed as means ± SD. *, p < 0.05, compared to the control.
3.2. Effect of KRV and AD on UVB-induced cell apoptosis and necrosis
UVB-induced apoptotic and necrotic HaCaT cells were counted using quantitative flow cytometry with Annexin V and PI staining. As shown in Fig. 3A, in the cells irradiated with 30 mJ/cm2 UVB, the percentage of living cells was significantly reduced to 69.12%. In contrast, KRV and AD treatment increased the viable cell count to 83.21%. The results indicated that pretreatment of cells with KRV and AD dramatically decreased the percentages of apoptotic cells as compared to UVB-induced cells. Quantification of apoptotic cells is shown in Fig. 3B.
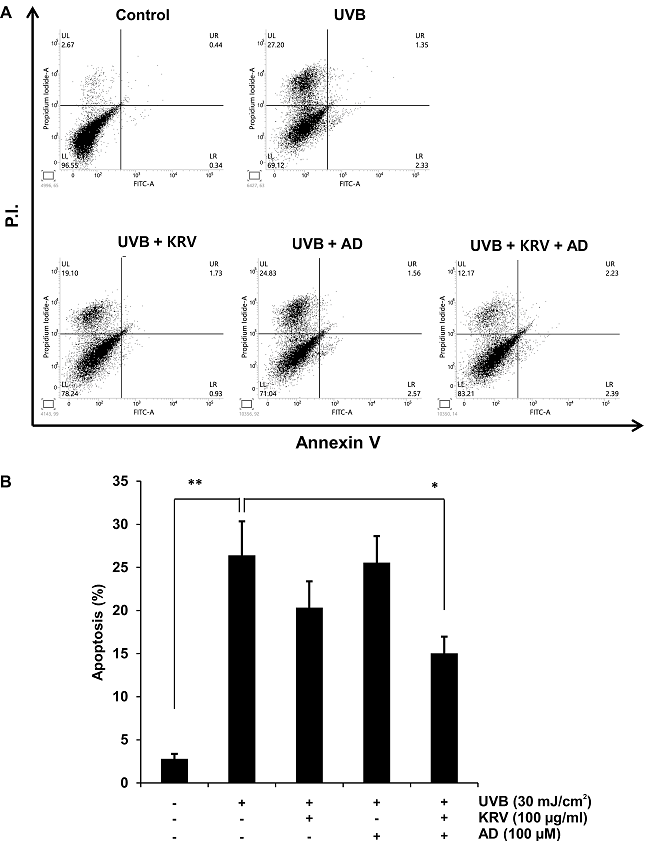
Fig. 3. Effect of KRV and AD on UVB-induced cell apoptosis and necrosis in HaCaT cells. (A) The apoptotic index (%) was determined by flow cytometry upon treatment of cells with KRV and AD, exposed to UVB radiation (30 mJ/cm2) 3 h later, incubated for another 24 h, and stained with Annexin V and propidium iodide (PI). (B) This graph shows statistical analysis of apoptosis. Data are representative of three independent experiments. *, p < 0.05, **, p < 0.001, compared to the control.
3.3. Effect of KRV and AD on intracellular ROS generation in UVB-irradiated HaCaT cells
To estimate whether KRV and AD function as a scavenger of UVB-induced ROS generation, intracellular ROS was determined via the DCF-DA assay. Cells were pretreated with KRV (100 µg/ml) and AD (100 µM) and then exposed to UVB radiation (30 mJ/cm2) 3 h later and incubated for another 24 h. Cells were stained with DCF-DA for 30 min at 37°C in a 5% CO2 incubator; after incubation, ROS generation was measured by fluorescence analysis via flow cytometry. UVB-irradiated cells showed a strong DCF fluorescence. In contrast, KRV and AD treatment significantly decreased DCF fluorescence to about 30% the level of UVB-irradiated cells (Fig. 4A). Quantification of ROS generation is presented in Fig. 4B.
In addition, fluorescence microscopic observation showed that UVB-irradiated cells had the highest level of green fluorescence (Fig. 4C).
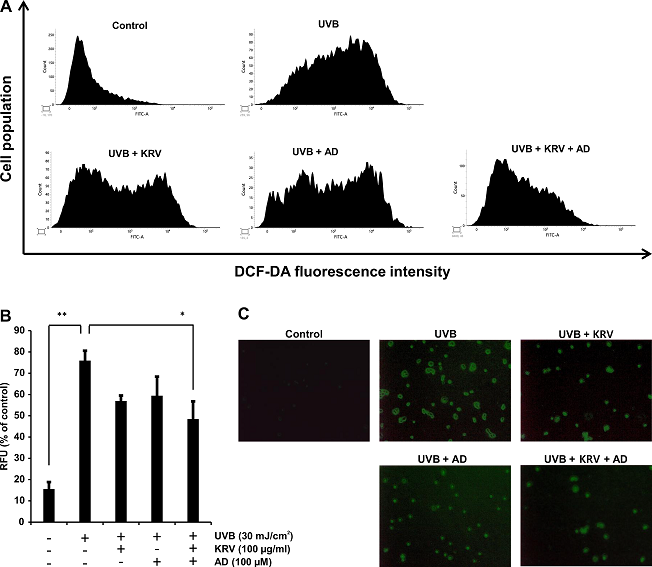
Fig. 4. Effect of KRV and AD on UVB-induced intracellular ROS generation in HaCaT cells. Cells were pretreated with KRV and AD, exposed to UVB radiation (30 mJ/cm2) 3 h later, and incubated for another 24 h. Later, cells were examined for intracellular accumulation of ROS, (A) as measured by flow cytometry. (B) Quantification and statistical analysis of ROS generation after KRV and AD treatment. The experiment was performed in triplicate and the data presented as mean ± SD. *, p < 0.05, **, p < 0.001. (C) Images were acquired with a fluorescence microscope using the DCF-DA fluorescence staining method (×200).
3.4. Effect of PA and AD on the regulation of UVB-mediated MMP
A pivotal contribution to photoaging is precipitated as the activated MMPs cause degradation and synthesis inhibition of extracellular matrix (ECM), and of collagen in connective tissues.[25] The effects of KRV and AD on MMP-9 in HaCaT cells were determined using zymography. UVB-induced HaCaT cells were pretreated with KRV and AD. Only UVB-exposed cells exhibited higher MMP-9 gene expression than UVB non-irradiated cells. However, MMP-9 gene expression of UVB-treated cells was appreciably decreased by KRV and AD (Fig. 5A). Quantification of MMP-9 is presented in Fig. 5B. From these experiments, KRV and AD exhibited the most potent protective effect on UVB-mediated photodamage.
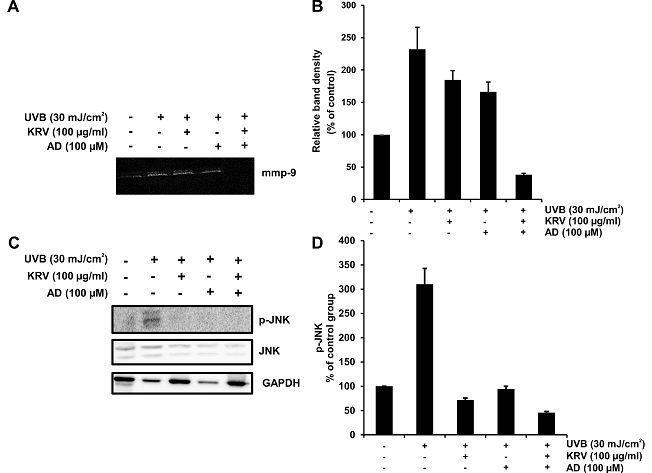
Fig. 5. Effect of KRV and AD on UVB-induced p-JNK and MMP-9 expression. HaCaT cells were pretreated with KRV and AD, exposed to UVB radiation (30 mJ/cm2) 3 h later, and incubated for another 24 h. (A) p-JNK levels were detected by western blot analysis. (B) The activity of MMP-9 was detected by gelatin zymography.
3.6. Effect on UVB-induced SA-β-gal activity in HaCaT cells
Levels of SA-β-gal, which represent the activity of a cellular senescence biomarker, in UVB-exposed HaCaT cells were examined by an SA-β-gal histochemical staining assay. The number of senescent cells in non-UVB irradiated HaCaT cells was low; however, a significant decrease in SA-β-gal activity was observed in non-UVB exposed cells. When the cells were exposed to UVB, SA-β-gal activity markedly increased; on the other hand, pretreatment with KRV and AD before UVB exposure blocked the increase in SA-β-gal activity (Fig. 6A). Quantification of SA-β-gal is presented in Fig. 6B.
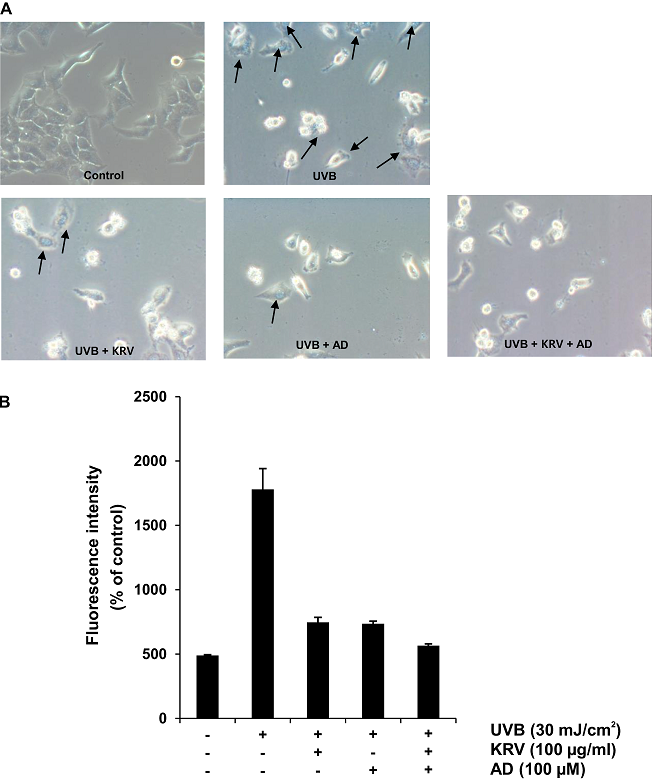
Fig. 6. Effect of KRV and AD on SA-ß-gal activity in HaCaT cells. HaCaT cells were pretreated with KRV and AD, exposed to UVB radiation (30 mJ/cm2) 3 h later, and incubated for another 24 h. Cellular senescence was assessed by SA-ß-gal activity staining.
Photoaging represents a group of mechanisms developed through an evolutionary process to minimize the oxidative damage that can occur in organs, including skin exposed to UV radiation. When the skin is exposed to UV, oxidative stress causes skin cells to undergo DNA damage, apoptosis, and MAPK dysregulation, owing to increased production of intracellular ROS. In addition, MMPs that degrade collagen and elastin in ECM are also activated through the UV-induced AP-1 pathway.[26-28]. This signal reduces skin elasticity and promotes skin aging, such as wrinkles.
Exposure of skin cells to UV can induce cell death. Pretreatment of skin cells with KRV and AD increased the cell viability of HaCaT cells irradiated with 30 mJ/cm2 (Fig. 2C). ROS perform an important role in inducing UV-mediated cell senescence through DNA alteration.[29] Excess production and elimination of ROS provoked skin aging induced by UV irradiation.[30] UVB irradiation significantly stimulted ROS production, but treatment with KRV and DA suppressed these consequences. Thus, ROS production is thought to be a key mediator of cell death caused by UVB exposure. Although UVB irradiation significantly stimulated ROS generation, KRV and AD treatment suppressed the effect (Fig. 4).
Based on previous studies, we measured the expression of MMPs and MAPK proteins markedly increased in UVB (30 mJ/cm2)-irradiated HaCaT cells (Fig. 5). MAPKs play an important role in regulating cell growth and differentiation and modulating cellular responses to cytokines and stressors.[31, 32] Exposure of HaCaT cells treated with UVB irradiation resulted in a substantial increase in the phosphorylation of JNK protein expression. When HaCaT cells were pretreated with KRV and AD, it was found to inhibit UVB-induced phosphorylation of JNK.
UVB-induced ROS generation is well known for its important role in UVB-initiated signaling pathways that lead to MMP induction to induce connective tissue destruction.[33] In particular, MMP-9 of the skin not only plays an important role in the final degradation of fibrin collagen after initial cleavage by collagenase but also in apoptotic development.[13, 34] In the present study, the inhibitory effect of KRV and AD on photodamage by UVB irradiation was also confirmed by its ability to block the expression of MMP-9.
Cellular senescence is thought to contribute to aging when the cells lose reproductivity in the body. SA-β-gal assays were performed in various cells and tissues to demonstrate the onset of celluar senescence.[35, 36] In this study, treatment with KRV and AD inhibited the increase in SA-β-gal activity in HaCaT cells exposed to UVB.
In summary, the results of this study demonstrated that KRV and AD inhibit the harmful effects caused by UVB irradiation of human keratinocytes. These findings demonstrate that KRV and AD protect against UVB-induced oxidative skin damage, including wrinkle formation, via the inhibition of ROS, MAPKs, and the MMP-9 signaling pathway.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Bravo K, Duque L, Ferreres F, et al. Passiflora tarminiana fruits reduce UVB-induced photoaging in human skin fibroblasts. J Photochem Photobiol B 2017; 168: 78-88. PMid:28189068
View Article PubMed/NCBIThiele JJ, Hsieh SN, Briviba K, et al. Protein oxidation in human stratum corneum: susceptibility of keratins to oxidation in vitro and presence of a keratin oxidation gradient in vivo. J Invest Dermatol 1999; 113: 335-339. PMid:10469330
View Article PubMed/NCBISubedi L, Lee TH, Wahedi HM, et al. Resveratrol-enriched rice attenuates UVB-ROS-induced skin aging via downregulation of inflammatory cascades. Oxid Med Cell Longev 2017; 2017: 8379539. PMid:28900534
View Article PubMed/NCBIZhang C, Wen C, Lin J, et al. Protective effect of pyrroloquinoline quinine on ultraviolet A irradiation-induced human dermal fibroblast senescence in vitro proceeds via the anti-apoptotic sirtuin 1/nuclear factor-derived erythroid 2-related factor 2/heme oxygenase 1 pathway. Mol Med Rep 2015; 12: 4382-4388. PMid:26126510
View Article PubMed/NCBIde Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol Appl Skin Physiol 2002; 15: 316-320. PMid:12239425
View Article PubMed/NCBIKulms D, Zeise E, Poppelmann B, et al. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene 2002; 21: 5844-5851. PMid:12185583
View Article PubMed/NCBIHeck DE, Vetrano AM, Mariano TM, et al. UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem 2003; 278: 22432-22436. PMid:12730222
View Article PubMed/NCBILopez-Torres M, Shindo Y, Packer L. Effect of age on antioxidants and molecular markers of oxidative damage in murine epidermis and dermis. J Invest Dermatol 1994; 102: 476-480. PMid:8151123
View Article PubMed/NCBIChen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermatol 2012; 67: 1013-1024. PMid:22406231
View Article PubMed/NCBIRhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 1999; 31: 53-59. PMid:10410302
View Article PubMed/NCBITorres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors 2003; 17: 287-296. PMid:12897450
View Article PubMed/NCBIJung YR, Kim DH, Kim SR, et al. Anti-wrinkle effect of magnesium lithospermate B from Salvia miltiorrhiza BUNGE: inhibition of MMPs via NF-kB signaling. PLoS One 2014; 9: e102689. PMid:25099178
View Article PubMed/NCBIYanti A, Hwang JK. Effects of macelignan isolated from Myristica fragrans Houtt. on UVB-induced matrix metalloproteinase-9 and cyclooxygenase-2 in HaCaT cells. J Dermatol Sci 2010; 57: 114-122. PMid:19914807
View Article PubMed/NCBIJibiki I, Hashimoto S, Maruoka S, et al. Apoptosis signal-regulating kinase 1-mediated signaling pathway regulates nitric oxide-induced activator protein-1 activation in human bronchial epithelial cells. Am J Respir Crit Care Med 2003; 167: 856-861. PMid:12623859
View Article PubMed/NCBIKim M, Park YG, Lee HJ, et al. Youngiasides A and C Isolated from Youngia denticulatum inhibit UVB-induced MMP expression and promote type I procollagen production via repression of MAPK/AP-1/NF-kappaB and activation of AMPK/Nrf2 in HaCaT cells and human dermal fibroblasts. J Agric Food Chem 2015; 63: 5428-5438. PMid:25994852
View Article PubMed/NCBISon Y, Kim S, Chung HT, et al. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol 2013; 528: 27-48. PMid:23849857
View Article PubMed/NCBISon Y, Cheong YK, Kim NH, et al. Mitogen-Activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J Signal Transduct 2011 2011; 792639. PMid:21637379
View Article PubMed/NCBIMauviel A. Cytokine regulation of metalloproteinase gene expression. J Cell Biochem 1993; 53: 288-295. PMid:8300745
View Article PubMed/NCBIQuan T, Qin Z, Xia W, et al. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc 2009; 14: 20-24. PMid:19675548
View Article PubMed/NCBIKim DY, Won KJ, Hwang DI, et al. Potential skin regeneration activity and chemical composition of absolute from Pueraria thunbergiana flower. Nat Prod Commun 2015; 10: 2009-2012. PMid:26749850
View Article PubMed/NCBIPark EK, Shin J, Bae EA, Lee YC, Kim DH. Intestinal bacteria activate estrogenic effect of main constituents puerarin and daidzin of Pueraria thunbergiana. Biol Pharm Bull, 2006; 29: 2432-2435. PMid:17142977
View Article PubMed/NCBIChatterjee B, Pancholi J. Prakriti-based medicine: A step towards personalized medicine. Ayu 2011; 32: 141-146. PMid:22408293
View Article PubMed/NCBILinden J, Adenosine in tissue protection and tissue regeneration. Mol Pharmacol 2005; 67: 1385-1387. PMid:15703375
View Article PubMed/NCBIFredholm BB. Adenosine--a physiological or pathophysiological agent? J Mol Med (Berl) 2014; 92: 201-206. PMid:24362516
View Article PubMed/NCBIHossen MJ, Hong YD, Baek KS, et al. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J Ginseng Res 2017; 41: 43-51. PMid:28123321
View Article PubMed/NCBIRittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev 2002; 1: 705-720. 00024-7
View ArticleNatarajan VT, Ganju P, Ramkumar A, et al. Multifaceted pathways protect human skin from UV radiation. Nat Chem Biol 2014; 10: 542-551. PMid:24937072
View Article PubMed/NCBILopez-Camarillo C, Ocampo EA, Casamichana ML. Protein kinases and transcription factors activation in response to UV-radiation of skin: implications for carcinogenesis. Int J Mol Sci 2012; 13: 142-172. PMid:22312244
View Article PubMed/NCBIChen QM, Prowse KR, Tu VC, et al. Uncoupling the senescent phenotype from telomere shortening in hydrogen peroxide-treated fibroblasts. Exp Cell Res 2001; 265: 294-303. PMid:11302695
View Article PubMed/NCBIHam SA, Hwang JS, Kang ES, et al. Ethanol extract of Dalbergia odorifera protects skin keratinocytes against ultraviolet B-induced photoaging by suppressing production of reactive oxygen species. Biosci Biotechnol Biochem 2015; 79: 760-766. PMid:25560618
View Article PubMed/NCBIRobinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 1997; 9: 180-186. 80061-0
View ArticleJohnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911-1912. PMid:12471242
View Article PubMed/NCBIBae JY, Choi JS, Choi YJ, et al. Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: involvement of mitogen-activated protein kinase. Food Chem Toxicol 2008; 46: 1298-1307. PMid:18226437
View Article PubMed/NCBIOnoue S, Kobayashi T, Takemoto Y, et al. Induction of matrix metalloproteinase-9 secretion from human keratinocytes in culture by ultraviolet B irradiation. J Dermatol Sci 2003; 33: 105-111. PMid:14581136
View Article PubMed/NCBIYang NC, Hu ML. A fluorimetric method using fluorescein di-beta-D-galactopyranoside for quantifying the senescence-associated beta-galactosidase activity in human foreskin fibroblast Hs68 cells. Anal Biochem 2004; 325: 337-343. PMid:14751269
View Article PubMed/NCBIPillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation - a review. Int J Cosmet Sci 2005; 27: 17-34. PMid:18492178
View Article PubMed/NCBI