Ji Eun Park
Phone: +82.2.539.5408
E-mail: n2616@naver.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 2
Page No: 193-202
Ji Eun Park
Phone: +82.2.539.5408
E-mail: n2616@naver.com
Ji Eun Park, Young Mi Kim
Theraderm Co., Ltd. R&D Department, 10-6, Yeondong-daero 86-gil, Gangnam-gu, Seoul 135-502, South Korea
Kim M, Park J, et al. Effects of Black Vinegar and Niacinamide on LPS-Induced Inflammation on Human Keratinocytes. Journal of Cellular and Molecular Physiolo-gy (2020); 3(2)pp:193–202
In this study, the effects of black vinegar (BA) and niacinamide on lipopolysaccharide (LPS)-treated human keratinocytes, HaCaT cells, were investigated. First of all, BA and niacinamide have no cytotoxicity in HaCaT cells even at high concentrations. LPS treatment triggers the phosphorylation of p38 mitogen-activated protein kinases (MAPK) and the expression of inflammatory enzymes, iNOS and COX-2. In contrast, BA and niacinamide weakened the expression of LPS-induced COX-2 and iNOS. Based on the results, we concluded that BA and niacinamide have effective anti-inflammatory properties in HaCaT cells. Therefore, BA and niacinamide may be used as new alternative treatments for inflammatory skin diseases.
Keywords: Black vinegar, HaCaT cell, Inflammation, Keratinocytes, Liposaccharides, Niacinamide
The skin is made up of three layers, the epidermis, dermis, and hypodermis. The epidermis, in particular, provides an effective immune and protective barrier that protects us from ultraviolet, mechanical, or chemical stress and colonization by microorganisms [1, 2]. Within the epidermis, keratinocytes play a role in physical barriers and the first immunity of the host [3]. The maintenance of skin homeostasis is affected by well-controlled interactions between the host and harmful environments [4].
Wound healing is achieved by the coordinated function of various cell types, cytokines, and growth factors [5]. The healing process consists of hemostasis, inflammation, proliferation, and remodeling [6]. Inflammation is a protective mechanism against harmful stimuli that promotes the healing process. Inflammation protects the body from a variety of irritants, including pathogens, damaged cells, or allergens [7]. Epidermal keratinocytes are involved in the inflammatory response to external stimuli and relay inflammatory signals. Epidermal keratinocytes are mostly induced by inflammatory stimulants, such as lipopolysaccharides (LPS) present in various pathogenic cells. LPS induces potent inflammation and immune responses with endotoxin, a membrane component of gram-negative bacteria [8-10]. Acute inflammation triggered by LPS induces the production of various cytokines, cell adhesion molecules, and inflammatory markers, such as nitric oxide (NO) [11].
NO plays a substantial role in the immune system, and NO production induced by LPS occurs via the activity of the inducible NO synthase (iNOS) [12, 13]. Furthermore, the signaling pathway of mitogen-activated protein kinases (MAPKs) is involved in the regulation of iNOS and COX-2 expression in LPS-stimulated HaCaT cells.
MAPKs play a definitive role in cell response to fundamental biological processes and external stress factors. MAPK is a family of proline-directed Ser/Thr kinases composed of the extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 MAPK. Among these, p38 MAPK is a typical inflammatory signal pathway [14-16]. MAPK activation is caused by cellular stresses, such as osmotic stress, DNA damage, ROS, and inflammatory cytokines. As a result, a variety of inflammatory reactions are accommodated by p38 MAPK, such as the expression of pro-inflammatory mediators, leukocyte adhesion, chemotaxis, oxidative burst, and degranulation, among others [17].
Inflammatory signals activate keratinocytes, epithelial cells, macrophages, mast cells, and Langerhans cells of the skin layer; a wide variety of inflammatory intermediates, such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-a; cytokines, IL-8, and monocyte chemotactic protein (MCP)-1 chemokines; and NO and prostaglandin E2 (PGE2) mediators [18]. IL-6 expression is highly and temporarily unregulated in almost all pathophysiological inflammatory conditions and in autoimmune diseases[19, 20]. Its trans-signaling is also critically included in the conservation of disease conditions by promoting the transfer of acute to chronic inflammation[19, 21]. Also, IL-8 is a well-known circulating inflammatory cytokine, which has many receptors on the surface membranes that can be bonded[22]. It is an effective neutrophil chemoattractant and activating factor produced by various cells, including keratinocytes[23].
Vinegar is a condiment made centuries ago from raw materials, such as fruits, rice, grains, and cocoa. Various types of vinegar, including special flavors, are popular in other parts of the world[24]. Among a variety of vinegar, rice vinegar is a traditional seasoning that has long been used in Asian countries[25]. Rice vinegar contains high levels of amino acids, minerals, organic materials, and so on. The main amino acids present in black vinegar (BA) are serine, alanine, valine, isoleucine, leucine, and γ- amino butyric acid[25, 26]. The anticancer effects of the brown rice vinegar were observed previously in vitro and in animal models of colon cancer. In this experiment, we used brown rice vinegar[27, 28].
Niacinamide (NA), the amide form of vitamin B3, is a safe, comprehensive, and inexpensive substance that may help prevent skin cancer[29]. NA confers a wide range of neuroprotective effects in response to various stimuli. Additionally, NA may help prevent stroke and cerebral ischemia by reducing oxidative stress and free radical generation[30, 31].
In the present study, we examined the effects of LPS on an inflammation model using HaCaT cells to determine the activation mechanism of epidermal keratinocytes. We further investigated the effect of BA and NA in LPS-stimulated HaCaT cells and the effects of BA and NA on the expression of iNOS, COX-2, NF-kb, p38 MAPK, IL-6, and IL-8 to understand the mechanism of both pro-inflammatory and anti-inflammatory actions of BA and NA in HaCaT cells.
2.1. Materials
Cell culture reagents were purchased from Gibco BRL (Rockville, MD, USA). Niacinamide antibodies for p38 were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA), and COX-2 and iNOS antibodies were obtained from Sigma Chemical Co. (St. Louis, MO, USA). β-Actin and p-p38 antibody were obtained from Sigma Chemical Co.(St. Louis, MO, USA)
2.2. Cell culture and treatment
Human keratinocytes (HaCaT cells) were obtained from the American Type Culture Collection and maintained in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin G and 100 mg/ml streptomycin) at 37°C in a humidified incubator containing 5% CO₂ and 95% air. In all experiments, cells were seeded in 1 × 106 cells/well and incubated with BA and NA at 80% confluence. All chemicals were dissolved in ethanol, and the final ethanol concentration was less than 0.1%.
2.3. Cell viability assay
The cell growth effect was measured by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (CellTiter 96® aqueous nonradioactive cell proliferation kit; Promega, Wisconsin, USA). HaCaT cells (2 × 103) were incubated in triplicate in a 96-well plate in the presence or absence of BA and NA in a final volume of 100 µl at different time intervals for 24 h at 37°C under 5% CO2. MTS solutions (5 mg/ml) were added to each well, and the cells were cultured for another 1 h, after which the optical density was read at 492 nm (Tecan Trading AG, Switzerland). Cell viability is presented as the relative percentage compared with the control.
2.4. Wound healing migration assay
For the wound healing assay, cells were cultured in a 6-well plate until cells reached 95%–100% confluence and were serum-starved for 24 h. Then, perpendicular or horizontal wounds were made by dragging the sterile yellow micropipette tip across each cell plate. Plates were washed with PBS and replenished with fresh medium alone or with medium containing BA and NA and incubated for 24h. Cell migration into the wound was monitored under a phase-contrast microscope.
2.5. Western blot analysis
Cells were harvested and lysed in RIPA buffer, and the resulting protein samples were quantified by using the bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Equal amounts of protein extracts were denatured by boiling at 100°C for 5 min in sample buffer. The proteins were separated by 8% to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween-20 buffer (TBS-T; 10 mM Tris, 150 mM NaCl, pH 7.5, and 0.1% Tween-20) for 1 h. The membranes were washed three times for 10 min each with TBS-T buffer and incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies. The membranes were washed three times for 10 min each with TBS-T buffer. Immunoblot membranes were incubated with SuperSignal West Pico Chemiluminescent Substrate or Dura Luminol Substrate (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instruction. Membranes were then photographed using ImageQuantTM LAS 4000 (Fujifilm Life Science, Japan).
2.6. Reverse transcriptase PCR
Total RNA was isolated using TRIzol (Invitrogen). and cDNA synthesis was performed using the AccuPower® RocketscriptTM cycle RT premix (Bioneer, Daejeon, Korea) according to the manufacture’s protocol. The relative expression of IL-6 and IL-8 was analyzed by quantitative RT-PCR with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The following pairs of forward and reverse primer sets were used: IL-6, sense; 5′-ATGAACTCCTTCTCCACAAGCGC-3′, antisense; 5′-GAAGAGCCCTCAGGCTGGAGTG-3′, IL-8, sense; 5′-TGTGCTCTCCAAATTTTTTTTAETG-3′, antisense; 5′-CTCTCTTTCCTCTTTAATGTCCAGC-3′, GAPDH, sense; 5′-CAGCCTCAAGATCATCAGCA-3′, antisense; 5′-GTCTTCTGGGTGGCAGTGAT-3′. The RT-PCR reaction mixture contained 2.5 μl of 10×Taq reaction buffer, 0.5 μl of each 10 mM dNTP, 1 μl each of forward and reverse primers, and 2 μl template DNA each of in a final volume of 25 μl. Amplification products were resolved by 1.5% agarose gel electrophoresis, stained with safe dye and photographed using ImageQuant LAS 4000.
2.7. Statistical analysis
Where appropriate, data are expressed as the mean ± SD of at least three independent experiments, and statistical analysis for single comparison was performed using the Student’s t-test; p values less than 0.05 were considered statistically significant. The software used for statistical analysis was Microsoft Excel 2010. [Microsoft Name, version, and manufacturer.]
3.1. Effect of BA and NA on cytotoxicity and proliferation in HaCaT cells
The cytotoxic effect of BA and NA on HaCaT cells was determined by the MTS assay (Fig. 1A, B). The cells were treated with various concentrations of BA (100, 500, and 1000 µg/ml) and NA (20, 50, and 100 µl) for 24 h. As cell viability was within 80%, it was considered that there was no effect on viability. Figure 1C shows that cell proliferation increased statistically significantly after 24 h when both BA and NA were processed at the same time. Based on these results, a BA concentration of 500 µg/ml and 50 µl of NA were used in further studies.
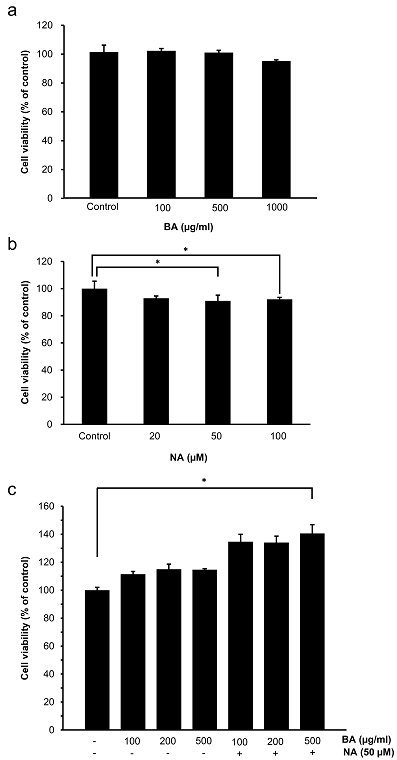
Fig. 1 Effects of BA and NA on the cell viability of HaCaT cells. (A) HaCaT cells were treated with the indicated concentrations of BA for 24 h. Cell viability was determined by the MTS assay. (B) HaCaT cells were treated with the indicated concentrations of NA for 24 h. Cell viability was determined by the MTS assay. (C) HaCaT cells were pretreated with BA and NA, and incubated for another 24 h. Cell viability was determined by the MTS assay. Values are expressed as means ± SD. *, p < 0.05, compared to the control.
3.2. Effect of BA and NA on the migration in HaCaT cells
We investigated BA and NA wound suture capabilities for keratinocyte cells. It is evident that this occurs at a faster rate under the presence of BA and NA relative to the negative control (Fig. 2). BA and NA, respectively, induced the stenosis of scratch wounds, but when both BA and NA were treated at the same time, the cells were significantly higher than the negative controls. Our results show that when both BA and NA are administered at the same time, they both affect the activity of the wound healing stimulation in HaCaT cells using scratch analysis.
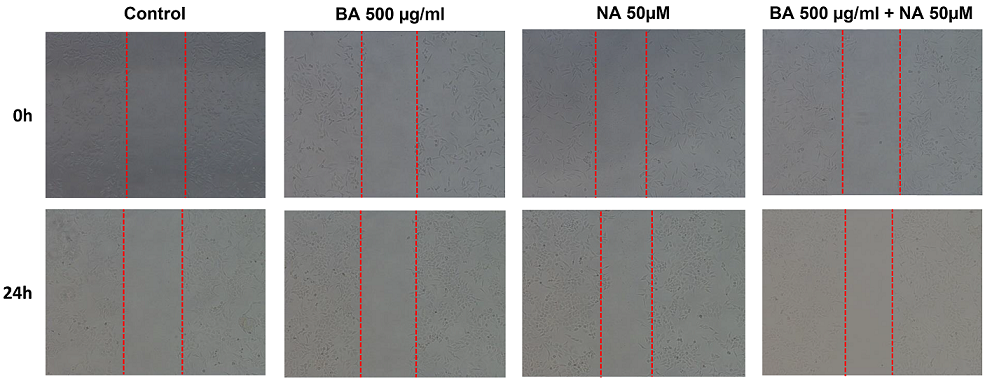
Fig. 2 Effect of BA and NA on HaCaT cells migration. Cell migration was evaluated using a scratch wound healing assay. Cells were grown to 95%–100% confluence in 6-well plate, and the wound was made by scratching the surface of the monolayer with standard 200 ml pipette tip. The detached cells were removed by washing them with PBS, and the attached cells were incubated in the fresh medium with BA and NA. Photographs were taken at after 24 h using an inverted microscope (x200).
3.3. Effect of BA and NA on LPS-induced COX-2 and iNOS expression in HaCaT cells
To evaluate the inflammatory effects of BA and NA, LPS was used as an inflammatory model, and COX-2 and iNOS were induced as inflammatory enzymes. Therefore, it was investigated whether BA and NA controlled COX-2 and iNOS expressions. As a result of LPS treatment, the levels of COX-2 and iNOS were increased. However, BA and NA inhibited LPS-induced COX-2 and iNOS expression (Fig. 3). Our results suggest that when both BA and NA are administered concomitantly, both BA and NA exert an anti-inflammatory effect on HaCaT cells.
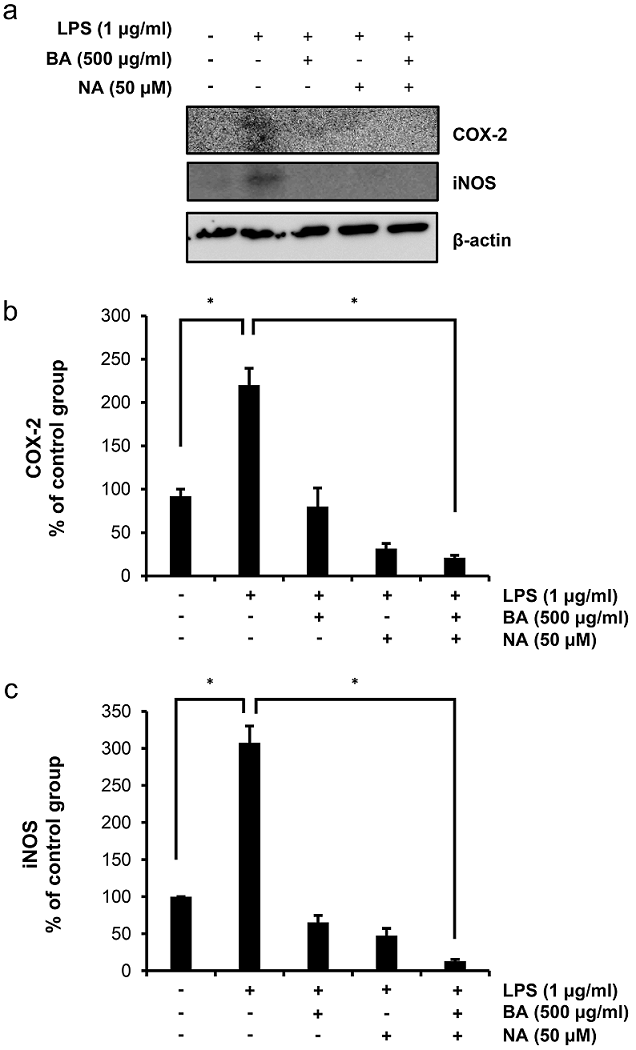
Fig. 3 Inhibition of LPS-induced iNOS and COX-2 protein expression by BA and NA. The expression levels of iNOS and COX-2 were determined by western blotting as described in Materials and Methods (A). HaCaT cells were pretreated with LPS (1 µg/ml) and then treated with BA and NA. β-actin was used as an internal control. The relative intensities of bands were calculated using Image J software (NIH, USA) (B and C). The experiment was performed in triplicate and the data presented as mean ± SD. *, p < 0.001.
3.4. Effect of BA and NA on LPS-induced p38 MPAK expression in HaCaT cells
MAPK activation is involved in controlling inflammatory reactions[32, 33]. Activation of LPS-induced p38 MAPK and the effect of LPS on the regulation of various genes involved in inflammation have been extensively documented. Thus, p38 MAPK signaling can be the basis of a new strategy for treating inflammatory diseases. p38 MAPK signaling is important for the synthesis of LPS-induced pro-inflammatory cytokines in the presence of BA and NA. HaCaT cells were treated with BA and NA under the presence or absence of LPS to investigate whether BA and NA inhibited phosphorylation of p38 MAPK. The result of our experiment indicated that both BA and NA inhibit p38 MAPK phosphorylation induced by LPS (Fig. 4).
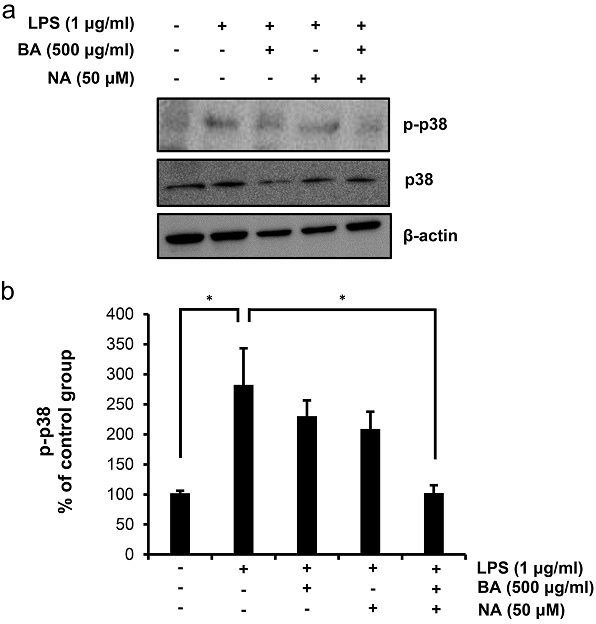
Fig. 4 Inhibition of LPS-induced p-p38 protein expression by BA and NA. The expression levels of p-p38 were determined by western blotting as described in Materials and Methods (A). HaCaT cells were pretreated with LPS (1 µg/ml) and then treated with BA and NA. β-actin was used as an internal control. The relative intensities of bands were calculated using Image J software (NIH, USA) (B). The experiment was performed in triplicate and the data presented as mean ± SD. *, p < 0.001.
3.5. Effect of BA and NA on LPS-induced IL-6 and IL-8 expression in HaCaT cells
IL-6 and IL-8 are two major pro-inflammatory cytokines produced by keratinocytes, mononuclear cells, and macrophages. Inflammation models using HaCaT cells were implemented to investigate the anti-inflammatory activity of BA and NA, and LPS were used as inflammatory factors. As shown in Fig. 5, when BA and NA were present, the release of IL-6 and IL-8 was reduced in relation to the LPS control. Conversely, the negative controls did not produce cytokines.
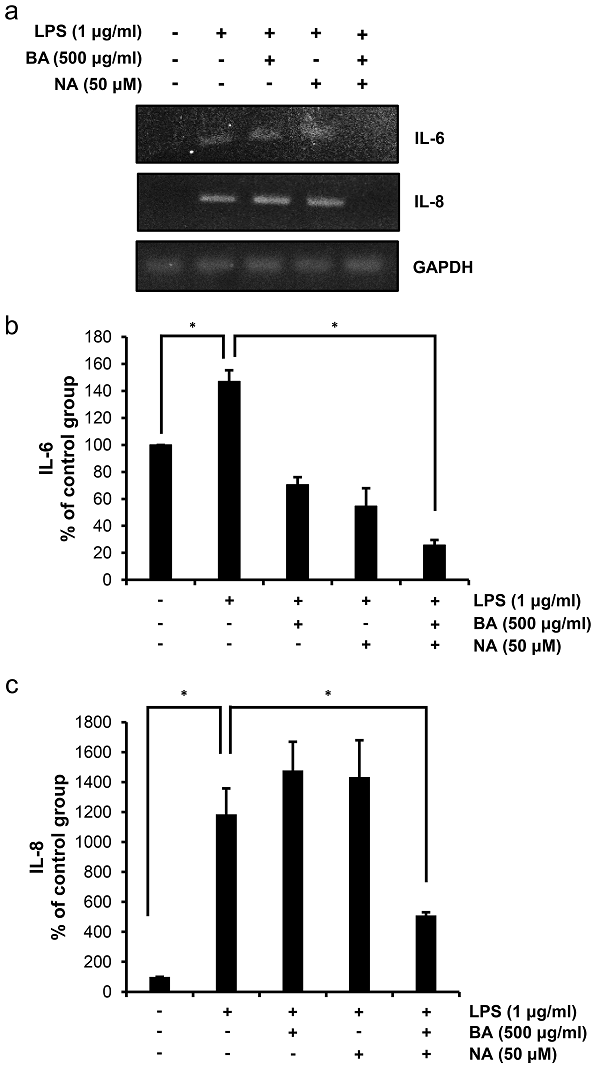
Fig. 5 Inhibition of LPS-induced IL-6 and IL-8 expression by BA and NA. The expression levels of IL-6 and IL-8 were determined by agarose gel electrophoresis as described in Materials and Methods (A). HaCaT cells were pretreated with LPS (1 µg/ml) and then treated with BA and NA. GAPDH was used as an internal control. The relative intensities of bands were calculated using Image J software (NIH, USA) (B and C). The experiment was performed in triplicate and the data presented as mean ± SD. *, p < 0.001.
Wound healing is a natural process of the body by which the skin and epidermal tissue regenerate. This is a complex process that involves hemostasis, inflammation, proliferation, formation, and remodeling of new tissue[34, 35]. Skin damage results in cellular responses, including the action of fibroblasts, keratinocytes, endothelial cells, and macrophages[36]. Keratinocytes are known to secrete numerous cytokines that can induce proliferation, migration, and differentiation via autocrine or paracrine signaling[37, 38]. The migration of keratinocytes is essential for wound re-epithelialization and re-establishment of skin remodeling[39]. Thus, inflammation is the first and most important step in wound healing. If inflammation persists, there is no other step that could affect wound healing.
LPS, the major component of the outer membrane of gram-negative bacteria, stimulates the production of NO and the expression of pro-inflammatory molecules, such as IL-6 and IL-8[40-43]. The production of NO is induced by iNOS and COX-2 products in inflammatory disease mechanisms[44]. Synthetic suppression measurements of iNOS and COX-2 are considered a universal method of testing anti-inflammatory effects. iNOS and COX-2 are remarkably expressed in inflammatory cells in response to stimuli with cytokines or in inflammatory or other immune responses[45, 46].
In this study, we have increased the potential anti-inflammatory action of BA and NA in HaCaT cells. In particular, it was investigated whether LPS-induced production of IL-6 and IL-8 mRNA was inhibited by inhibiting the expression of COX-2 proteins. It was found that the inhibitory effect of BA and NA on the production of inflammatory mediators was accompanied by a concentration-dependent reduction at the levels of protein and mRNA expression in IL-6, IL-8, and COX-2. These data show that the expression of IL-6, IL-8, and COX-2 in HaCaT cells is inhibited by BA and NA.
To further clarify the anti-inflammatory mechanism of BA and NA, p38 MAPK signaling was analyzed. p38 MAPK plays an important role in cell growth regulation and differentiation and control of cellular responses to cytokines and stressors. In the present study, we found that BA and NA inhibited the LPS-induced phosphorylation of p38 MAPK in HaCaT cells.
In conclusion, we have demonstrated that LPS-induced IL-6 and IL-8 generation in HaCaT cells was inhibited by BA and NA. In addition, this effect was mediated by the suppression of COX-2 expression and p38 phosphorylation. The results of this study suggest that simultaneous use of BA and NA may have anti-inflammatory effects. Based on these findings and those of previous studies, BA and NA are relatively safe and effective treatment options for inflammation, including human inflammatory skin disorders.
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jieun Park. The first draft of the manuscript was written by Jieun Park and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Eckhart, Leopold, Saskia Lippens, Erwin Tschachler, and Wim Declercq. 2013. Cell death by cornification. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1833(12): 3471-3480. doi:10.1016/j.bbamcr.2013.06.010. PMid:23792051
View Article PubMed/NCBIKupper, Thomas S., and Robert C. Fuhlbrigge. 2004. Immune surveillance in the skin: mechanisms and clinical consequences. Nature Reviews Immunology. doi:10.1038/nri1310. PMid:15039758
View Article PubMed/NCBIAfshar, Maryam, and Richard L. Gallo. 2013. Innate immune defense system of the skin. Veterinary Dermatology. doi:10.1111/j.1365-3164.2012.01082.x. PMid:23331677
View Article PubMed/NCBIAkira, Shizuo, and Hiroaki Hemmi. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunology Letters. doi:10.1016/s0165-2478(02)00228-6. 00228-6
View ArticleSinger, Adam J., and Richard A. F. Clark. 1999. Cutaneous Wound Healing. New England Journal of Medicine. doi:10.1056/nejm199909023411006. PMid:10471461
View Article PubMed/NCBIEllis, Samantha, Elaine J. Lin, and Danielle Tartar. 2018. Immunology of Wound Healing. Current Dermatology Reports. doi:10.1007/s13671-018-0234-9. PMid:30524911
View Article PubMed/NCBIPasparakis, Manolis, Ingo Haase, and Frank O. Nestle. 2014. Mechanisms regulating skin immunity and inflammation. Nature Reviews Immunology. doi:10.1038/nri3646. PMid:24722477
View Article PubMed/NCBIKarima, Risuke, Shigeru Matsumoto, Hidemitsu Higashi, and Kouji Matsushima. 1999. The molecular pathogenesis of endotoxic shock and organ failure. Molecular Medicine Today. doi:10.1016/s1357-4310(98)01430-0. 01430-0
View ArticleO'Neill, Gary P., and Anthony W. Ford-Hutchinson. 1993. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Letters. doi:10.1016/0014-5793(93)80263-t. 80263-T
View ArticleCao, Jackie Q., Mark H. Einstein, Patrick S. Anderson, Carolyn D. Runowicz, Raluca Balan, and Joan G. Jones. 2002. Expression of COX-2, Ki-67, Cyclin D1, and P21 in Endometrial Endometrioid Carcinomas. International Journal of Gynecological Pathology. doi:10.1097/00004347-200204000-00007. PMid:11917224
View Article PubMed/NCBIOni-Orisan, Akinyemi, Yangmei Deng, Robert N. Schuck, Katherine N. Theken, Matthew L. Edin, Fred B. Lih, Kimberly Molnar, et al. 2013. Dual modulation of cyclooxygenase and CYP epoxygenase metabolism and acute vascular inflammation in mice. Prostaglandins & Other Lipid Mediators. doi:10.1016/j.prostaglandins.2012.09.003. PMid:23000418
View Article PubMed/NCBIMoncada, S. 2007. A2. Nitric oxide and bioenergetics: Physiology and pathophysiology. Nitric Oxide. doi:10.1016/j.niox.2007.09.007.
View ArticleAktan, Fugen. 2004. iNOS-mediated nitric oxide production and its regulation. Life Sciences. doi:10.1016/j.lfs.2003.10.042. PMid:15172174
View Article PubMed/NCBIJohnson, G. L. 2002. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and p38 Protein Kinases. Science. doi:10.1126/science.1072682. PMid:12471242
View Article PubMed/NCBIChen, Weixing, Qingbo Tang, Melissa S. Gonzales, and G. Tim Bowden. 2001. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene. doi:10.1038/sj.onc.1204530. PMid:11439356
View Article PubMed/NCBIRoux, Philippe P., and John Blenis. 2004. ERK and p38 MAPK-Activated Protein Kinases: a Family of Protein Kinases with Diverse Biological Functions. Microbiology and Molecular Biology Reviews. doi:10.1128/mmbr.68.2.320-344.2004. PMid:15187187
View Article PubMed/NCBIHerlaar, Ellen, and Zarin Brown. 1999. p38 MAPK signalling cascades in inflammatory disease. Molecular Medicine Today. doi:10.1016/s1357-4310(99)01544-0. 01544-0
View ArticleHahn, Beth L., and Peter G. Sohnle. 1988. Characteristics of Dermal Invasion in Experimental Cutaneous Candidiasis Leucopenic Mice. Journal of Investigative Dermatology. doi:10.1111/1523-1747.ep12470359. PMid:3045209
View Article PubMed/NCBIRose-John, Stefan. 2015. The soluble interleukin-6 receptor and related proteins. Best Practice & Research Clinical Endocrinology & Metabolism. doi:10.1016/j.beem.2015.07.001. PMid:26522462
View Article PubMed/NCBIHashizume, Misato, and Masahiko Mihara. 2011. The Roles of Interleukin-6 in the Pathogenesis of Rheumatoid Arthritis. Arthritis. doi:10.1155/2011/765624. PMid:22046525
View Article PubMed/NCBIScheller, Jürgen, and Stefan Rose-John. 2006. Interleukin-6 and its receptor: from bench to bedside. Medical Microbiology and Immunology. doi:10.1007/s00430-006-0019-9. PMid:16741736
View Article PubMed/NCBIRabinovich, Alex, Jacqueline. M. Cohen, and Susan R. Kahn. 2015. Predictive value of markers of inflammation in the postthrombotic syndrome: a systematic review. Thrombosis Research. doi:10.1016/j.thromres.2015.06.024. PMid:26139086
View Article PubMed/NCBIFeliciani, C., A. K. Gupta, and D. N. Saucier. 1996. Keratinocytes and Cytokine/Growth Factors. Critical Reviews in Oral Biology & Medicine. doi:10.1177/10454411960070040101. PMid:8986393
View Article PubMed/NCBIBudak, Nilgün H., Elif Aykin, Atif C. Seydim, Annel K. Greene, and Zeynep B. Guzel-Seydim. 2014. Functional Properties of Vinegar. Journal of Food Science. doi:10.1111/1750-3841.12434. PMid:24811350
View Article PubMed/NCBINanda, K., M. Taniguchi, S. Ujike, N. Ishihara, H. Mori, H. Ono, and Y. Murooka. 2001. Characterization of Acetic Acid Bacteria in Traditional Acetic Acid Fermentation of Rice Vinegar (Komesu) and Unpolished Rice Vinegar (Kurosu) Produced in Japan. Applied and Environmental Microbiology. doi:10.1128/aem.67.2.986-990.2001. PMid:11157275
View Article PubMed/NCBIKong, Yan, Li-Li Zhang, Ying Sun, Yu-Yu Zhang, Bao-Guo Sun, and Hai-Tao Chen. 2017. Determination of the Free Amino Acid, Organic Acid, and Nucleotide in Commercial Vinegars. Journal of Food Science. doi:10.1111/1750-3841.13696. PMid:28369909
View Article PubMed/NCBIShimoji, Yumi, Hiroyuki Kohno, Kumiko Nanda, Yasushi Nishikawa, Hajime Ohigashi, Kazuo Uenakai, and Takuji Tanaka. 2004. Extract of Kurosu, a Vinegar From Unpolished Rice, Inhibits Azoxymethane-Induced Colon Carcinogenesis in Male F344 Rats. Nutrition and Cancer. doi:10.1207/s15327914nc4902_8. PMid:15489210
View Article PubMed/NCBIBaba, Naoko, Yuko Higashi, and Takuro Kanekura. 2013. Japanese Black Vinegar "Izumi" Inhibits the Proliferation of Human Squamous Cell Carcinoma Cells Via Necroptosis. Nutrition and Cancer. doi:10.1080/01635581.2013.815234. PMid:23914757
View Article PubMed/NCBISurjana, Devita, Gary M. Halliday, Andrew J. Martin, Fergal J. Moloney, and Diona L. Damian. 2012. Oral Nicotinamide Reduces Actinic Keratoses in Phase II Double-Blinded Randomized Controlled Trials. Journal of Investigative Dermatology. doi:10.1038/jid.2011.459. PMid:22297641
View Article PubMed/NCBIHoane, Michael R., Shelby A. Kaplan, and Amy L. Ellis. 2006. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Research. doi:10.1016/j.brainres.2006.10.019. PMid:17109832
View Article PubMed/NCBIRolfe, Heidi M. 2014. A review of nicotinamide: treatment of skin diseases and potential side effects. Journal of Cosmetic Dermatology. doi:10.1111/jocd.12119. PMid:25399625
View Article PubMed/NCBIBranger, Judith, Bernt van den Blink, Sebastiaan Weijer, Jeffrey Madwed, Carina L. Bos, Abhya Gupta, Chan-Loi Yong, et al. 2002. Anti-Inflammatory Effects of a p38 Mitogen-Activated Protein Kinase Inhibitor During Human Endotoxemia. The Journal of Immunology. doi:10.4049/jimmunol.168.8.4070. PMid:11937566
View Article PubMed/NCBIOno, Koh, and Jiahuai Han. 2000. The p38 signal transduction pathway Activation and function. Cellular Signalling. doi:10.1016/s0898-6568(99)00071-6. 00071-6
View ArticleSilver, I. A. 1994. The physiology of wound healing. Journal of Wound Care. doi:10.12968/jowc.1994.3.2.106. PMid:27922399
View Article PubMed/NCBIMonsuur, Hanneke N., Mireille A. Boink, Ester M. Weijers, Sanne Roffel, Melanie Breetveld, Amit Gefen, Lenie J. van den Broek, et al. 2016. Methods to study differences in cell mobility during skin wound healing in vitro. Journal of Biomechanics. doi:10.1016/j.jbiomech.2016.01.040. PMid:26903411
View Article PubMed/NCBIAslam, R., H. Scheuenstuhl, H. Hopf, S. Beckert, Z. Hussain, and T. K. Hunt. 2008. 061 Lactate, Oxygen, and Wound Healing. Wound Repair and Regeneration. doi:10.1111/j.1067-1927.2005.130215bi.x.
View ArticleKirfel, G., and V. Herzog. 2004. Migration of epidermal keratinocytes: mechanisms, regulation, and biological significance. Protoplasma. doi:10.1007/s00709-003-0031-5. PMid:15221512
View Article PubMed/NCBIMitev, V., and L. Miteva. 1999. Signal transduction in keratinocytes. Experimental Dermatology. doi:10.1111/j.1600-0625.1999.tb00355.x. PMid:10232399
View Article PubMed/NCBISivamani Raja K., Miki S. Garcia, and R. Rivkah Isseroff1. 2007. Wound re-epithelialization: modulating kerationcyte migration in wound healing. Frontiers in Bioscience. doi:10.2741/2277. PMid:17485264
View Article PubMed/NCBINathan, C. and Xie, Q.W., 1994. Regulation of biosynthesis of nitric oxide. Journal of Biological Chemistry, 269(19), pp.13725-13728.
Tracey, Kevin J., Yuman Fong, David G. Hesse, Kirk R. Manogue, Annette T. Lee, George C. Kuo, Stephen F. Lowry, et al. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. doi:10.1038/330662a0. PMid:3317066
View Article PubMed/NCBIAkira, Shizuo, Tetsuya Taga, and Tadamitsu Kishimoto. 1993. Interleukin-6 in Biology and Medicine. Advances in Immunology Volume 54. doi:10.1016/s0065-2776(08)60532-5. 60532-5
View ArticleZhang, X. 1993. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. Journal of Experimental Medicine. doi:10.1084/jem.177.2.511. PMid:8426119
View Article PubMed/NCBIKim, Ji-Hee, Gwangsoo Lee, Young-Lai Cho, Chun-Ki Kim, Sanghwa Han, Hansoo Lee, Jae Sue Choi, et al. 2009. Desmethylanhydroicaritin inhibits NF-κB-regulated inflammatory gene expression by modulating the redox-sensitive PI3K/PTEN/Akt pathway. European Journal of Pharmacology. doi:10.1016/j.ejphar.2008.10.062. PMid:19027002
View Article PubMed/NCBIKharitonov, S. A., Deborah. Yates, R. A. Robbins, Peter J. Barnes, Ronald Logan-Sinclair, and Elliot A. Shinebourne. 1994. Increased nitric oxide in exhaled air of asthmatic patients. The Lancet. doi:10.1016/s0140-6736(94)90931-8. 90931-8
View ArticleParente, Luca, and Mauro Perretti. 2003. Advances in the pathophysiology of constitutive and inducible cyclooxygenases: two enzymes in the spotlight. Biochemical Pharmacology. doi:10.1016/s0006-2952(02)01422-3. 01422-3
View Article