KD Ibrahim (Email: kardio_ib@outlook.fr)
HS Senay (Email: senay.sanlier@gmail.com)
© 2019 Sift Desk Journals. All Rights Reserved
KD Ibrahim (Email: kardio_ib@outlook.fr)
HS Senay (Email: senay.sanlier@gmail.com)
Ibrahim KARIDIO DIORI1* and Senay HAMARAT SANLIER 1,2,*
1Department of Biochemistry, Faculty of Science, Block E, Ege University, Erzene Mahallesi, Bornova/Izmir 35040, Turkey.
2ARGEFAR, Faculty of Medicine, Ege University, Bornova/ Izmir 35040, Turkey
Agnieszka Bia%c5%82ek(agnieszka.bialek@wum.edu.pl)
Binxing Zhou(bxzhou01@126.com)
Diori I.K., Sanlier SH, Cytotoxic potentials of the culture extract of the endophytic Aspergillus niger strain karmalı isolated from Punica granatum against SKOV3 and A549 cancer cell lines (2020) Journal of Plant Science 4(1) pp: 199-207
Endophytes encompass the widespread taxa of yet under-explored endosymbionts that are believed to bestow, to the benefit of their host plants bioactive compounds enabling the later to withstand biotic and/ or abiotic stresses. Some of these molecules are pharmacologically valuable compounds. Herein, we do report the isolation of a newly identified endophytic strain of Aspergillus niger named karmali from roots and twigs of Punica granatum. Aspergillus niger strain karmali has been used in a solid-state fermentation on rice medium enriched with peptone and artificial seawater. The polar phase of the culture extract was fractionated using Dry Column Vacuum Chromatography (DCVC). The cancer treatment potential of the major fraction of the endophytic compounds was determined in vitro as its cytotoxicity against ovarian cancer cell line SKOV-3 and lung cancer cell line A549. As a result of cell culture experiments, IC50 values were determined to be 1.08 µg / ml for SKOV3 cells and 11.7 µg / ml for A549 cells at 24 hours. The cancer treatment potential of the purified endophytic compound was found to be more active on SKOV-3 cell. Biocompatibility tests were performed in order to determine its effectiveness in vivo conditions, and it was determined to be biocompatible.
Keywords: Aspergillus niger strain karmali, cytotoxic, endophyte, Punica granatum, A459, SKOV3, ovarian cancer, lung cancer
Highlights
Considering that plants threating factors such as global warming, desertification, recurrent episodes of floods and droughts, in parallel with the rapid global population growth with its associated increasing food demands, medicines (mostly derived from plant sources), favor the widespread nowadays phenomenon of biodiversity scarce; it is emergent to overcome frontiers of current knowledge about the accurate and efficient use of endophytes for agricultural, industrial and pharmaceutical purposes to avoid the permanent disappearance of many species even before scientists explore their scope of usefulness. Following the production of Taxol from the endophytic fungi Taxomyces andreanae isolated from the inner bark of Taxus brevifolia, scientists developed interests in the yet-poorly known diversified genera of the endophytic microbiota of plants.
Herein, we do report the isolation of a newly identified endophytic strain of Aspergillus niger named karmali from roots and twigs of P. granatum. Aspergillus niger strain karmali has been used in a solid-state fermentation on rice medium enriched with peptone and artificial seawater. The polar phase of the culture extract was fractionated using Dry Column Vacuum Chromatography (DCVC). The cancer treatment potential of the major fraction of the endophytic compounds was determined in vitro as its cytotoxicity against ovarian cancer cell line SKOV3 and lung cancer cell line A549.
Etymologically thought, endophyte is derived from the Greek words “Endon” meaning “inside” and “phyto” meaning “plant”. So literally, endophyte means “inside the plant.” The term was first introduced by De Bary (1, 2). Broadly, endophyte refers to the endosymbiotic microbiota that comprises bacteria and fungi that asymptomatically live within and/ or between plant cells (3,5). Many species of the same or different genera could be hosted by the same or distinct plant tissues. Endophytes are widely spread among and within plant species with remarkable diversity. To date, all the plants investigated have been individually proven to be associated with at least one kind of endophytic microbe either in its phyllosphere and/or rhizosphere (4,6-9). Endophytes are mostly located in relatively privileged niches. Therein, they establish an interrelationship with their host that could range from commensalism to mutualism. Endophytes are broad taxa of unexplored; thus, poorly known functional bacterial and fungal communities that, depending on their genotype, the environmental conditions, and the genotype of their host plant, from within, alter its biochemistry, physiology and evolutionary ecology.
Sometimes endophytes produce among others, secondary metabolites that favor plant growth, increase yield and resistance against abiotic stresses (10-16). Moreover, the widely spread endophytes could also produce secondary metabolites with antimicrobial activities against pathogens, as well as toxic and noxious compounds against insect and vertebrate herbivores thereby reflecting a “barrier effect” against biotic stressors. Some of these bioactive compounds are as well rare as valuable novel compounds that could have medical significance (17-20).
Considering that plants threating factors such as global warming, desertification, recurrent episodes of floods and droughts, in parallel with the rapid global population growth with its associated increasing food demands, medicines (mostly derived from plant sources), favour the widespread nowadays phenomenon of biodiversity scarce; it is emergent to overcome frontiers of current knowledge about the accurate and efficient use of endophytes for agricultural, industrial and pharmaceutical purposes to avoid the permanent disappearance of many species even before scientists explore their scope of usefulness(21-24). Following the production of Taxol from the endophytic fungi Taxomyces andreanae isolated from the inner bark of Taxus brevifolia, scientists developed interests in the yet-poorly known diversified genera of the endophytic microbiota of plants (23).
Our interest firstly, focused on the research on endophytes from Punica granatum (nar ağacı), at the Faculty of Science of Ege University, then the anticancer potential of their eventually produced metabolites. The historical use of P. granatum for its natural and holistic medical significance dates far back to ancient times as noted in Egyptian mythology and art. The plant represents an enormous treasure of pharmacophoric compounds. Extracts from different parts of P. granatum have been proven useful for relieving from various ailments such as throat inflammation, coughs, periodontal disease, urinary infection, bronchitis, diarrhoea, tapeworm parasitism, arthritis, diabetes, cancer, cardiopathy etc. (25-31).
Research data evidenced the high content of phytochemicals in P. granatum, mainly polyphenols such as ellagitannins/punicalagins, delphinidin, cyaniding, pelargonidin glycosides, cathechins, gallocatechins, prodelphinidins etc. (32-35).
Despite the tremendous interests’ researchers manifest towards pomegranate for its nutraceutical potentials, there are very few reports about its endophytic microbiota. Of recent, antimicrobial producing fungal endophytes named as IPG3-1 and IPG3-3 were isolated from P. granatum (36). (37) reported, the production of novel styrylpyrones from the endophytic fungi Penicillium glabrum isolated from P. granatum. In late 2017, Q. cyanescens was reported for the first time as isolated endophytes from the Lythraceae family (24). Considering the significance of phytochemical reported from P. granatum and the fewer availability of data about its eventual endophytic microbiota, focusing on the later would tell us more about their eventual potentials with regard to bioactive compounds production.
Every year 18.1 million new cases of cancer are diagnosed, and 9.6 million people die due to cancer. In 2015, according to estimations of the World Health Organization (W.H.O.), cancer is the first or second cause of premature deaths in 91 countries out of 172. Additionally, cancer is the third or fourth cause of premature deaths in 22 countries. One type of cancer, the most commonly diagnosed and major cause of cancer deaths, i.e. lung cancer, alone is the fourth global deadly disease after ischemic heart disease, stroke, and diabetes mellitus. The global maps of cancer types, incidence, prevalence, and mortality appear as variegated pictures linked to the geographical region, socioeconomic development and/ or life-style of the population. For instance, ovarian cancer, the eighth commonest female cancer, and the third gynaecological cancer is common in developed and transitioning countries while it is rare in low income- African countries (38-41).
Herein, we do report the isolation of a newly identified endophytic strain of Aspergillus niger named karmali from roots and twigs of P. granatum. Aspergillus niger strain karmali has been used in a solid-state fermentation on rice medium enriched with peptone and artificial seawater. The polar phase of the culture extract was fractionated using Dry Column Vacuum Chromatography (DCVC). The cancer treatment potential of the major fraction of the endophytic compounds was determined in vitro as its cytotoxicity against ovarian cancer cell line SKOV3 and lung cancer cell line A549.
Isolation and purification procedures
Collection and surface sterilization of plant samples
Samples from fresh roots, twigs, leaves, flowers, and unripe fruits were collected from a P. granatum tree at the Faculty of Science of Ege University (38°27'34.2"N 27°13'51.8"E), and brought to the laboratory of Biotechnology (Department of Biochemistry), in sterile plastic bags. Samples were chosen based on their appearance of being healthy, i.e. not showing disease symptoms, brought into the laboratory and processed within an hour to lower risks of contamination by ambient microorganisms. The collected samples were exhaustively washed by means of running tap water so as to remove eventually, sand and dust on their surface. Roots and twigs’ samples were cut into small segments with sterile bistoury. Next, the plant materials were then processed to remove epiphytes through surface sterilization i.e. a combination of a sequential immersion of the sample into a different solution at each step. The flower, leaves and fruits’ samples were immersed in 70% EtOH for 30 seconds, then in 2% NaOCl for 10-15 seconds, then in 70% EtOH again for 30 seconds and finally in sterile-distilled water (sdH2O) for 15 seconds twice. Roots and twigs’ samples were passed through the same process, but with relatively long periods i.e. 3 min in 70% EtOH, 1 min in NaOCl, 1 min in EtOH, and 1 min in sdH2O twice. The surface-sterilized samples were dried under aseptic conditions in the folds of sterile tissue papers. The roots and twigs’ samples were sliced longitudinally while others were cut into more or less forms and placed into media (PDA + chloramphenicol, LB-agar), containing Petri dishes so that the freshly cut edges were in direct contact with the agar surface. To check for the efficiency of the procedure, 0.1 ml of the final rinsing water was inoculated on LB-agar, PDA + chloramphenicol, and incubated under the same conditions as the samples (16, 42-47).
Isolation and purification of the endophytes
The controls were monitored for up three weeks without showing any organismal growth. Many fungal strains grew from the same samples. Each individual strain was transferred into a new Petri according to the hyphae tips method. Observation of the cultures’ morphologies, colours, and down-side culture image uniformity helped in the purification process. Many cultures, media were used, some served, as well for purification as for the screening process for the eventual potentiality to produce specific valuable secondary metabolites. In the course of the conduct of the present study, an endophytic fungal species isolated from the roots of P. granatum have been selected.
Identification of the endophytic fungal strain
The genomic DNA of the endophytic fungi was extracted and isolated using the QuickDNA Fungal/Bacterial kit purchased from ZYMO RESEARCH. The ITS region of the extracted genomic DNA was amplified using the primers ITS1 and ITS4 and sequenced for molecular biological identification purpose. The sequenced ITS region was submitted to the National Centre for Biotechnology Information (NCBI) GenBank database and was attributed to the accession number MH886512.1
The phylogenic tree from the internal spacer’s sequence of Aspergillus niger strain karmali is presented in figure 1 below.
Fermentation and extraction
Prior to the solid-state fermentation, the endophytic fungus was cultured on Potato Dextrose Agar (PDA), for one week. The culture surfaces of the Petri dishes were cut into small pieces and transferred into Erlenmeyer flasks containing sterilized solid rice medium. The rice medium contained 100g (per 1000 ml flask), of commercially available rice enriched with 5.5g peptone, 100ml artificial seawater, and 900 ml distilled water in 1000ml Erlenmeyer flasks. Prior to sterilization, the so prepared medium was kept at room temperature, overnight under static conditions. The inoculated prepared rice media (cultures), were kept at room temperature, under static conditions in a closed cupboard for 40 days. The fermentation process was brought into an end by adding 250ml of EtOAc into each culture flask. The cultures, so-submerged with EtOAc, were hermetically covered with an adhesive plastic and incubated at 40º C, 150 rpm overnight. The culture was again exhaustively extracted again with EtOAc, at 40ºC, 150 rpm for 2 hours. The concentrated crude extract was exhaustively extracted with MeOH: n-hexane solvent system, and the polar phase concentrated for purification.
Purification
Dry column vacuum chromatography (DCVC) was used for the purification process coupled with silica gel TLC plates that served as to determine the eventual fractions under UVfluorescent detector (48). The solvent system used as eluent/ mobile phase for the DCVC was nhexane: EtOAc. After loading the celite-bound-sample on the column, it was then eluted with nhexane thrice, then n-hexane -EtOAc with increasing polarity, i.e. 19:1; 18:2; until 0:20 was achieved. At this point, the 0:20 hexane-EtOAc was repeated four to five times, before the EtOAc- 90% MeOH system was introduced. The later system was also eluted with an increasing polarity, i.e. EtOAc- 90% MeOH 19:1; 18:2 and so on (48,49).
MTT assay
The cytotoxicity of the sample was assessed according to the MTT assay developed by Hansen (50). The cell viability was determined by comparing the spectroscopic analysis data of the treated and untreated samples. The cytotoxicity was tested against two cancer cell lines, namely A549 and Sköv3. The tests were performed in triplicates and the averages of the separate experiments served on the statistical analysis of the data to determine the significance of the drug’s eventual effect against the cancer cell lines.
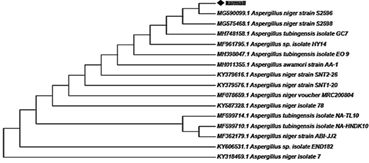
Figure 1 The phylogenic tree from the internal spacer’s sequence of Aspergillus niger strain karmali
Cancer Treatment Potential of Isolated Endophytic culture’s extract in vitro
Research
Cytotoxicity Studies
The MTT test was used for cytotoxicity analysis and cell viability was measured at 24 h, 48 h and 72 h. The amount of viability detected in the cells incubated in the medium was accepted as 100% and the calculation was made in the cells where the drug samples were applied. Cell viability graphs were plotted with Excel.
The cytotoxicity produced by the secondary metabolite in the concentration range (400.625 µg / mL) against A459 cell lines is shown for 24 hours in Figure 2, for 48 hours in Figure 3 and for 72 hours in Figure 4, respectively. Likewise, the cytotoxicity produced by the same endophytic metabolic against SKOV3 is shown by figure 5, figure 6 and figure 7 for 24, 48 and 72 incubation time after the sample is added.
It was found that the secondary metabolite increased cytotoxicity with increasing drug concentration in 24th hour results. The results recorded after 48 hours and 72 hours firstly reflected a proportional increment of the cytotoxicity of the drug with respect to its concentration before the concentration of 10 µg/ml was reached. For 10 µg /ml and above the cytotoxicity was inversely proportional to the concentration of the drug.
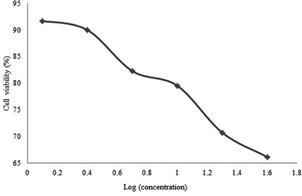
Figure 2: Percentage of cell viability versus drug’s (secondary metabolite) doses administered to A549 cells for 24 hours
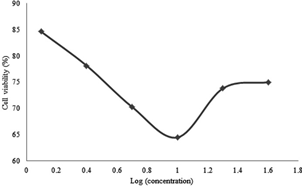
Figure 3: Cell Viability percentages versus secondary metabolite doses administered to A549 cells for 48 hours
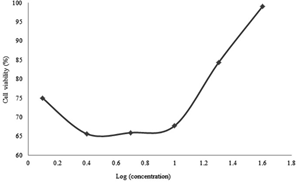
Figure 4: Percentages of cell viability versus secondary metabolite doses administered to A549 cells for 72 hours.
For the SKOV-3 cell lines, 24 hours incubation effects are shown in Figure 5, 48 hours in Figure 6 and 72 hours in Figure 7. It was found that the metabolite expressed a proportional cytotoxicity with respect to its concentration in 24th hour results. At the 48th and 72nd hour results, the concentration of the drug was proportional to its cytotoxicity below 10 µg / ml. For concentration equal or above 10µg/ml, the concentrations seemed inversely proportional to the toxicity of the drug.
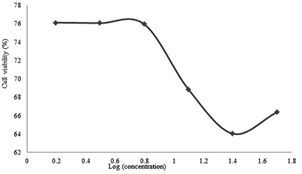
Figure 5: Percentage of viability versus secondary metabolite doses administered to SKOV-3 cells for 24 hours.
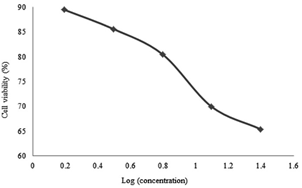
Figure 6: Cell Viability percentages versus secondary metabolite doses administered to SKOV-3 cells for 48 hours
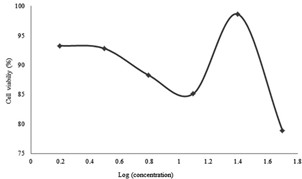
Figure 7: Cell Viability percentages versus secondary metabolite doses administered to SKOV-3 cells for 48 hours
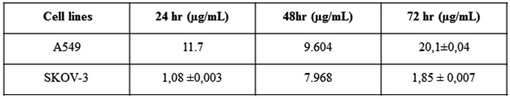
IC50 values of the drugs on A549 and SKOV-3 cells at 24, 48 and 72 hours were calculated by Graphpad Prism 5.0 program using percent viability of the cells against the log (concentration) of the drugs. The values obtained are shown in Table I below
Table 1: A549 and SKOV-3 on 24, 48hours and 72 hours after incubation of free drug groups and drug IC50 values
Determination of Biocompatibility of Isolated Endophytic metabolic (drug sample)
Protein Binding
It is known that adsorption to plasma proteins significantly determines the in vivo behaviour of particles (51). Phagocytosis is facilitated by proteins adsorbed to the drug’s surface, and the drug’s surface chemistry affects the amount and type of binding proteins (52). For example, adsorption of opsonins facilitates phagocytosis and causes the removal of drugs from the systemic circulation by the mononuclear phagocytic system.
Conversely, the binding of disopsonins such as albumin increases the circulation time in the blood (51). In the study, the amount of protein binding in fetal bovine serum of the secondary metabolite was determined. Since the amount of plasma protein may vary from one person to another, experiments were performed at different concentrations of substance and serum. In Table II, protein binding amounts and binding percentages are given.
Table 2: Quantity and percentage of binding of endophyte compound to serum proteins.
Hemolysis
Hemolysis experiments were performed by incubating the secondary metabolite with erythrocytes at different concentrations. Figure 8 shows the photographs of the experiment. PBS was used as the negative control and Triton X, which was known to cause lysis of the cells, was used as the positive control. According to the results, the secondary metabolite did not cause hemolysis compared to the positive and negative controls. When all of the protein binding and hemolysis results are considered, it can be said that the drug sample was biocompatible.
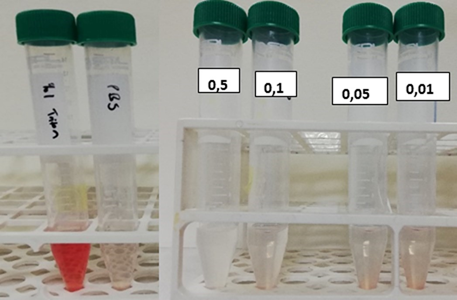
Figure 8: Image of tubes after hemolysis assay centrifugation (left to right respectively): Positive control, negative control, drug concentrations of 0.5; 0.1; 0.05; 0.01 mg/ml
The cancer treatment potential of the endophytic compound produced by A. niger karmali, in vitro, was determined as its cytotoxicity against ovarian cancer cell SKOV3 and lung cancer cell A549. As a result of cell culture experiments, IC50 values were determined as 1.08 µg / ml for SKOV3 cell and 11.7 µg / ml for A549 cell at 24 hours. The cancer treatment potential of the purified endophyte compound was found to be more effective on SKOV3 cell. Biocompatibility tests were performed in order to determine its effectiveness in vivo conditions and it was determined to be biocompatible.
We would like to express our profound gratitude to Prof.Dr. Nurdan KASIKARA PAZARLIOGLU for her valuable and constructive contributions during the course of this research work.
Funding
This research work has been funded by “Ege University BAP Project” through the project number “18-FEN-009”
Author contributions: This study has been conceived, designed, the experiments performed, the data analysis and the writing of the manuscript were done by Dr KARIDIO DIORI Ibrahim under the supervision of Prof.Dr. HAMARAT SANLIER Senay.
Gouda, S., Das, G., et al. Endophytes: a treasure house of bioactive compounds of medicinal importance. Frontiers in microbiology 2016, (7), p.1538,. PMid:27746767
View Article PubMed/NCBIRajamanikyam, M., Vadlapudi, V. et al. Endophytic fungi as novel resources of natural therapeutics. Brazilian Archives of Biology and Technology 2017, (60).
View ArticleClay K and Schardl C: Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. The American naturalist 2002, 160 Suppl 4: S99-S127,. PMid:18707456
View Article PubMed/NCBIHardoim PR, van Overbeek LS, Berg G, et al: The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiology and molecular biology reviews 2015, MMBR 79: 293-320. PMid:26136581
View Article PubMed/NCBISuman, A., Yadav, A. N., & Verma, P.: Endophytic microbes in crops: Diversity and beneficial impact for sustainable agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity 2016. (pp. 117- 143). Springer India.
View ArticleBehie, S.W., Jones, S.J. and Bidochka, M.J.: Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecology 2015, (13), pp.112119.
View ArticleKnief C, Delmotte N, Chaffron S, et al: Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. The ISME journal 2012, (6) 13781390. PMid:22189496
View Article PubMed/NCBIWeyens N, van der Lelie D, Taghavi S and Vangronsveld J: Phytoremediation: plantendophyte partnerships take the challenge. Current opinion in biotechnology, 2009, (20) 248-254. PMid:19327979
View Article PubMed/NCBIWeyens N, van der Lelie D, Taghavi S, Newman L and Vangronsveld J: Exploiting plantmicrobe partnerships to improve biomass production and remediation. Trends in biotechnology 2009, 27: 591-598. PMid:19683353
View Article PubMed/NCBIDastogeer KMG, Li H, Sivasithamparam K, Jones MGK and Wylie SJ: Host Specificity of Endophytic Mycobiota of Wild Nicotiana Plants from Arid Regions of Northern Australia. Microbial ecology 2018, (75) 74-87. PMid:28702707
View Article PubMed/NCBIDeng Z and Cao L: Fungal endophytes and their interactions with plants in phytoremediation: A review. Chemosphere 2017, (168) 1100-1106. PMid:28029384
View Article PubMed/NCBIEberl, F., Uhe, C. and Unsicker, S.B.: Friend or foe? The role of leaf-inhabiting fungal pathogens and endophytes in tree-insect interactions. Fungal Ecology, 2018.
View ArticleHiruma K, Kobae Y and Toju H: Beneficial associations between Brassicaceae plants and fungal endophytes under nutrient-limiting conditions: evolutionary origins and hostsymbiont molecular mechanisms. Current opinion in plant biology 2018, (44), 145-154. PMid:29738938
View Article PubMed/NCBIHoffman, M.T. and Arnold, A.E: Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Applied and environmental microbiology 2010, 76(12), pp.4063-4075. PMid:20435775
View Article PubMed/NCBIHoffman, M.T., Gunatilaka, M.K., Wijeratne, K., Gunatilaka, L. and Arnold, A.E: Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLoS One 2013, 8(9), p.e73132. PMid:24086270
View Article PubMed/NCBIJalgaonwala, R.E., Mohite, B.V. and Mahajan, R.T. A review: natural products from plantassociated endophytic fungi. Journal of microbiology and biotechnology research 2017, 1(2), pp.21-32.
Bahgat, M.M.M., Kawasthy, S.A. and Bous, M.M.E. Characterization of endophytic bacteria isolated from medicinal plant Capparissinaica Veill. and analyze its bioactive flavonoid. Indian Journal of Applied Research 2014, 4(11), pp.5-13.
Muzzamal, H., Sarwar, R., Sajid, I. and Hasnain, S.: Isolation, identification and screening of endophytic bacteria antagonistic to biofilm formers. Pakistan J. Zool 2012, 44(1), pp.249-257.
Nisa H, Kamili AN, Nawchoo IA, Shafi S, Shameem N and Bandh SA. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microbial pathogenesis 2015, (82) 50-59. PMid:25865953
View Article PubMed/NCBISatari, A.H., Zargar, M.I., Shah, W.A., Bansal, R. and Bhat, M.F. Isolation, molecular identification, phytochemical screening and in vitro anti-oxidant activity of endophytic fungi from Achilea millefolium Linn. Journal of Pharmacognosy and Phytochemistry 2018, 7(4), pp.87-92.
Huang LH, Yuan MQ, Ao XJ, Ren AY, Zhang HB and Yang MZ: Endophytic fungi specifically introduce novel metabolites into grape flesh cells in vitro. PLoS One 2018, (13), e0196996. PMid:29734364
View Article PubMed/NCBIJia M, Chen L, Xin HL, et al: A Friendly Relationship between Endophytic Fungi and Medicinal Plants: A Systematic Review. Frontiers in microbiology 2016, (7) 906.
View ArticleStrobel G and Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiology and molecular biology reviews : MMBR 2003, 67: 491-502. PMid:14665674
View Article PubMed/NCBIVAHEDI-DARMIYAN, M.E., JAHANI, M., MIRZAEE, M.R. and ASGARI, B.: A noteworthy record of endophytic Quambalaria cyanescens from Punica granatum in Iran. Czech Mycology 2017, 69(2).
View ArticleBhowmik, D., Gopinath, H., Kumar, B.P. and Kumar, K.: Medicinal uses of Punica granatum and its health benefits. Journal of Pharmacognosy and Phytochemistry 2013, 1(5).
Bogdan, C., Iurian, S., Tomuta, I. and Moldovan, M.: Improvement of skin condition in striae distensae: Development, characterization and clinical efficacy of a cosmetic product containing Punica granatum seed oil and Croton lechleri resin extract. Drug design, development and therapy 2017, (11), p.521. PMid:28280300
View Article PubMed/NCBIEl-Awady, M.A., Awad, N.S. and El-Tarras, A.E.: Evaluation of the anticancer activities of pomegranate (Punica granatum) and harmal (Rhazya stricta) plants grown in Saudi Arabia. Int. J. Curr. Microbiol. App. Sci 2015, 4(5), pp.1158-1167.
Jurenka JS: Therapeutic applications of pomegranate (Punica granatum L.): a review. Alternative medicine review : a journal of clinical therapeutic 2008, (13) 128-144.
Kiraz Y, Neergheen-Bhujun VS, Rummun N and Baran Y. Apoptotic effects of non-edible parts of Punica granatum on human multiple myeloma cells. Tumour biology. the journal of the International Society for Oncodevelopmental Biology and Medicine 2016, (37) 18031815. PMid:26318303
View Article PubMed/NCBINicolson, G.L., Veljkovic, V., Glisic, S., Perovic, V. and Veljkovic, N.: Pomegranate (Punica granatum): a natural source for the development of therapeutic compositions of food supplements with anticancer activities based on electron acceptor molecular characteristics. Functional Foods in Health and Disease 2016, 6(12), pp.769787.
View ArticleSingh B, Singh JP, Kaur A and Singh N: Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem 2018, (261) 75-86. PMid:29739608
View Article PubMed/NCBIHernandez, F., Melgarejo, P., Tomas-Barberan, F.A. and Artes, F.: Evolution of juice anthocyanins during ripening of new selected pomegranate (Punica granatum) clones. European Food Research and Technology 1999, 210(1), pp.39-42.
View ArticleBen Nasr C, Ayed N and Metche M: Quantitative determination of the polyphenolic content of pomegranate peel. Zeitschrift fur Lebensmittel-Untersuchung und -Forschung 1996, (203) 374-378. PMid:9123975
View Article PubMed/NCBIPlumb GW, de Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC and Williamson G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox report : communications in free radical research 2002, (7) 41-46. PMid:11981454
View Article PubMed/NCBISingh RP, Chidambara Murthy KN and Jayaprakasha GK: Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem 2002, (50) 81-86. PMid:11754547
View Article PubMed/NCBIKumala, S. and Izzati, H.: Isolation IPG3-1 and IPG3-3, endophytic fungi from Delima (Punica granatum Linn.) twigs and in vitro assessment of their antimicrobial activity. Int Res J Pharmacy 2013, 4(6), pp.49-53.
View ArticleHammerschmidt, L., Wray, V., Lin, W., Kamilova, E., Proksch, P. and Aly, A.H.: New styrylpyrones from the fungal endophyte Penicillium glabrum isolated from Punica granatum. Phytochemistry letters 2012, 5(3), pp.600-603.
View ArticleBray, F., Ferlay, J., Soerjomataram, I., Siegel, R.L., Torre, L.A. and Jemal, A.: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2018, 68(6), pp.394-424. PMid:30207593
View Article PubMed/NCBIBray, F., Jemal, A., Grey, N., Ferlay, J. and Forman, D.: Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. The lancet oncology 2012, 13(8), pp.790-801. 70211-5
View ArticleFerlay, J., Colombet, M., Soerjomataram, I., Mathers, C., Parkin, D.M., Piñeros, M., Znaor, A. and Bray, F.: Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International journal of cancer 2019, 144(8), pp.1941-1953. PMid:30350310
View Article PubMed/NCBIFerlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D.M., Forman, D. and Bray, F.: Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer 2015, 136(5), pp.E359-E386. PMid:25220842
View Article PubMed/NCBIAly AH, Edrada-Ebel R, Wray V, et al: Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides. Phytochemistry 2008, (69) 1716-1725. PMid:18400237
View Article PubMed/NCBIJouda JB, Tamokou JD, Mbazoa CD, Sarkar P, Bag PK and Wandji J: Anticancer and antibacterial secondary metabolites from the endophytic fungus Penicillium sp. CAM64 against multi-drug resistant Gram-negative bacteria. African health sciences 2016, (16) 734-743. PMid:27917206
View Article PubMed/NCBIKalyanasundaram, I., Nagamuthu, J. and Muthukumaraswamy, S.: Antimicrobial activity of endophytic fungi isolated and identified from salt marsh plant in Vellar Estuary. Journal of microbiology and antimicrobials 2015, 7(2), pp.13-20.
View ArticleKusari S, Lamshoft M, Zuhlke S and Spiteller M: An endophytic fungus from Hypericum perforatum that produces hypericin. J Nat Prod 2008, (71) 159-162. PMid:18220354
View Article PubMed/NCBITeiten MH, Mack F, Debbab A, et al: Anticancer effect of altersolanol A, a metabolite produced by the endophytic fungus Stemphylium globuliferum, mediated by its proapoptotic and anti-invasive potential via the inhibition of NF-kappaB activity. Bioorganic & medicinal chemistry 2013, (21) 3850-3858. PMid:23664494
View Article PubMed/NCBIZhao LX, Xu LH and Jiang CL: Methods for the study of endophytic microorganisms from traditional Chinese medicine plants. Methods in enzymology 2012, (517) 3-21. PMid:23084931
View Article PubMed/NCBIPedersen, D.S. and Rosenbohm, C.: Dry column vacuum chromatography. Synthesis 2001, 2001(16), pp.2431-2434.
View ArticleLi G, Kusari S, Golz C, Laatsch H, Strohmann C and Spiteller M: Epigenetic Modulation of Endophytic Eupenicillium sp. LG41 by a Histone Deacetylase Inhibitor for Production of Decalin-Containing Compounds. J Nat Prod 2017, (80) 983-988. PMid:28333449
View Article PubMed/NCBIHansen, M.B., Nielsen, S.E. and Berg, K.. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. Journal of immunological methods 1989, 119(2), pp.203-210. 90397-9
View ArticleGöppert, T.M. and Müller, R.H.: Adsorption kinetics of plasma proteins on solid lipid nanoparticles for drug targeting. International Journal of Pharmaceutics 2005, 302(1-2), pp.172-186. PMid:16098695
View Article PubMed/NCBIPerry, J.L., Reuter, K.G., Kai, M.P., Herlihy, K.P., Jones, S.W., Luft, J.C., Napier, M., Bear, J.E. and DeSimone, J.M. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano letters 2012, 12(10), pp.5304-5310. PMid:22920324
View Article PubMed/NCBI