Juan Peragón
Telephone #: +34 953212523; Fax #: + 34 953 211875
E-mail: jperagon@ujaen.es
ORCID ID: orcid.org/0000-0002-7502-1119
© 2019 Sift Desk Journals. All Rights Reserved
Juan Peragón
Telephone #: +34 953212523; Fax #: + 34 953 211875
E-mail: jperagon@ujaen.es
ORCID ID: orcid.org/0000-0002-7502-1119
MANUEL HERNÁNDEZ-YERA , JUAN PERAGÓN *
Biochemistry and Molecular Biology Section, Department of Experimental Biology, University of Jaén, Jaén, Spain
Juan Jos%c3%a9 Acevedo-Fern%c3%a1ndez(juan.acevedo@uaem.mx)
Hernandez-Yera and Peragón (2020), TIME COURSE PROFILE OF PENTACYCLIC TRITERPENES FROM STEM AND ROOT OF CV. PICUAL OLIVE TREE (Olea europaea, L.) ALONG RIPENING, Journal of Plant Science 4(1) p:186-193
Background: Pentacyclic triterpenes are plant secondary metabolites that have many applications for health of the people.
Methods: In this work, we determine by a HPLC-UV/Vis and HPLC-MS what pentacyclic triterpenes are found in stem and root of cv. Picual olive tree, a variety typically grown in the area of Jaén (Spain). Moreover, the concentration of these compounds along fruit ripening also has been determined in both organs.
Results: Maslinic acid (MA), oleanolic acid (OA), erythrodiol (EO), and uvaol (UO) have been found in stem. OA is the compound that shown the highest concentration in this organ. MA, betulinic acid (BA), OA and ursolic acid have been found in root. In general, BA has the highest concentration in this organ. BA had not been detected in other olive tree organs.
Conclusion: The evolution showed by the concentrations of these compounds during ripening suggests the existence of two different phases: the first related with the increment in weight of fruit and the second related with the ripening following. The evolution found in stem is different to the found in root and we believe that are related with the functions of these compounds in each organ.
Keywords: Betulinic acid, erythrodiol, maslinic acid, oleanolic acid, ripening, ursolic acid, uvaol.
Abbreviations
BA: betulinic acid, EO: erythrodiol, HPLC-UV/Vis: ultraviolet-visible high performance liquid chromatography; HPLC-MS: mass high-performance liquid chromatography; MA: maslinic acid, OA: oleanolic acid, OS: (3S)-2,3-oxidoescualene, UA: ursolic acid, UO: uvaol
Pentacyclic triterpenes are plant secondary metabolites amply distributed in plants that have important biological properties related with health and disease prevention. They are found in higher concentration in the cuticle wax and stem bark (Pensec et al., 2016). Chemically, they are molecules of 30 carbons grouped in five cycles of 6 carbons that have several characteristics substituents. These compounds proceed from squalene, a 30 carbon lineal terpene that is synthesized from isopentenyl pyrophosphate [1]. The biosynthesis of pentacyclic triterpenes proceed form (3S)-2,3-oxidoescualene (OS) that is synthesized via acetate/mevalonate cytoplasmatic pathway. OS is the substrate of several OS cyclases or triterpene cyclases that produced different pentacyclic triterpenes. The main triterpenes found in Olea europaea are maslinic acid [(2α,3β)-2,3-dihydroxyoleane-12-en-28-oic acid, C30H50O4; Mw: 472.7; MA], oleanolic acid (3β-hydroxyoleane-12-en-28-oic acid, C30H48O3; Mw: 456.7; OA), ursolic acid (3β-hydroxyursane-12-en-28-oic acid, C30H48O3; Mw: 456.7; UA), erythrodiol (oleane-12-en-3β,28-diol, C30H50O2; Mw: 442.7; EO) and uvaol (12-ursene-3β,28-diol, C30H50O2; Mw: 442.7; UO) [2] (Fig. 1). During biosynthesis, after OS cyclation, several cations are produced, between them oleanil cation, produced by the activity of β-amyrin synthase, that is the precursor of EO from which OA is synthesized. MA can be form by isomerization from OA. By the action of α-amyrin synthase, ursanil cation can be also produced and it is the precursor of UO, and from it, UA is synthesized [3].
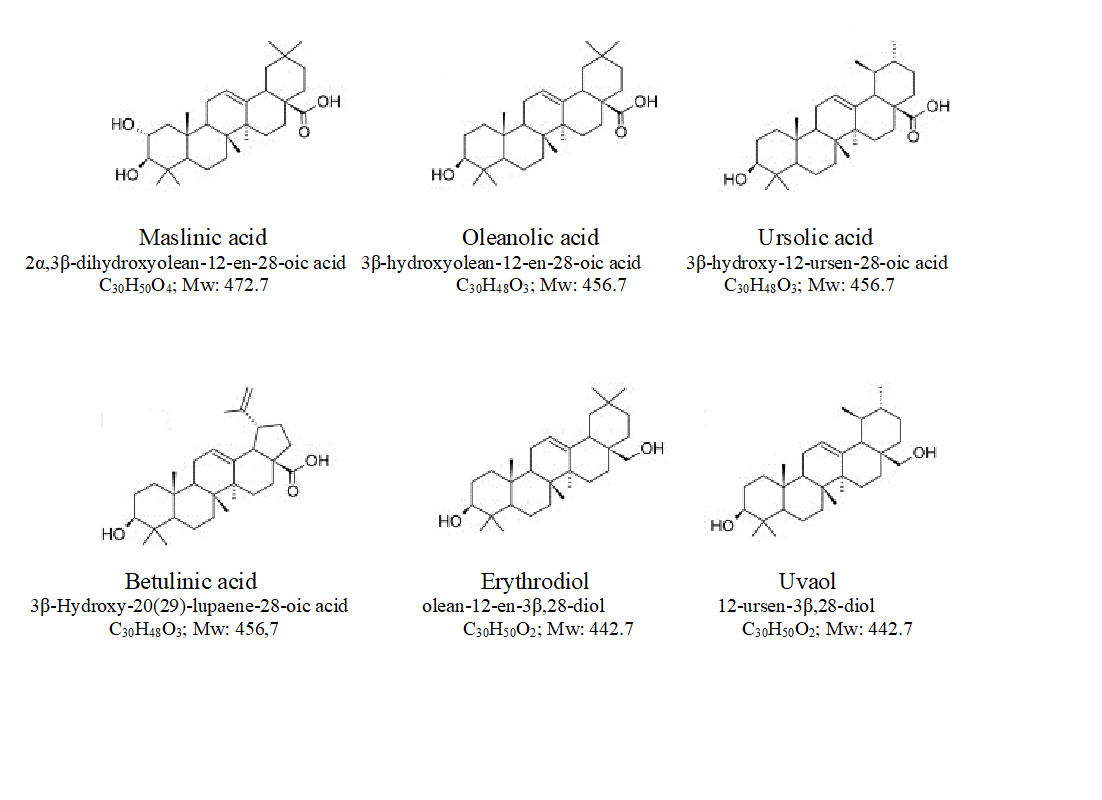
Fig. 1. Chemical structure of the main pentacyclic triterpenoids found in cv. Picual olive tree.
Stem and root are two differential organs of vascular plants or spermatophytes. They are form from embryo epicotyl bud or radicle bud, respectively [4]. Stem can be woody or herbaceous appearance and, usually, contain leaves or some modifications of its, for example, spines. The main functions of stems are the support of the plant and the transport of compounds between different parts of the plant, although, can also accumulated and assimilated different compounds and have photosynthetic function [4]. Differently, root has positive geotropism and has not leaves. Their functions are the fixation to the ground, water and mineral salts absorption or the store of substances [4].
The presence of these compounds has been described in several plants species. Chen et al., [5] identified three triterpenic compounds in the stem and roots of Rubus alceaefolius, a Chinese herbaceous plants used by the treatment of carcinome and osteoma. The three compounds were finally related with the antitumoural activity of this plant. Pai et al., [6] show the presence of BA, OA and UA in leaf, stem and root of Achyranther aspera, a medicinal plant used for the treatment of dysentery, gonorrhea, pneumonia, and others. Mawa et al., [7] identified three new triterpenes in the stem of Ficus aurantiaca related with the inhibition of quimiotaxis in neutrophils and the inhibition of reactive oxygen species production. Callies et al., [8] isolated several lupane type pentacyclic triterpenes in the stem of Cassine xylocarpa and Maytenus cuzcoina that inhibit the replication of Type 1 VIH viruses. In olive tree (Olea europaea) several pentacyclic triterpenes have been identified in organs such fruit and leaf [2]. MA, OA, EO and UO are found in leaves and MA and OA in fruits and many studies described important activities of these compounds for the health and disease prevention. Nevertheless there is not knowledge about the identity, presence and concentration of pentacyclic triterpenes in stem and root of olive tree. So, the aim of this work is to determine the pentacyclic triterpene profile typical of stem and root of this species and how change their concentration along the ripening of the fruit, an annually periodic situation that determine the interest of this tree for the economy of the region in which it is cultivated. Picual is the main variety cultivated in Jaén (Spain), the main province in the olive tree ground of the South-Est of Spain.
2.1. Drugs
Assays were made with chemical compounds supplied by Sigma Chemical Co. (St. Louis, USA) and Fluka Chemie GmbH (Buchs, Swizertland). MA, OA, UA, BA, EO and UO standard were purchased from Extrasynthèse (Z.I. Lyon-Nord, Genay, France). All the reagents used were of analytical or HPLC grade.
2.2. Stem and root olive tree sampling during ripening
Forty-year-old (Olea europaea L. cv. Picual) olive trees, in an orchard at Torredonjimeno (Jaén, Spain, 37º45’61’’N, 3º57’12’’W, 655 m above sea level) were used in this study. Three trees randomly distributed in the orchard were selected and, for each orientation of each tree, five 25-cm segments of branches with fruits near the apical end were collected. Fruits and leaves were removed, and stems were cut in some pieces and put on liquid nitrogen until analysis. Fruits were used for the evaluation of the ripeness index as proposed by Uceda and Frias [9]. Adventitious and 2 mm no lignified roots samples located near to the trunk were extracted with a hone. Seven different stem and root samples were collected in the same trees throughout the ripening period of olive fruit, from July to December. Specifically, sample 1 and 2 were picked at days 2 and 30 of July, sample 3 at 10 of September, samples 4 and 5 at 7 and 22 of October, sample 6 at 6 of November and sample 7 at 3 of December of 2015. Table 1 shows the time course of the ripeness index and water content over the experiment.
|
Table 1. Fruit ripeness index (RI) and humidity of stem and root during ripening |
|||||||
|
# Sample |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
Date |
2/07/2015 |
30/07/2015 |
10/09/2015 |
7/10/2015 |
22/10/2015 |
6/11/2015 |
3/12/2015 |
|
Days |
0 |
28 |
70 |
97 |
112 |
134 |
154 |
|
RI |
0 |
0 |
0.3 |
2.5 |
3.9 |
4.5 |
4.5 |
|
Humidity |
|||||||
|
Stem (%) |
41.96±2.10a |
38.94±1.95a |
40.20±2.01a |
37.28±1.86a |
39.77±1.99a |
43.15±2.16a |
41.34±2.067a |
|
Root (%) |
35.06±1.75ab |
31.55±1.58a |
35.68±1.78a |
31.96±1.60a |
40.89±2.04b |
55.84±2.79c |
57.10±2.855c |
|
Humidity of each organ is expressed as the mean ± S.E.M. Values followed by different letters are significantly different (P < 0.05). |
|||||||
2.3. Extraction and analysis of triterpenic compounds
We followed the procedure described in [2] and [10] with some modifications. Determination of water content. For each ripening stage, samples of stems and roots were finely pulverized with liquid nitrogen. The water content was determined weighing 3 g of the organ and then oven drying at 55ºC to constant weight. Afterwards, samples were cooled for 30 min in a dryer and reweighed.
Extraction of triterpenic compounds. For each sample, 0.125 g of dry tissue was mixed with 1.5 mL of methanol/ethanol (1:1, v:v). The mixture was vigorously vortexed for 1 min and, after sedimentation, was centrifuged at 7700xg for 5 min at 4ºC. The supernatant was collected in other tube. The extraction was repeated 5 times. All supernatants were pooled, and the resulting mixture was evaporated with a SpeedVac concentrator (Thermo Scientific, USA). The residue was dissolved in 1 mL of methanol and, after filtration (PTFE filters, 4 mm x 0.2 µm, Supelco Analytical, USA), was used for HPLC analysis of triterpenic compounds.
HPLC Analysis. We used two chromatographic systems: a HPLC-UV/Vis and a HPLC-MS system. The HPLC-UV/Vis chromatographic system was used for the analysis of stem samples. A reverse-phase Spherisorb ODS-2 (Waters Corporation, Milford, USA) (25 cm-4.6 mm, 5 µm) column was used in a Shimadzu HPLC system (Shimadzu Corporation, Kyoto, Japan). Separation was achieved by an isocratic elution for 20 min. The solvent used for separation was methanol/water with acetic acid (pH 3.1) at a proportion of 92:8 (v:v). A flow rate of 0.8 mL min-1 was used. Absorbance at 210 nm was recorded. A typical chromatogram for stem samples is shown in Fig. 2. MA, OA, EO and UO were identified and the concentration of each compound was determined on the basis of their peak area. Standard calibration curves were previously constructed. The equations formulated relating peak areas (y in arbitrary units) and concentrations (x in mg mL-1) were
y=9734990 x +58384.5 for MA;
y = 12653586.789 x + 35217.214 for OA;
y = 117741666.971 x + 73364.964 for EO;
y = 11774166.971 x for UO. In all cases, correlation coefficient (r2) was equal to 0.99.
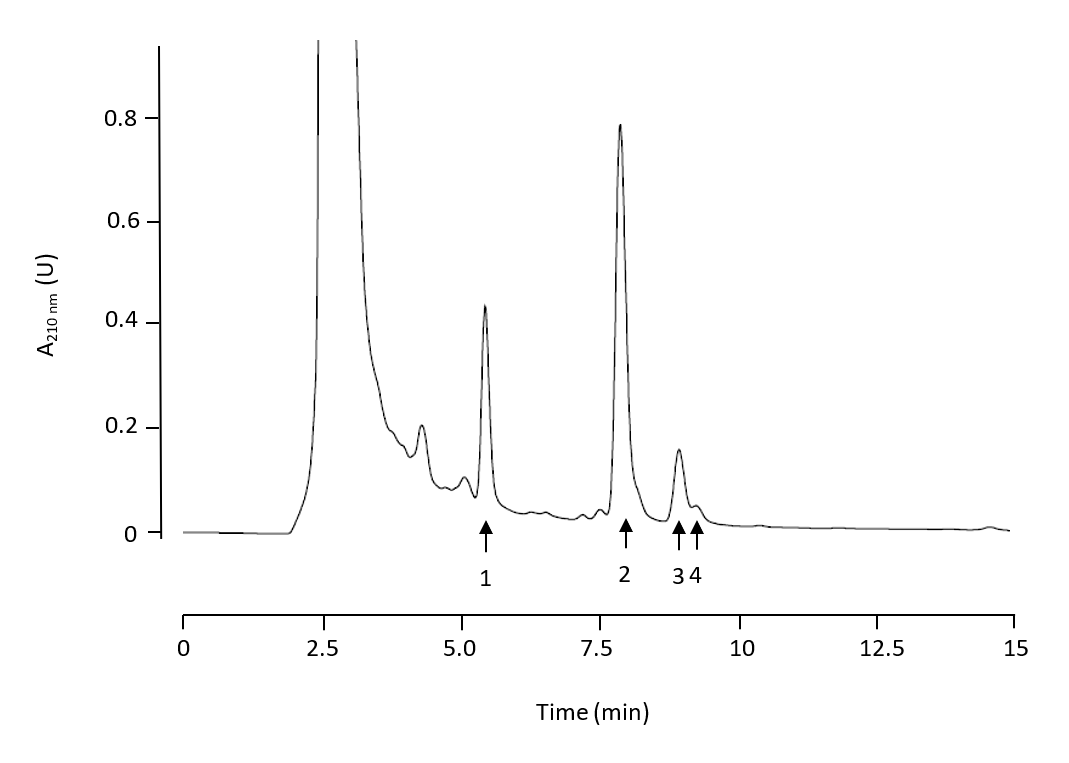
Fig. 2. HPLC-UV/Vis chromatogram of stem olive tree triterpenoid extracts. Compound 1: maslinic acid, compound 2: oleanolic acid, compound 3: erythrodiol, compound 4: uvaol.
We used a HPLC-MS system for the analysis of roots samples because the concentration of triterpenic compounds are so low and can not be appropriately detected and determine with a HPLC-UV/Vis system. A HPLC Agilent Series 1100 system (Agilent Technologies, Santa Clara, U.S.A) constituted by a vacuum degasser, an auto sampler, a quaternary pump, a diode-array detector, and a Esquire 6000 ion-trap mass spectrometer (Bruker Daltonics, Billerica, U.S.A) equipped with an electrospray ionization (ESI) source operating in negative ion mode was used. Separation was achieved by an isocratic elution as previously described for HPLC-UV/Vis system using also a reverse phase Spherisorb ODS-2 (Waters Corporation, Milford, USA) (25 cm-4.6 mm, 5 µm) column was used in. The injection volume was 2 μL. The solvent used for separation was methanol/water containing 0.1% formic acid (pH 3.1) at a proportion of 92:8 (v:v). A flow rate of 0.8 mL min-1 was used and a temperature of 35ºC. Ions were detected in an ion-charged control (ICC) (target: 2500 ions) with a variable accumulation time (detected in μs), using the following operation parameters: capillary exit voltage (fragmenter): −300.0 V; capillary voltage: 4000 V; nebulizer pressure: 60 psig, drying gas: 11 L·min−1, gas temperature: 350 °C. This chromatographic system operates with Bruker Daltonics Data Analysis Software (Bruker Daltonics). The fragmentation options used for the MS/MS analyses were: Energy 0.8 V, width m/z = 4, and time 40 ms. A typical chromatogram for the roots samples is shown in Fig. 3. MA, BA, OA and UA were identified and quantified following this method. MA is characterized by a retention time of 5.70 ± 0.01 min (Panel A). HPLC-MS analysis showed a peak of m/z = 471.0 corresponding with the negative ion of MA (Panel B). BA, OA, and UA are characterized by a retention time of 7.8, 8.4 and 8.7 min respectively. HPLC-MS analysis showed a peak of m/z = 455.0 corresponding with the negative ion of each compound (Panel B). Due to their chromatographic behavior, MA, BA, OA, and UA were quantified on the basis of retention time and the presence of 471 and 455 ion and their integrates areas, respectively. We constructed standard calibration curves with 6 concentrations of each compound (from 0.001 to 0.025 mg mL-1). The equations formulated relating concentration (x, mg·mL-1) and peak areas (y, arbitrary units) were: y = 317987991.71146 x for MA; y = 2293640505.391x +24492206.764 for OA; y = 5839461405.136 x + 32638130.696 for BA; y = 1350191687.212 x + 3856830.68 for UA. In all cases r2 was higher than 0.95.
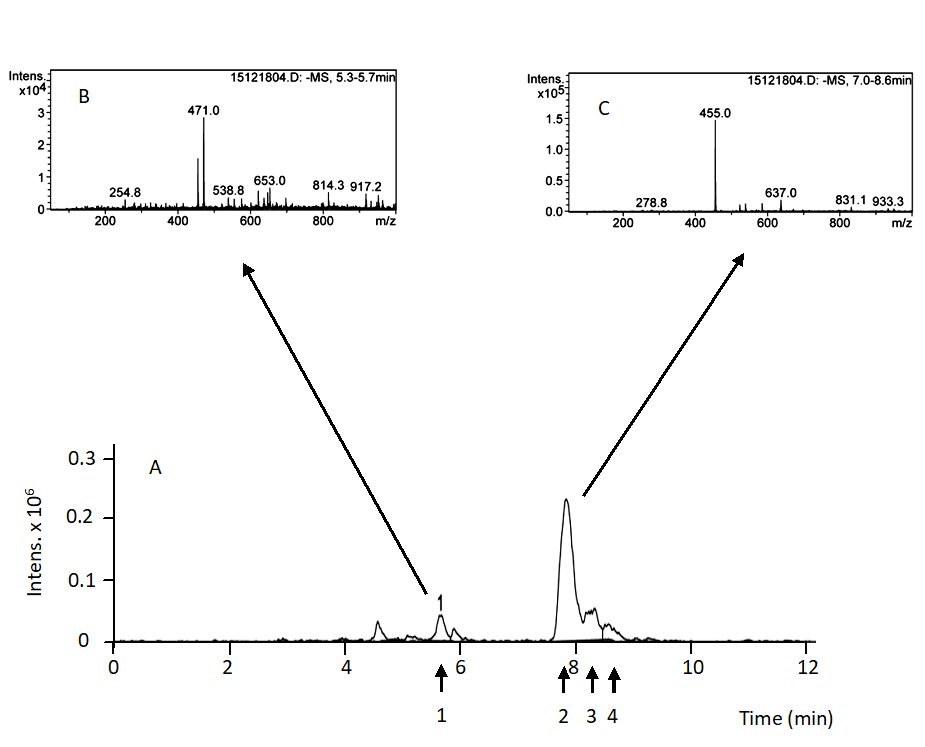
Fig. 3. HPLC-MS analysis of root olive tree triterpenoid extracts. Panel A: Extracted ion chromatogram. Panel B: MS spectra of peak 1 eluted at 5.7 min. Panel C: MS spectra of peaks 2, 3, 4 eluted at 7.8, 8.4 and 8.7 min, respectively. Compound 1: maslinic acid, compound 2: betulinic acid, compound 3: oleanolic acid, compound 4: ursolic acid.
2.4. Statistical Analysis
Results are expressed as the mean followed by the standard error of the mean (S.E.M.). Data were analysed by a one-way analysis of variance. The differences between means were analysed using a Student’s t-test. The criterion of significance was taken as P < 0.05.
In the present work we determined the pentacyclic triterpenoid profile found in cv. Picual olive tree stem and root. MA, OA, EO and UO are present in stem (Fig. 2). Considering all the samples peak at the different ripening stages, OA was found with a concentration between 3-6 mg/g of dry weight, MA between 1-2.5 mg/g of dry weight, EO between 0.25-0.6 mg/g of dry weight and UO with a concentration of 0.1-0.2 mg/g dry weight (Fig. 4). OA is that show a higher concentration in this organ, representing a 0.62% of the dry weight in sample 3. MA follows OA, with a 0.26% of the dry weight in sample 3. The total concentration obtained as the sum of all these compounds range from 4.35 to 9.3 mg/g dry weight. Results of this study demonstrated that the method of extraction used is appropriated for the quantification of the concentration of these compounds in stem samples. The profile described for stem is likely to the found in leaf in which, also this four triterpenic compounds are found [11] although with concentration in leaf higher that in stem. The presence of MA, OA, EO and UO indicate that β-amyrin synthase is present in this organ and it is implied in the biosynthesis pathway of MA, OA and EO and that α-amyrin synthase is also present in this organ and it is implied in biosynthesis of UO. This similar patter between stem and leaf can be related with that both organs are photosynthetic organs.
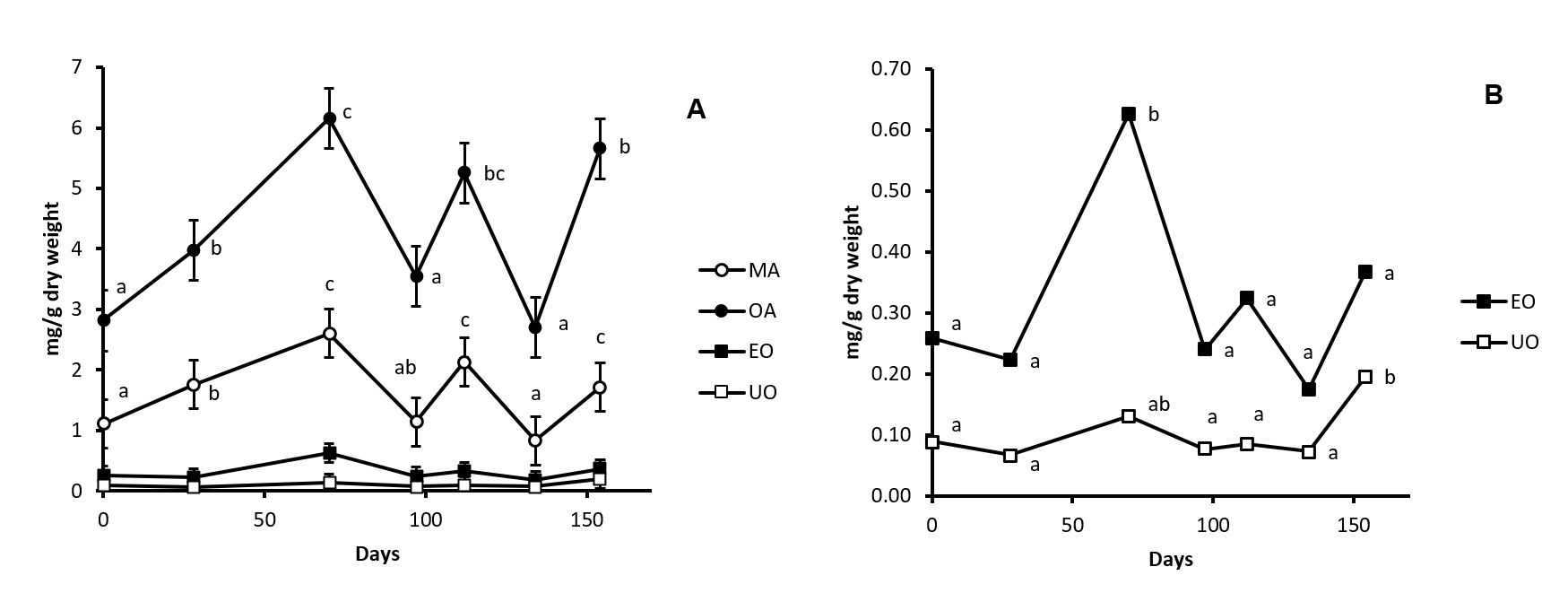
Fig. 4. Concentration of maslinic acid (MA, Panel A), oleanolic acid (OA, Panel A), erythrodiol (EO, Panel A and B) and uvaol (UO, Panel A and B) in cv. Picual olive tree stem samples along fruit ripening. Day 0 was considered the sample peak at 02/07/2015. Values followed by different letter are significantly (P<0.05) different.
In roots, the triterpenic compounds identified are MA, BA, OA and UA (Fig. 3) with a concentration that range between 0.1 and 0.4 mg/g dry weight for BA, 0.025 and 0.3 for MA, 0.0025 and 0.022 for OA, and 0.001 and 0.022 for UA (Fig. 5). The total concentration obtained as the sum of all these compounds range from 0.13 to 0.74 mg/g dry weight. This value is between 32.46 and 11.54-fold lower than the found in stem and demonstrated the difference in the importance of triterpenoids in the physiology of both organs. Also, the presence of BA in root samples merit to be mentioned because it has not been previously described in other organ of this species. The presence of BA in olive tree samples must implied the existence of a lupeol synthase in olive root sample responsible of the biosynthesis of the intermediates that lead to BA.
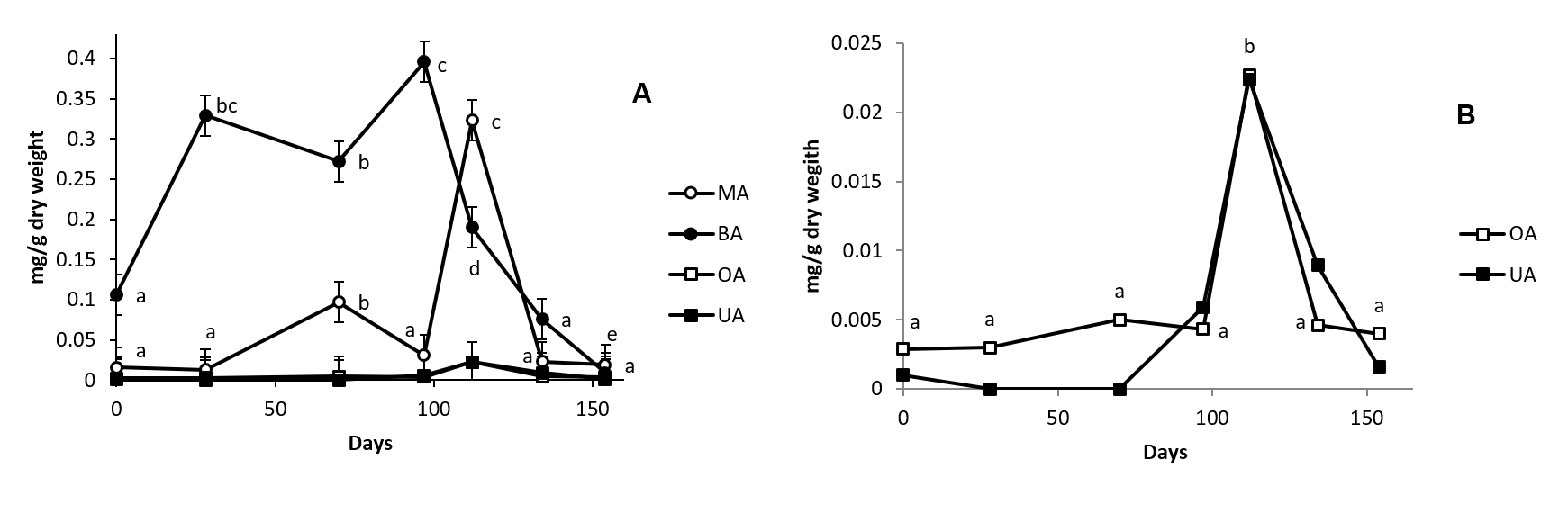
Fig. 5. Concentration of maslinic acid (MA, Panel A), betulinic acid (BA, Panel A), oleanolic acid (OA, Panel A and B) and ursolic acid (UA, Panel A and B) in cv. Picual olive tree root samples along fruit ripening. Day 0 was considered the sample peak at 02/07/2015. Values followed by different letter are significantly (P<0.05) different.
The changes in the concentration of the four triterpenoids found in stem during olive fruit ripening are plotted in Fig. 4. The concentration of OA and MA changed as in two phases along this processes. In the first phase, that comprised samples 1, 2 and 3 -75 days after beginning of the experiment-, a significant increment is produced. The concentration of both major triterpenes is duplicated in sample 3 with respect sample 1. This is the time in which the fruit increase in size and weight and is name as “green ripening”. So, coinciding with the accumulation of compounds in fruit, a significant increment in the synthesis and concentration of OA and MA is found in stem. It can be probably related with the supply of both compounds to the fruit. In the second phase, that comprises samples 4, 5, 6 and 7 – 79 days after sample 3-, a fluctuating tendency to the decrease in the concentration of OA and MA can be observed. This is the time in which change the composition of fruit that do not increase in size and weight, yet. This phase is name as “black ripening”.
The concentration of EO in stem is practically maintained along fruit ripening although a significant one-fold increase is produced in sample 3. UO concentration also is maintained around 0.1 mg/g dry weight increasing only in sample 7 until reach 0.2 mg/g dry weight.
The concentration of triterpenoids found in roots during olive fruit ripening are plotted in Fig. 5. BA, the triterpenoid that show higher concentration in this organ, significantly increment their concentration 3-fold from sample 1 to sample 4. After, their concentration fall until reach, in sample 7, values below to the showed in sample 1. MA, OA and UA maintain their levels until sample 5 in which, the tree acids, significantly increase their levels several folds. MA also shows a previous enhance in their concentration in sample 3.
With respect to the physiological function of pentacyclic triterpenoids these compounds are considered as part of a defense system which defends plant against environmental stress, wound and injuries repair mechanism and enhanced the resistance against plant pathogens [12, 13]. A variety of infectious organisms were reported inhibited by triterpenoids compounds, including fungi, viruses, bacteria, protozoa and parasites [14]. So, in these sense it has been shown as UA is an efficient antifungal against the infection of apple by Alternaria alternata that could be an useful alternative for this disease management [13]. Also OA and BA exert a inhibitory activity against influenza virus and hepatitis C virus infection binding to viral fusion proteins and disrupting viral entry into the host cells [14]. In root olive tree, pentacyclic triterpenes can be also part of a defense system that protects plant against different agents. As is reported in other plants, these molecules can act avoiding the growth of invasive cells acting on their viability or avoiding the entry of virus inside the cells. These compounds have a non-polar nature that permits its interaction with apolar alpha-helix avoiding the normal effect of its interaction and avoiding the membrane fusion or the normal function of calcium channels [14].
Concluding, the pentacyclic triterpenes found in stem and root of cv. Picual olive tree (Olea europaea) have been identified and quantifed in this work. The profile reported in stem is likely with the previously described in leaf while in root it is different to previously described and characterize the metabolism of these compounds in this organ. Moreover, it has been showed how the concentration of each triterpene changes during fruit ripening. A differential behavior is showed by the different triterpenes in both organs that can be related with the specific functions of each one in stem or root.
The authors thank the Unit of Chromatography of the Scientific and Technical Instrumentation Center, University of Jaén, for the technical and instrumental assistance.
Funding
This research was funded by the University of Jaén (Plan Propio de Investigación, Grant UJA2014/07/13), and by the Junta de Andalucía (Plan Andaluz de Investigación, Junta de Andalucía, Spain, Grant BIO-341 “Enzymes and Metabolism”).
Author’s contributions
JP conceived and designed research, MH-Y conducted experiments and analyzed data. JP and MH-Y wrote the manuscript. All authors read and approved the final manuscript.
Pensec, F., Szakiel, A., Paczkowski, C., Wonzniak, A., Grabarczyk, M., Bertsch, C., Fischer M.J.C., Chong, J., 2016. Characterization of triterpenoid profiles and triterpene synthase expression in the leaves of eight Vitis vinifera cultivars grown in the Upper Rhine Valley. J. Plant. Res. 129, 499-512. PMid:26879930
View Article PubMed/NCBIPeragón, J., 2013. Time course of pentacyclic triterpenoids from fuit and leaves of olive tree (Olea europaea L.) cv. Picual and cv. Cornezuelo during Ripening. J. Agric. Food Chem. 61, 6671-6678. PMid:23768136
View Article PubMed/NCBIGiménez, E., Juan, M.E., Calvo-Melià, S., Barbosa, J., Sanz-Nebot, V., Planas, J.M., 2015. Pentacyclic triterpene in Olea europaea L: A simultaeous determination by high-performance liquid chromatography coupled to mass spectrometry. J. Chromatogr. A. 1410, 68-75. PMid:26210113
View Article PubMed/NCBIIzco, J., Barreno, E., Brugués, M., Costa, M., Devesa, J.A., Fernández, F., Gallardo, T., Llimona, X., Prada, C., Talavera, S., Valdés, B., 2009. Botánica. McGraw-Hill Interamericana. Second edition.
Chen, P., Yan, H.J., Mei, Q.X., Chen, Y.F., Wang, X.L., Ming, L., Fang, Z.J., 2016. Triterpenoids from the Roots and Stems of Rubus alaceaefolius. Chem. Nat. Comp. 52, 248-251.
View ArticlePai, S.R., Upadhya, V., Hegde, H.V., Joshi, R.K., Kholkate, S.D., 2016. Determination of betulinic acid, oleanolic acid and ursolic acid from Achyranthes aspera L. using RP-UFLC-DAD analysis and evaluation of various parameters for their optimum yield. Ind. J. Exp. Biol. 54, 196-202.
Mawa, S., Jantan, I., Husain, K., 2016. Isolation of triterpenoids from the stem of Ficus aurantiaca Griff and their effects on reactive oxygen species production and chemotactic activity of neutrophils. Molecules. 21, 1-14. PMid:26742027
View Article PubMed/NCBICallies, O., Bedoya, L.M., Beltran, M., Munoz, A., Calderon, P.O., Osorio, A.A., Jimenez, I.A., Alcami, J., Bazzocchi, I.L., 2015. Isolation, structural modification, and HIV inhibition of pentacyclic lupane-type triterpenoids from Cassie xylocarpa and Maytenus cuzcoina. J. Nat. Prod. 78, 1045-1055. PMid:25927586
View Article PubMed/NCBIUceda, M., Frías, L., 1975. Harvest dates: Evolution of the fruit oil content, oil composition and oil quality. Proceedings of the II Seminario Oleícola Internacional, International Olive Oil Council, Cordoba, 1975, pp. 125130.
Peragón, J., Rufino-Palomares, E.E., Muñoz-Espada, I., Reyes-Zurita, F.J., Lupiáñez, J.A., 2015. A new HPLC method for measuring maslinic acid and oleanolic acid in HT29 and HepG2 human cancer cells. Int. J. Mol. Sci. 16, 21681-21694. PMid:26370984
View Article PubMed/NCBIJiménez-Herrera, R., Pacheco-López, B., Peragón, J., 2019. Water stress, irrrigation and concentrations of pentacyclic triterpenes and phenols in Olea europaea L. cv. Picual olive trees. Antioxidants. 8: 294, 14 pages. PMid:31398872
View Article PubMed/NCBIDomingo, V., Arteaga, J.F., Quílez Del Moral, J.F., Barrero, A.F., (2009). Unusually cyclized triterpenes: ocurrence, biosynthesis and chemical synthesis. Nat. Prod. Rep. 26, 115-134. PMid:19374125
View Article PubMed/NCBIShu, C., Zhao, H., Jiao, W., Liu, B., Cao, J., Jiang, W., 2019. Antifungal efficacy of ursolic acid in control of Alternario alternata causing black spot rot on apple fruit and possible mechanisms involved. Sci. Hortic. 256, 108636, 9 pages.
View ArticleSi, L., Meng, L., Tian, Z., Sun, J., Li, H., Zhang, Z., Soloveva, V., Li, H., Fu, G., Xia, Q., Xiao, S., Zhang, L., Zhou, D., 2018. Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelopes. Sci. Adv. 4, eaau8408. PMid:30474060
View Article PubMed/NCBI