Faiçal Brini
Email: faical.brini@cbs.rnrt.tn
© 2019 Sift Desk Journals. All Rights Reserved
Faiçal Brini
Email: faical.brini@cbs.rnrt.tn
Walid Saibi and Faiçal Brini*
Biotechnology and Plant Improvement Laboratory, Center of Biotechnology of Sfax, (CBS)-University of Sfax, 3018 Sfax, Tunisia.
Walid Saibi, Brini faiçal, Oxidative stress and antioxidant defense in Brassicaceae plants under abiotic stresses (2021) Journal of Plant Science 5(1) p:232-244
Brassicaceae plants, as an important source of primary and secondary metabolites, are becoming a research model in plant science. Plants have developed different ways to ward off environmental stress factors. This is lead to the activation of various defense mechanisms resulting in a qualitative and/or quantitative change in plant metabolite production. Reactive oxygen species (ROS) is being continuously produced in cell during normal cellular processes. Under stress conditions, there are excessive production of ROS causing progressive oxidative damage and ultimately cell death. Despite their destructive activity, ROS are considered as important secondary messengers of signaling pathway that control metabolic fluxes and a variety of cellular processes. Plant response to environmental stress depends on the delicate equilibrium between ROS production, and their scavenging. This balance of ROS level is required for performing its dual role of acting as a defensive molecule in signaling pathway or a destructive molecule. Efficient scavenging of ROS produced during various environmental stresses requires the action of several non-enzymatic as well as enzymatic antioxidants present in the tissues. In this review, we describe the ROS production and its turnover and the role of ROS as messenger molecules as well as inducers of oxidative damage in Brassicaceae plants. Further, the antioxidant defense mechanisms comprising of enzymatic and non-enzymatic antioxidants have been discussed.
Keywords: Abiotic stress, Antioxidant defence, Brassicaceae, Oxidative stress, ROS
One of the most damage effects of the abiotic stress in plants is the production of toxic ROS in different cellular and sub-cellular compartments [1]. Some ROS are considered to be the most potent reactive ions known. They are generated due to the decreased content of intracellular CO2, this results in the transfer to O2 of one, two or three electrons, to form superoxide (O2-), hydrogen peroxide (H2O2) or the hydroxyl radical (HO-), respectively [2]. Due to their reactivity with various key cellular components, their excess can lead to irreparable metabolic dysfunction and cell death [3,4]. At normal conditions, the small amounts of ROS are by-products of normal cell metabolism, formed in vital processes such as respiration, photorespiration and photosynthesis [5,6]. Abiotic stress increases their production resulting of oxidative damage [7,8]. The presence of high concentrations of ROS in the cell leads to major disturbance of ionic homeostasis by depressing cytosolic K+ concentrations followed by activation of proteases and endonucleases [9,10], alteration of the cell membrane integrity, inhibition of enzyme activities and the function of the photosynthetic apparatus and of DNA lesions. The collective effect can lead to cell death [11,12]. Under non-stressful conditions, ROS at low cellular concentrations play a key role as signaling molecules involved in plant growth, development, hormonal action and many other physiological processes [5,8,13-16]. Such low-level ROS functions include triggering of antioxidant defense mechanisms for adapting to abiotic stress [12,16-18]. In fact, ROS at concentrations much lower than those causing cellular damage, can activate different Na+- and K+-permeable ion channels [19-21] that help maintain the cytosolic K+/Na+ ratios needed for salinity tolerance [22]. The production of ROS is the outcome of a plant metabolism that needs to be controlled to safeguard its cellular components [23]. Under stress condition, plants activate enzymatic and non-enzymatic antioxidant systems. The latter include antioxidant compounds such as ascorbic acid, glutathione, flavonoids, β-carotenes or other phenolic compounds. Among enzymatic antioxidant systems are superoxide dismutases (SOD), catalase (CAT), ascorbate peroxidase (APX) or redox regulatory enzymes such as glutathione reductase (GR), among many others [24]. Under stress conditions, the biosynthesis and the activities of these antioxidant molecules are altered [14,25-27].
The genus Brassica includes economically important oilseed and vegetable plants. This group comprising about 100 species, including mustard (Brassica juncea L.), rapeseed (B. napus L.), turnip rape (B. rapa L.) and cabbage (B. oleracea L.) that are grown mainly for oil and vegetables [28]. Brassicaceae is grown in both arid and semiarid regions and is severely affected by both biotic stresses, including bacteria, viruses and fungi, and abiotic stresses, including cold, heat, salinity and drought. Brassica is a good source of antioxidants due to the presence of high phenolics and glucosinolate content [29].
In this review, the effect of oxidative stress on Brassicaceae and the role played by ROS as signaling molecule in the mechanism of response of Brassicaceae to abiotic stress are discussed in detail.
2. Family Brassicaceae
The Brassicaceae (or cruciferae/mustard family) is considerably a large angiosperm family of dicots belonging to order brassicales with 10–19 tribes comprising of 338–360 genera and 3709 species [30]. The largest genera are Draba (365 species) followed by Lepidium (230 species), Erysimum (225 species), Cardamine (200 species) and Alysum (195 species) [31]. The family comprises of several plant species with agronomic and economic significance including model species (e.g. Arabidopsis and Brassica), developing model systems (e.g. Brassica and Cradamine) as well as various cultivated plant species (e.g. cauliflower, horseradish, cabbage, turnip, etc.) [32]. In 1934, Morinaga [33], using cytological work demonstrated the relationships among the cultivated Brassica species. According to his hypothesis, the high chromosome number of species B. napus (2n = 38, AACC), B. juncea (2n = 36, AABB), and B. carinata (2n = 34, BBCC) are amphidiploids combining in pairs the chromosome sets of the low chromosome number species B. nigra (2n = 16, BB), B. oleracea (2n = 18, CC), and B. rapa (2n = 20, AA) (Figure 1). The family Brassicaceae serves as a good source of oils, vegetables, weeds, and ornamentals of huge economic importance. For example, locally known as shagsoo, Christolea is used as vegetable in combination with milk in addition to the edible Meeacarpea species. Mustards (B. nigra and B. juncea) are used as condiments. Seeds of B. campestris, B. juncea, B. nigra and B. pekinensis are crushed in preparation of edible oils. In different regions, Camelina sativa, Eruca sativa and Sinapis alba which are odiferous are also cultivated as oil plants along with the seeds of Capsella which contain about 15–20% oil. The seeds of Conringia orientalis contain fatty oil and its young sprouts are also comestible. Fresh leaves of Lepidium sativum are used as salad, and its seeds contain 5% fatty oil, making it worthy for illumination. Some species like Arabis are cultivated as ornamentals in rock gardens. Parrya exscapa grows at high altitudes laced with beautiful flowers. Different pigments from Isatis tinctoria serve as a dying agent besides being a honey-producing plant. Erysimum Perofskianum seeds serve as crude material for the formulation of cardiac drugs in pharmaceutical industry.
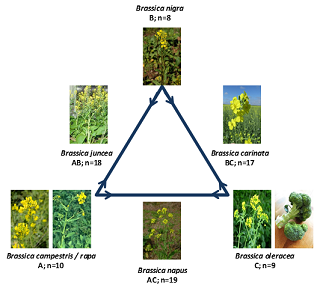
Figure 1. The U-triangle showing derivation of the high chromosome Brassica species from low chromosome species
3. Oxidative Stress and Generation of ROS
During abiotic stresses, plants undergo several mechanisms to combat increased ROS production. The balance between production and scavenging of ROS may be disturbed by a number of biotic and abiotic factors, which may increase the intracellular levels of ROS [13]. When the level of ROS exceeds the defense mechanisms, the cell is in a state of oxidative stress [4,13]. Oxidative stress leads to the loss of physiological capacity and eventual cell death. Therefore, defense mechanisms against oxidative damage are activated during stress to regulate toxic levels of ROS [34]. ROS are a group of free radicals, reactive molecules, and ions derived from oxygen. The most common ROS include singlet oxygen (1O2), superoxide radical (O2⋅−), hydrogen peroxide (H2O2), and hydroxyl radical (OH⋅). These substances are highly reactive and toxic and can lead to oxidative destruction of the cell [13,14,27]. ROS are generated mainly by mitochondrion via electron transport. However, it can found in others various subcellular compartments such as chloroplasts via the Mehler reaction, and peroxisomes via photorespiration [14]. The oxidative stress usually results from excessive ROS production, mitochondrial dysfunction, impaired antioxidant system, or a combination of these factors. Abiotic and biotic stresses can severely disturbed the balance between production and elimination of ROS [5]. These disturbances in the ROS equilibrium (redox homeostasis) can lead to a rapid increase in intracellular ROS levels, which can cause significant damage to cell structures [35]. However, when ROS production overcomes the cellular scavenging capacity, there occurs an unbalancing of the cellular redox homeostasis resulting in a rapid and transient excess of ROS, known as oxidative stress [4,34]. Thus, the antioxidant defense imbalance disrupts metabolic activities [36], causing severe oxidative damages to cellular constituents, which can lead to loss of function and even cell death (Figure 2) [34].
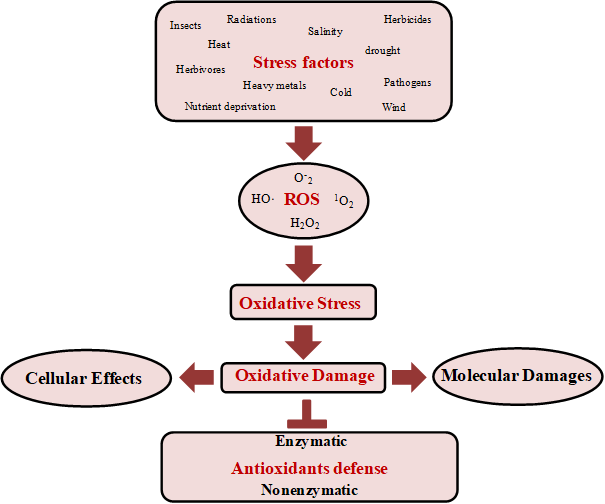
Figure 2. Stress factors, ROS generation, oxidative damage, and antioxidant defense. Several stress factors increased the ROS production, such as HO⋅, O-2, 1O2, and H2O2. The increased ROS levels lead to oxidative stress. Consequently, oxidative damage at the molecular and cellular levels occurs. Defense mechanisms against oxidative stress are activated to neutralize toxic levels of ROS. Singlet oxygen (1O2), superoxide radical (O2•-), hydrogen peroxide (H2O2), and hydroxyl radical (OH⋅).
Enhanced level of ROS can cause damage to biomolecules such as lipids (lipid peroxidation), proteins (fragmentation of the peptide chain) and DNA (deoxyribose oxidation, strand breakage, removal of nucleotides, variety of modifications in the organic bases of the nucleotides, and DNA-protein crosslinks), and so forth ultimately resulting in cell death [4]. To avoid potential damage caused by ROS and to maintain growth, development and metabolism, the balance between production and elimination of ROS must be regulated. Plants possess complex antioxidative defense system comprising of non-enzymatic and enzymatic components to scavenge ROS [5,14]. Nonenzymic components of the antioxidative defense system include the major cellular redox buffers ascorbate (AsA) and glutathione (γ-glutamyl-cysteinyl-glycine, GSH) as well as tocopherol, carotenoids, and phenolic compounds [5,14,36]. The enzymatic components of the antioxidative defense system comprise of several antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX), enzymes of ascorbate glutathione (AsA-GSH) cycle ascorbate peroxidase (APX), monodehydro ascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) [5,36]. Various components of antioxidative defense system involved in ROS scavenging have been already well characterized into plant models, and disturbances or alterations in this system are an excellent strategy to understand the different signaling pathways involving ROS.
3.1. Nonenzymatic antioxidants
Nonenzymatic antioxidants interact with numerous cellular components and play key roles in defense and as enzyme cofactors. Moreover, these antioxidants influence plant growth and development, cell elongation and cell death [37].
Ascorbate (AsA) is found in organelles of most plant cell types and in the apoplast. AsA has a key role in defense against oxidative stress caused by enhanced level of ROS because of its ability to donate electrons in a number of enzymatic and nonenzymatic reactions. AsA can directly eliminate O2⋅−, OH⋅, and 1O2, and thus reduce H2O2 to water via the ascorbate peroxidase reaction [38]. AsA is generally maintained in its reduced state by a set of NAD(P)H-dependent enzymes, including monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase [14,27]. Moreover, AsA is involved in the regulation of cell division, cell elongation and it participates in multiple functions in photosynthesis [39].
Glutathione (γ-glutamylcysteinyl- glycine, GSH) is one of the crucial thiol that plays an important role in intracellular defense against ROS-induced oxidative damage. GSH is oxidized by ROS to form oxidized glutathione (GSSG), which is present in all cellular compartments. The balance between the GSH and glutathione disulfide (GSSG) is a central component in maintaining cellular redox state [5,40,41]. Due to its reducing power, GSH plays an important role in diverse biological processes, including cell growth/division, enzymatic regulation, regulation of sulfate transport, conjugation of metabolites, synthesis of proteins and nucleic acids, signal transduction and the expression of the stress responsive genes [42].
Tocopherols (α, β, γ, and δ) is a group of lipophilic antioxidants involved in scavenging of oxygen free radicals, lipid peroxyradicals, and 1O2 [4]. The α-tocopherol with its three methyl substituents has the highest antioxidant activity of tocopherols [43]. The α-tocopherol present in the membrane of chloroplasts protects them against photooxidative damage [38,44]. Accumulation of α-tocopherol has been shown to induce tolerance to water deficit, salinity and chilling, in different plant species [45,46]. Chemical investigation of Brassicaceae has revealed the presence of tocopherols, of which α-tocopherol is the most abundant [47].
Phenolic compounds are abundantly found in plant tissues, such as flavonoids, lignin, tannins, hydroxycinnamate esters, and possess antioxidant properties [48]. They have been shown to outperform well-known antioxidants, AsA and α-tocopherol, in in vitro antioxidant assays because of their strong capacity to donate electrons or hydrogen atoms. They also modify lipid packing order and decrease fluidity of the membranes [49]. Species within the Brassicaceae family are also rich in phenolics, including simple phenolic acids, flavonoids, anthocyanins and lignans as the major chemical classes [50,51].
3.2. Enzymatic antioxidants
Enzymatic components of the antioxidative defense system comprise several antioxidant enzymes such as catalase (CAT, EC 1.11.1.6), superoxide dismutase (SOD, EC 1.15.1.1), glutathione peroxidase (GPX, EC 1.11.1.9), guaiacol peroxidase (POX, EC 1.11.1.7), peroxiredoxins (Prxs, EC 1.11.1.15), enzymes of ascorbateglutathione (AsA-GSH) cycle ascorbate peroxidase (APX, EC 1.1.11.1), monodehydroascorbate reductase (MDHAR, EC 1.6.5.4), dehydroascorbate reductase (DHAR, EC 1.8.5.1), and glutathione reductase (GR, EC 1.8.1.7) [5,14,36,27]. This antioxidant system plays an important role in the maintenance of cell homeostasis and in the antioxidant response in plants.
Catalases catalyze the dismutation of two molecules of H2O2 into water and oxygen. CATs are largely, but not exclusively, localized to peroxisomes. Plants possess multiple CATs encoded by specific genes, which respond differentially to various stresses that are known to generate ROS [52,53]. Overexpression of a CAT gene from Brassica juncea introduced into tobacco, enhanced its tolerance to Cd induced oxidative stress [54].
Superoxide dismutases catalyze the dismutation of O2⋅ to H2O2. These enzymes may be attached to a metal ion (Mn, Fe, Cu/Zn, and Ni); thus, they are classified according to their subcellular location and metal cofactor. SOD activity has been reported to increase in plants exposed to various environmental stresses, including drought and metal toxicity [53].
Ascorbate peroxidases are enzymes that play a key role in catalyzing the conversion of H2O2 in to H2O and use ascorbate as a specific electron donor. Plants have different APX isoforms that are distributed in distinct subcellular compartments, such as mitochondria, chloroplasts, peroxisomes, and the cytosol. The APX genes are differentially modulated by several abiotic stresses in plants [55-57]. Overexpression of the tApx gene in either tobacco or in Arabidopsis increased tolerance to oxidative stress [58]. The balance between SODs, CATs, and APXs is crucial for determining the effective intracellular level of O2⋅ and H2O2, and changes in the balance of these appear to enhance compensatory mechanisms [13,52,53].
Glutathione peroxidases are nonheme thiol peroxidases that catalyze the reduction of H2O2 or organic hydroperoxides to water. The GPX proteins have been identified in many life species [59]. In plants, the GPX proteins are localized to mitochondria, chloroplasts, and cytosol.
Peroxiredoxins are a family of thiol-specific antioxidant enzymes that are involved in cell defense and protection from oxidative damage. The peroxiredoxins are a group of peroxidases that have reducing activity in their active sites via cysteine residues. They do not possess a prosthetic group and catalyze the reduction of H2O2, peroxynitrite, and a wide variety of organic hydroperoxides to their corresponding alcohols [60]. The peroxiredoxins are widely distributed in plant cells and are important proteins in chloroplast ROS detoxification [61].
Guaiacol peroxidases are involved in H2O2 detoxification. The POX proteins are hemecontaining enzymes that belong to class III or the “secreted plant peroxidases”. Theses enzymes are able to undertake a second cyclic reaction, called the hydroxylic reaction, which is distinct from the peroxidative reaction. Due to the use of both cycles, class III peroxidases are known to participate in many different plant processes, from germination to senescence, cell wall elongation, auxin metabolism, and protection against pathogens [62].
Monodehydroascorbate reductase is a flavin adenine dinucleotide enzyme that catalyzes the regeneration of AsA from the monodehydroascorbate radical using NAD(P)H as an electron donor. Thereby, MDAR plays an important role in the plant antioxidant system by maintaining the AsA pool [63]. Isoforms of MDAR have been reported to be present in the cytosol, chloroplasts, peroxisomes, and mitochondria [64]. Overexpression of Arabidopsis MDHAR gene in tobacco confers enhanced tolerance to salt stress [65]. Tomato chloroplastic MDHAR overexpressed in transgenic Arabidopsis enhanced its tolerance to temperature and methyl viologen-mediated oxidative stresses [66].
Dehydroascorbate reductase is a thiol enzyme that maintains AsA in its reduced form. DHAR catalyzes the reduction of dehydroascorbate to AsA using GSH as a reducing substrate [36,63]. It is present in various plant tissues, and its modulation activity has been reported in various plant species [67]. Transgenic potato overexpressing Arabidopsis cytosolic AtDHAR1 showed higher tolerance to salt, drought and herbicide stresses [68].
Glutathione reductase a NAD(P)H-dependent enzyme catalyzes the reduction of GSSG to GSH and, thus, maintains high cellular GSH/GSSG ratio. It is a key enzyme of the AsA-GSH cycle; it protects cells against oxidative damage; and it maintains adequate levels of reduced GSH. A high GSH/GSSG ratio is essential for protection against oxidative stress [15].
4. Effect of Environmental Stress on Family Brassicaceae
The plant families Brassicaceae, also known as Cruciferae, contain high phenolics and glucosinates levels thereby considered them a good source of antioxidants [69,70]. These compounds are known to have a preventive role against different types of diseases [69]. However, presence of some polyphenols, tannins, glucosinolates, erucic acid and S-methylcysteine sulfoxide, in Brassicaceae vegetables, reflected its anti-nutritional effects [71]. Under abiotic and biotic stress, plants respond through activation of their defense system to ensure their growth and development [72,73]. In Brassica plants, these stress factors affect the primary and secondary metabolism, increasing the metabolite level production, e.g. amino acids, sugars and indoles [69,74]. Abiotic and biotic stresses enhance some specific secondary metabolite production which activate a number of signal pathways like abscisic acid (ABA), salicylic acid (SA), ethylene (ET) or jasmonic acid (JA) pathways [75]. These pathways are known to have a well-defined role in the plant defense responses [72,75].
4.1. Effect of Stress on Primary Metabolites
Abiotic stress affects plant metabolite production [76]. In B. napus leaves drought stress lead to a distinctive increase of amino acids, followed by a reduction in concentration upon rehydration of the plants [77]. The same stress has been proved to increase sugar contents in cabbage seedlings as compared to their control ones [78]. Similarly, metal exposure caused rapid increase in the levels of photosynthetic pigments, free amino acids, proteins and sugar content compared to the unstressed plants [79]. In Arabidopsis plants, ROS production were caused by cadmium stresses and this generate oxidative damage resulting in a significant decrease of chlorophyll content [80]. In B. pekinensis plants, increased total free amino acid content was noticed after exposure to copper stress, where free amino acids are reported to play a role in the detoxification of the copper stress [81]. Metal stress is also known to accumulate low-molecular compounds with chelating properties in Brassica [82]. Also, the ascorbic acid content was reported to be largely decreased after boiling of brussels sprouts, broccoli kale, and white cauliflower [83], whereas UV light exposure of broccoli (B. oleracea var. italica) caused an increased levels of ascorbic acid [84,85].
4.2. Effect of Stress on Secondary Metabolites
Brassicaceae family is known for some metabolites like glucosinolates, which are derived from amino acid biosynthesis (e.g. tryptophan, methionine, phenylalanine, etc.) [76]. These compounds are benefic for human health including anti-carcinogenic, cholesterol-reducing and other pharmacological effects [69,86,87] with some known anti-nutritional effects as well [88]. In addition, these secondary metabolites play a key role in plant defense after exposure to salt stress [89], wounding and/or pathogen attack [90], insect herbivory [91,92], other environmental stresses [93] or by plant signaling molecules [94], viz. SA, JA and MeJA [95].
Brassicaceae is attributed also for a group of naturally occurring plant steroidal compounds, brassinosteroids (BRs), with a broad range of biological activities and the capacity to make these Brassica plants to confer resistance against a wide range of both abiotic and biotic stresses [96], viz. salt stress, water stress, low and high temperatures, pathogen attack [96,97] and heavy metal stress [98]. These steroid compounds not only function as the precursors of brassinosteroids and membrane constituents but are known to have an essential role in plant growth and development [99]. For example, in radish seedlings, brassinosteroids were able to keep the membrane intact during Cd stress, thus checking ROS production by increasing levels of antioxidant enzyme activities [100].
5. Development of Plants from Family Brassicaceae Tolerant to Oxidative Stress
Understanding of the oxidative mechanism of action may contribute to the development of plants most well adapted to the environment. The increase of stress tolerance of crop plants is related to the maintenance of high antioxidant capacity to remove toxic levels of ROS. Maintaining high level of antioxidant enzymes will help a plant to protect itself against oxidative damage by rapidly scavenging the toxic levels of ROS in its cells and restoring redox homeostasis. Using transgenic approaches, several species were studied aiming at the improvement of tolerance to stress enhancing antioxidant capacity of antioxidant genes. Some examples of the successful and positive responses were obtained for Brassicaceae with increased tolerance to salt, drought, cold, heat, hydrogen peroxide, methyl viologen, and metals stresses (Table 1).
Table 1. Some examples of the transgenic Brassicaceae plants with potential stress tolerance expressing antioxidant genes.
|
Gene |
Native specie |
Target specie |
Stress tolerance |
Reference |
|
Catalase |
Brassica oleracea |
Arabidopsis thaliana |
Heat |
[101] |
|
Catalase |
B. juncea |
Nicotiana tabacum |
Cadmium |
[54]
|
|
Ascorbate peroxidase |
Hordeum vulgare |
Arabidopsis thaliana |
Zinc, Cadmium |
[102] |
|
Ascorbate peroxidase |
B. compestris |
Arabidopsis thaliana |
Heat |
[103] |
|
Ascorbate peroxidase |
Puccinellia tenuiflora |
Arabidopsis thaliana |
Salinity, Hydrogen peroxide |
[104]
|
|
Glutathione peroxidase |
Triticum aestivum |
Arabidopsis thaliana |
Salinity, Hydrogen peroxide |
[105] |
|
Peroxiredoxins |
Suaeda salsa |
Arabidopsis thaliana |
Salinity, Cold |
[106] |
|
Glutathione reductase |
B. compestris |
Nicotiana tabacum |
Methyl viologen |
[107] |
|
Ascorbate peroxidase / Superoxide dismutase |
Rheum austral / Potentilla astrisanguinea |
Arabidopsis thaliana |
Cold |
[108] |
Plants overexpressing one or more antioxidant genes have more antioxidant capacity and more efficient ROS elimination; consequently, plants can protect their cellular components against toxic effects of ROS produced during the exposure to stress. As a consequence, plants suffer less oxidative injury and can tolerate a stress condition more effectively. Recently, a numbers of transgenic plants have been developed with disposed expression of antioxidant enzymes that enhanced increased tolerance to salinity, drought, and extreme temperatures [109]. In fact, overexpression of Chinese cabbage (B. campestris) BcAPX2 and BcAPX3 in Arabidopsis enhanced seed germination rate and improved high temperature tolerance via efficient scavenging of cellular H2O2 [110]. Notably, overexpression of a single gene could increase plant tolerance to different stresses such as the overexpression of SOD for enhancing stress tolerance [111]. Nevertheless, the stress tolerance can develop markedly by applying the simultaneous coexpression of genes involved in metabolic pathways. For example, coexpression of B. rapa BrMDHAR and BrDHAR genes via hybridization conferred tolerance to freezing [112]. Coexpression of PaSOD and RaAPX genes from Potentilla atrosanguinea and Rheum austral, respectively, in transgenic Arabidopsis showed increased salt tolerance [113].
Many research aiming to increase the tolerance of plants to environmental stresses using antioxidant genes. However, due to the complexity of the antioxidant system and plant stress tolerance, it will be difficult to state that ROS scavenging is the only pathway that determines the level of tolerance. Furthermore, stresses often occur in combination; thus, the relation between ROS signaling mechanisms in different stress responses is very complex. Additionally, since Brassica plants are considered to be important staple food, it is important to understand, how different environmental factors triggering mechanisms and pathways affect their metabolic profile, since these will ultimately affect its quality, functional properties, and attributes such as taste and aroma, which will influence consumer acceptability.
This work was supported by the Ministry of Higher Education and Scientific Research of Tunisia.
Vaahtera, L. and Brosché, M. (2011) More than the sum of its parts - how to achieve a specific transcriptional response to abiotic stress. Plant Science, 180, 421-430. PMid:21421388
View Article PubMed/NCBIRodrigo-Moreno, A., Poschenrieder, C. and Shabala, S. (2013) Transition metals: a double edge sward in ROS generation and signaling. Plant Signaling Behavior, 8, e23245. PMid:23333964
View Article PubMed/NCBIKaruppanapandian, T., Wang, H.W., Prabakaran, N., Jeyalakshmi, K., Kwon, M., Manoharan, K. and Kim, W. (2011) 2,4- dichlorophenoxyacetic acid-induced leaf senescence in mung bean (Vigna radiata L. Wilczek) and senescence inhibition by co-treatment with silver nanoparticles. Plant Physiology and Biochemistry, 49, 168-177. PMid:21144762
View Article PubMed/NCBISharma, P., Jha, A.B., Dubey, R.S. and Pessarakli, M. (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 1-26.
View ArticleMittler, R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends In Plant Sciences, 7(9), 405-410. 02312-9
View ArticleUchida, A., Jagendorf, A.T., Hibino, T. and Takabe, T. (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Science, 163, 515-523. 00159-0
View ArticleAsada, K. (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology, 141, 391-396. PMid:16760493
View Article PubMed/NCBIBose, J., Rodrigo-Moreno, A. and Shabala, S. (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. Journal of Experimental Botany, 65, 1241-1257. PMid:24368505
View Article PubMed/NCBIShabala, S. (2009) Salinity and programmed cell death: unraveling mechanisms for ion specific signalling. Journal of Experimental Botany, 60, 709-712. PMid:19269993
View Article PubMed/NCBIDemidchik, V., Cuin, T.A., Svistunenko, D., Smith, S.J., Miller, A.J., Shabala, S., Sokolik, A. and Yurin, V. (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Sciences, 123, 1468-1479. PMid:20375061
View Article PubMed/NCBIYu, T., Jhun, B.S. and Yoon, Y. (2011) High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen- activated protein kinase-mediated activation of mitochondrial fission. Antioxidant Redox Signaling, 14, 425-437. PMid:20518702
View Article PubMed/NCBIKumari, A., Das, P., Parida, A.K. and Agarwal, P.K. (2015) Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Frontiers in Plant Sciences, 6, 537. PMid:26284080
View Article PubMed/NCBIApel, K. and Hirt, H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55, 373-399. PMid:15377225
View Article PubMed/NCBIMittler, R., Vanderauwera, S., Gollery, M. and Van Breusegem, F. (2004) Reactive oxygen gene network of plants. Trends In Plant Sciences, 9(10), 490-498. PMid:15465684
View Article PubMed/NCBIFoyer, C.H. and Noctor, G. (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell, 17(7), 1866-1875. PMid:15987996
View Article PubMed/NCBIMiller G, Shulaev V, Mittler R. (2008) Reactive oxygen signaling and abiotic stress. Physiologia Plantarum, 133, 481-489. PMid:18346071
View Article PubMed/NCBIAbogadallah, G.M (2010) Antioxidative defense under salt stress. Plant Signaling Behavior, 5, 369-374. PMid:20118663
View Article PubMed/NCBIJaspers, P. and Kangasjearvi, J. (2010) Reactive oxygen species in abiotic stress signaling. Physiologia Plantarum, 138, 405-413. PMid:20028478
View Article PubMed/NCBIDemidchik, V. and Maathuis, F.J.M. (2007) Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist, 175, 387-404. PMid:17635215
View Article PubMed/NCBIDemidchik, V., Shabala, S.N. and Davies, J.M. (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. The Plant Journal, 49, 377-386. PMid:17181775
View Article PubMed/NCBIRichards, S.L., Laohavisit, A., Mortimer, J.C., Shabala, L., Swarbreck, S.M., Shabala, S. and Davies, J.M. (2014) Annexin 1 regulates the H2O2-induced calcium signature in Arabidopsis thaliana roots. The Plant Journal, 77, 136-145. PMid:24180429
View Article PubMed/NCBIAnschutz, U., Becker, D. and Shabala, S. (2014) Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. Journal of Plant Physiology, 171, 670-687. PMid:24635902
View Article PubMed/NCBISaeidnejad, A.H. and Rajaei, P. (2015) Antioxidative responses to drought and salinity stress in plants, a comprehensive review. International Journal of Life Sciences, 9, 1-8.
View ArticleOzgur, R., Uzilday, B., Sekmen, A.H. and Turkan, I. (2013) Reactive oxygen species regulation and antioxidant defence in halophytes. Functional Plant Biology, 40, 832-847. PMid:32481154
View Article PubMed/NCBIHasanuzzaman, M., Hossain, M.A., Silva, J.A.T. and Fujita, M. (2012) Plant Responses and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In: Bandi V, Shanker AK, Shanker C, Mandapaka M (Eds) Crop Stress and its Management: Perspectives and Strategies. Springer, New York. pp. 261-316.
View ArticleHasanuzzaman, M., Nahar, K., Alam, M.M., Roychowdhury, R. and Fujita, M. (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. International Journal of Molecular Sciences, 14(5), 9643-9684. PMid:23644891
View Article PubMed/NCBIHasanuzzaman, M., Bhuyan, M.H.M.B., Anee, T.I., Parvin, K., Nahar, K., Mahmud, J.A. and Fujita, M. (2019) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants, 8(9), 384. PMid:31505852
View Article PubMed/NCBIAshraf, M. and McNeilly, T. (2004) Salinity tolerance in Brassica oilseeds. Critical Review in Plant Sciences, 23, 157-174.
View ArticleJahangir, M., Abdel-Farid, I.B., Kim, H.K., Choi, Y.H. and Verpoorte, R. (2009) Healthy and unhealthy plants: the effect of stress on the metabolism of Brassicaceae. Environmental and Experimental Botany, 67, 23-33.
View ArticleAl-Shehbaz, I.A., Beilstein, M.A. and Kellogg, E.A. (2006) Systematics and phylogeny of the Brassicaceae (Cruciferae): an overview. Plant Systematics and Evolution, 259, 89-120.
View ArticleKoch, M.A. and Mummenhoff, K. (2006) Editorial: evolution and phylogeny of the Brassicaceae. Plant Systematics and Evolution, 259(2), 81-83.
View ArticleBailey, C.D., Koch, M.A., Mayer, M., Mummenhoff, K., O'kane, S.L., Warwick, S.I. and Al-Shehbaz, I.A. (2006) Toward a global phylogeny of the Brassicaceae. Molecular Biology and Evolution, 23(11), 2142-2160. PMid:16916944
View Article PubMed/NCBIMorinaga, T. (1934) Interspecific hybridization in Brassica. VI. The cytology of F1 hybrids of Brassica juncea and B. nigra. Cytologia, 6, 62-67.
View ArticleMullineaux, P.M. and Baker, N.R. (2010) Oxidative stress: antagonistic signaling for acclimation or cell death. Plant Physiology, 154(2), 521-525. PMid:20921177
View Article PubMed/NCBIGill, S.S. and Tuteja, N. (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909-930. PMid:20870416
View Article PubMed/NCBIGratão, P.L., Polle, A., Lea, P.J. and Azevedo, R.A. (2005) Making the life of heavy metal-stressed plants a little easier. Functional Plant Biology, 32(6), 481-494. PMid:32689149
View Article PubMed/NCBIDe Pinto, M.C. and De Gara, L. (2004) Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. Journal of Experimental Botany, 55, 2559-2569. PMid:15475379
View Article PubMed/NCBIBlokhina, O., Virolainen, E. and Fagerstedt, K.V. (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annal of Botany 91(2):179-194 PMid:12509339
View Article PubMed/NCBISmirnoff, N. (2011) Vitamin C: the metabolism and functions of ascorbic acid in plants. Advances in Botanical Research. In: Rebeille F, Douce R, editors. Biosynthesis of Vitamins in Plants: Vitamins B6, B8, B9, C, E, K, Part 2. 1st edn. USA: Academic Press; 107-177. ISBN 978-0-12-385853-5
Hasanuzzaman, M., Nahar, K., Rohman, M.M., Anee, T.I., Huang, Y. and Fujita, M. (2018a) Exogenous silicon protects Brassica napus plants from salinity-induced oxidative stress through the modulation of AsA-GSH pathway, thiol-dependent antioxidant enzymes and glyoxalase systems. Gesunde Pflanzen 70, 185-194.
View ArticleHasanuzzaman, M., Nahar, K., Rahman, A., Mahmud, J.A., Alharby, H.F. and Fujita, M. (2018b) Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. Journal of Plant Interaction, 13(1), 203-212.
View ArticleFoyer, C.H. and Noctor, G. (2000) Oxygen processing in photosynthesis: regulation and signalling. New Phytologist, 146, 359-388.
View ArticleKamal-Eldin. and Appelqvist, L.A. (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids, 31, 671-701. PMid:8827691
View Article PubMed/NCBIHasanuzzaman, M., Nahar, K. and Fujita, M. (2014) Role of tocopherol (vitamin E) in plants: Abiotic stress tolerance and beyond. In: Parvaiz A, Rasool S (eds) Emerging technologies and management of crop stress tolerance. Vol 2-A Sustainable Approach. Academic Press and Elsevier, New York, pp 267-289.
View ArticleBafeel, S.O. and Ibrahim, M.M. (2008) Antioxidants and accumulation of α-tocopherol induce chilling tolerance in Medicago sativa. International Journal of Agriculture Biology, 10, 593-598.
Guo, P.G., Baum, M., Grando, S., Ceccarelli, S., Bai, G.H., Li, R.H., Von Korff, M., Varshney, R.K., Graner, A. and Valkoun, J. (2009) Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. Journal of Experimental Botany, 60, 3531- 3544. PMid:19561048
View Article PubMed/NCBIWarwick, S.I. (2011) Brassicaceae in agriculture. In: Schmidt R, Bancroft I (eds) Genetics and genomics of the Brassicaceae. Springer, Heidelberg, pp 33-65
View ArticleGrace, S.C. and Logan, B.A. (2000) Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philosophical Transactions of the Royal Society of London. Series B 2355(1402):1499-1510 PMid:11128003
View Article PubMed/NCBIArora, A., Byrem, T.M., Nair, M.G. and Strasburg, G.M. (2000) Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Archives of Biochemistry and Biophysics, 373,102-109. PMid:10620328
View Article PubMed/NCBIBjörkman, M., Klingen, I., Birch, A.N.E., Bones, A.M., Bruce, T.J.A., Johansen, T.J., Meadow, R., Mølmann, J., Seljåsen, R., Smart, L.E. and Stewart, D. (2011) Phytochemicals of Brassicaceae in plant protection and human health-influences of climate, environment and agronomic practice. Phytochemistry, 72, 538-556. PMid:21315385
View Article PubMed/NCBIAres, A.M., Nozal, M.J. and Bernal, J. (2013) Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. Journal of Chromatography A, 1313, 78-95. PMid:23899380
View Article PubMed/NCBIScandalios, J.G. (2002) The rise of ROS. Trends in Biochemical Sciences, 27(9), 483-486. 02170-9
View ArticleScandalios, J.G. (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Brazilian Journal of Medical and Biological Research, 38(7), 995-1014. PMid:16007271
View Article PubMed/NCBIGuan, Z., Chai, T., Zhang, Y., Xu, J. and Wei, W. (2009) Enhancement of Cd tolerance in transgenic tobacco plants overexpressing a Cd-induced catalase cDNA. Chemosphere, 76(5), 623- 630. PMid:19473687
View Article PubMed/NCBIRosa, S.B., Caverzan, A., Teixeira, F.K., Lazzarotto, F., Silveira, J.A.G., Ferreira-Silva, S.L., Abreu-Neto, J., Margis, R. and Margis-Pinheiro, M. (2010) Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry 71(5-6), 548-558. PMid:20129631
View Article PubMed/NCBICaverzan, A., Passaia, G., Rosa, S.B., Ribeiro, C.W., Lazzarotto, F. and Margis-Pinheiro, M. (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genetic and Molecular Biology, 35(4), 1011-1019. PMid:23412747
View Article PubMed/NCBICaverzan, A., Bonifacio, A., Carvalho, F.E.L., Andrade, C.M.B., Passaia, G., Schünemann, M., Maraschin, F.S., Martins, M.O., Teixeira, F.K., Rauber, R., Margis, R., Silveira, J.A.G. and Margis-Pinheiro, M. (2014) The knockdown of chloroplastic ascorbate peroxidases reveals its regulatory role in the photosynthesis and protection under photo-oxidative stress in rice. Plant Science, 214(NaN), 74-87. PMid:24268165
View Article PubMed/NCBIYabuta, Y., Motoki, T., Yoshimura, K., Takeda, T., Ishikawa, T. and Shigeoka, S. (2002) Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. The Plant Journal, 32, 915- 925. PMid:12492834
View Article PubMed/NCBIMargis, R., Dunand, C., Teixeira, F.K. and Margis-Pinheiro, M. (2008) Glutathione peroxidase family - an evolutionary overview. FEBS Journal, 275(15), 3959-3970. PMid:18616466
View Article PubMed/NCBIWood, Z.A., Schroder, E., Harris, J.R. and Poole, L.B. (2004) Structure, mechanism and regulation of peroxiredoxins. Trends in Biochemical Sciences, 28(1), 23-40. 00003-8
View ArticleFoyer, C.H. and Shigeoka, S. (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology, 155(1), 93-100. PMid:21045124
View Article PubMed/NCBIPassardi, F., Longet, D., Penel, C. and Dunand, C. (2004) The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry, 65(13), 1879-1893. PMid:15279994
View Article PubMed/NCBIAsada, K. (1999) The water-water cycle in chloroplasts: scavening of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology, 50(NaN), 601-639. PMid:15012221
View Article PubMed/NCBILeterrier, M., Corpas, F.J., Barroso, J.B., Sandalio, L.M. and del Rıo, L.A. (2005) Peroxisomal monodehydroascorbate reductase. Genomic clone characterization and functional analysis under environmental stress conditions. Plant Physiology, 138(4), 2111-2123. PMid:16055677
View Article PubMed/NCBIEltayeb, A.E., Kawano, N., Badawi, G.H., et al. (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta, 225, 1255- 1264. PMid:17043889
View Article PubMed/NCBILi, Y., Zhou, Y., Wang, Z., Sun, X. and Tang, K. (2010) Engineering tocopherol biosynthetic pathway in Arabidopsis leaves and its effect on antioxidant metabolism. Plant Science, 178, 312-320.
View ArticleAnjum, N.A., Gill, S.S., Gill, R., Hasanuzzaman, M., Duarte, A.C., Pereira, E., Ahmad, I., Tuteja, R. and Tuteja, N. (2014) Metal/metalloid stress tolerance in plants: role of ascorbate, its redox couple, and associated enzymes. Protoplasma, 251(5), 1265-1283. PMid:24682425
View Article PubMed/NCBIEltayeb, A.E., Yamamoto, S., Habora, M.E.E., Yin, L., Tsujimoto, H. and Tanaka, K. (2011) Transgenic potato overexpressing Arabidopsis cytosolic AtDHAR1 showed higher tolerance to herbicide, drought and salt stresses. Breeding Sciences, 61, 3-10.
View ArticleMoreno, D.A., Carvajal, M., Lopez-Berenguer, C. and Garcia-Viguera, C. (2006) Chemical and biological characterisation of nutraceutical compounds of broccoli. Journal of Pharmaceutical and Biomedical Analysis, 41, 1508-1522. PMid:16713696
View Article PubMed/NCBIJahangir, M., Kim, H.K., Choi, Y.H. and Verpoorte, R. (2008) Metabolomic response of Brassica rapa submitted to pre-harvest bacterial contamination. Food Chemistry, 107, 362-368.
View ArticleLotito, S.B. and Frei, B. (2006) Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radical Biology & Medicine, 41, 1727-1746. PMid:17157175
View Article PubMed/NCBIZhao, T.J., Liu, Y., Yan, Y.B., Feng, F., Liu, W.Q. and Zhou, H.M. (2007) Identification of the amino acids crucial for the activities of drought responsive element binding factors (DREBs) of Brassica napus. FEBS Letters, 581, 3044-3050. PMid:17560577
View Article PubMed/NCBIHayat, S., Ali, B., Hasan, S.A. and Ahmad, A. (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environmental and Experimental Botany, 60, 33-41.
View ArticlePetersen, I.L., Hansen, H.C.B., Ravn, H.W., Sorensen, J.C. and Sorensen, H. (2007) Metabolic effects in rapeseed (Brassica napus L.) seedlings after root exposure to glyphosate. Pesticides Biochemistry and Physiology, 89, 220-229.
View ArticleSudha, G. and Ravishankar, G.A. (2002) Involvement and interaction of various signalling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell, Tissue and Organ Culture, 71, 181-212.
View ArticleBellostas, N., Sorensen, A.D., Sorensen, J.C. and Sorensen, H. (2007) Genetic variation and metabolism of glucosinolates. Advances in Botanical Research, 45, 369-415. 45013-3
View ArticleGood, A.G. and Zaplachinski, S.T. (1994) The effects of drought stress on free amino-acid accumulation and protein-synthesis in Brassica napus. Physiologia Plantarum, 90, 9-14.
View ArticleSasaki, H., Ichimura, K., Okada, K. and Oda, M. (1998) Freezing tolerance and soluble sugar contents affected by water stress during cold-acclimation and de-acclimation in cabbage seedlings. Scientia Horticulturae, 76, 161-169. 00143-5
View ArticleSingh, S. and Sinha, S. (2005) Accumulation of metals and its effects in Brassica juncea (L.) Czern. (cv. Rohini) grown on various amendments of tannery waste. Ecotoxicology and Environmental Safety, 62, 118-127. PMid:15978297
View Article PubMed/NCBIZawoznik, M.S., Groppa, M.D., Tomaro, M.L. and Benavides, M.P. (2007) Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana. Plant Science, 173, 190-197.
View ArticleXiong, Z.T., Liu, C. and Geng, B. (2006) Phytotoxic effects of copper on nitrogen metabolism and plant growth in Brassica pekinensis Rupr. Ecotoxicology and Environmental Safety, 64, 273-280. PMid:16616956
View Article PubMed/NCBISeth, C.S., Chaturvedi, P.K. and Misra, V. (2008) The role of phytochelatins and antioxidants in tolerance to Cd accumulation in Brassica juncea L. Ecotoxicology and Environmental Safety, 71, 76-85. PMid:18082263
View Article PubMed/NCBISikora, E., Cieslik, E., Leszczynska, T., Filipiak-Florkiewicz, A. and Pisulewski, P.M. (2008) The antioxidant activity of selected cruciferous vegetables subjected to aquathermal processing. Food Chemistry, 107, 55-59.
View ArticleLemoine, M.L., Civello, P.M., Martinez, G.A. and Chaves, A.R. (2007) Influence of postharvest UV-C treatment on refrigerated storage of minimally processed broccoli (Brassica oleracea var. italica). Journal of Sciences and Food Agriculture, 87, 1132-1139.
View ArticleSchonhof, I., Klaring, H.P., Krumbein, A., Claussen, W. and Schreiner, M. (2007) Effect of temperature increase under low radiation conditions on phytochemicals and ascorbic acid in greenhouse grown broccoli. Agriculture, Ecosystems & Environment, 119, 103-111.
View ArticleCieslik, E., Leszczynska, T., Filipiak-Florkiewicz, A., Sikora, E. and Pisulewski, P.M. (2007) Effects of some technological processes on glucosinolate contents in cruciferous vegetables. Food Chemistry, 105, 976-981.
View ArticleSong, L.J. and Thornalley, P.J. (2007) Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chemistry and Toxicology, 45, 216-224. PMid:17011103
View Article PubMed/NCBIGriffiths, D.W., Birch, A.N.E. and Hillman, J.R. (1998) Antinutritional compounds in the Brassicaceae: analysis, biosynthesis, chemistry and dietary effects. Journal of Horticultural Science and Biotechnology, 73, 1-18.
View ArticleLopez-Berenguer, C., Martinez-Ballesta, M.C., Garcia-Viguera, C. and Carvajal, M. (2008) Leaf water balance mediated by aquaporins under salt stress and associated glucosinolate synthesis in broccoli. Plant Science, 174, 321-328.
View ArticleCole, R.A. (1997) The relative importance of glucosinolates and amino acids to the development of two aphid pests Brevicoryne brassicae and Myzus persicae on wild and cultivated Brassica species. Entomologia Experimentalis et Applicata, 85, 121-133.
View ArticleMartin, N. and Muller, C. (2007) Induction of plant responses by a sequestering insect: relationship of glucosinolate concentration and myrosinase activity. Basic and Applied Ecology, 8, 13-25.
View ArticleBurow, M., Zhang, Z.Y., Ober, J.A., Lambrix, V.M., Wittstock, U., Gershenzon, J. and Kliebenstein, D.J. (2008) ESP and ESM1 mediate indol-3-acetonitrile production from indol-3-ylmethyl glucosinolate in Arabidopsis. Phytochemistry 69, 663-671. PMid:17920088
View Article PubMed/NCBIVallejo, F., Garcia-Viguera, C. and Tomas-Barberan, F.A. (2003) Changes in broccoli (Brassica oleracea L. var. italica) health-promoting compounds with inflorescence development. Journal of Agriculture and Food Chemistry, 51, 3776-3782. PMid:12797743
View Article PubMed/NCBIMikkelsen, M.D., Petersen, B.L., Glawischnig, E., Jensen, A.B., Andreasson, E. and Halkier, B.A. (2003) Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiology, 131, 298-308. PMid:12529537
View Article PubMed/NCBIMithen, R. (2001) Glucosinolates-biochemistry, genetics and biological activity. Plant Growth Regulation, 34, 91-103.
View ArticleKrishna, P. (2003) Brassinosteroid-mediated stress responses. The Journal of Plant Growth Regulation, 22, 289-297. PMid:14676968
View Article PubMed/NCBIKagale, S., Divi, U.K., Krochko, J.E., Keller, W.A. and Krishna, P. (2007) Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta, 225, 353-364. PMid:16906434
View Article PubMed/NCBIJaneczko, A., Koscielniak, J., Pilipowicz, M., Szarek-Lukaszewska, G. and Skoczowski, A. (2005) Protection of winter rape photosystem 2 by 24-epibrassinolide under cadmium stress. Photosynthetica, 43, 293-29.
View ArticleFujioka, S. and Yokota, T. (2003) Biosynthesis and metabolism of brassinosteroids. Annual Review of Plant Biology, 54, 137-164. PMid:14502988
View Article PubMed/NCBIAnuradha, S. and Rao, S.S.R. (2007) The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil and Environment, 53, 465-472.
View ArticleChiang, C.M., Chen, S.P., Chen, L.F.O., Chiang, M.C., Chien, H.L. and Lin, K.H. (2014) Expression of the broccoli catalase gene (BoCAT) enhances heat tolerance in transgenic Arabidopsis. Journal of Plant Biochemistry and Biotechnology, 23(3), 266-277.
View ArticleXu, W., Shi, W., Liu, F., Ueda, A. and Takabe, T. (2008) Enhanced zinc and cadmium tolerance and accumulation in transgenic Arabidopsis plants constitutively overexpressing a barley gene (HvAPX1) that encodes a peroxisomal ascorbate peroxidase. Botany, 86(6), 567-575.
View ArticleChiang, C.M., Chien, H.L., Chen, L.F.O., Hsiung, T.C., Chiang, M.C., Chen, S.P. and Lin, K.H. (2015) Overexpression of the genes coding ascorbate peroxidase from Brassica campestris enhances heat tolerance in transgenic Arabidopsis thaliana. Biologia Plantarum, 59(2), 305-315.
View ArticleGuan, Q., Wang, Z., Wang, X., Takano, T. and Liu, S. (2015) A peroxisomal APX from Puccinellia tenuiflora improves the abiotic stress tolerance of transgenic Arabidopsis thaliana through decreasing of H2O2 accumulation. Journal of Plant Physiology, 175(1), 183-191. PMid:25644292
View Article PubMed/NCBIZhai, C.Z., Zhao, L., Yin, L.J., Chen, M., Wang, Q.Y., Li, L.C., Xu, Z.S. and Ma, Y.Z. (2013) Two wheat glutathione peroxidase genes whose products are located in chloroplasts improve salt and H2O2 tolerances in Arabidopsis. PLoS One, 8(10), e73989. PMid:24098330
View Article PubMed/NCBIJing, L.W., Chen, S.H., Guo, X.L., Zhang, H. and Zhao, Y.X. (2006) Overexpression of a chloroplast-located peroxiredoxin Q gene, SsPrxQ, increases the salt and low-temperature tolerance of Arabidopsis. Journal of Integrative Plant Biology, 48(10), 1244-1249.
View ArticleLee, H. and Jo, J. (2004) Increased tolerance to methyl viologen by transgenic tobacco plants that over-express the cytosolic glutathione reductase gene from Brassica campestris. Journal of Plant Biology, 47(2), 111-116.
View ArticleShafi, A., Dogra, V., Gill, T., Ahuja, P.S. and Sreenivasulu, Y. (2014) Simultaneous over-expression of PaSOD and RaAPX in transgenic Arabidopsis thaliana confers cold stress tolerance through increase in vascular lignifications. PLoS One, 9(10), e110302. PMid:25330211
View Article PubMed/NCBIChakradhar, T., Mahanty, S., Reddy, R.A. et al. (2017) Biotechnological perspective of reactive oxygen species (ROS)-mediated stress tolerance in plants. In: Khan M., Khan N., editors. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress. Singapore: Springer; 53-87
View ArticleChiang, C.M., Chien, H.L., Chen, L.F.O., Hsiung, T.C., Chiang, M.C., Chen, S.P. and Lin, K.H. (2015) Overexpression of the genes coding ascorbate peroxidase from Brassica campestris enhances heat tolerance in transgenic Arabidopsis thaliana. Biologia Plantarum 59(2), 305-315.
View ArticleCaverzan, A., Casassola, A. and Brammer, S.P. (2016) Antioxidant responses of wheat plants under stress. Genetic and Molecular Biology, 39(1), 1-6. PMid:27007891
View Article PubMed/NCBIShin, S.Y., Kim, M.H., Kim, Y.H., Park, H.M. and Yoon, H.S. (2013) Co-expression of monodehydroascorbate reductase and dehydroascorbate reductase from Brassica rapa effectively confers tolerance to freezing-induced oxidative stress. Molecular Cells, 36(4), 304-315. PMid:24170089
View Article PubMed/NCBIShafi, A., Chauhan, R., Gill, T. et al. (2015) Expression of SOD and APX genes positively regulates secondary cell wall biosynthesis and promotes plant growth and yield in Arabidopsis under salt stress. Plant Molecular Biology, 87(6), 615-63. PMid:25754733
View Article PubMed/NCBI