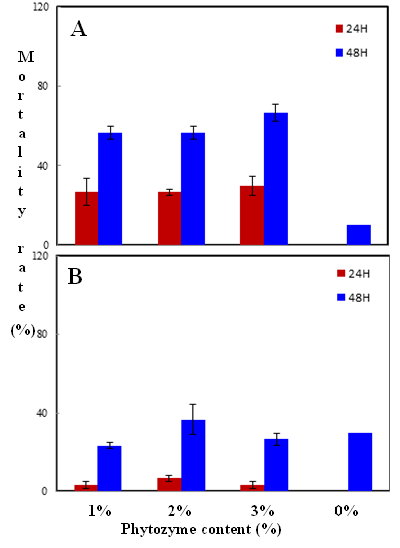
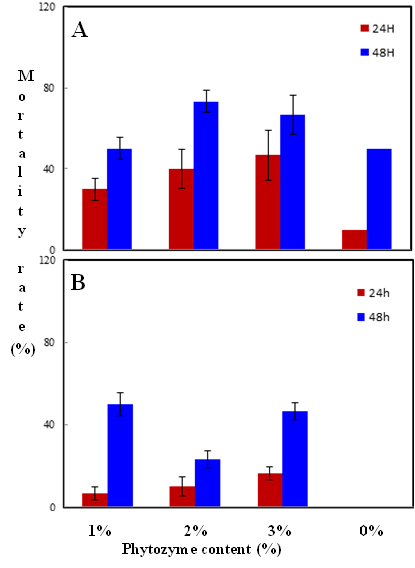
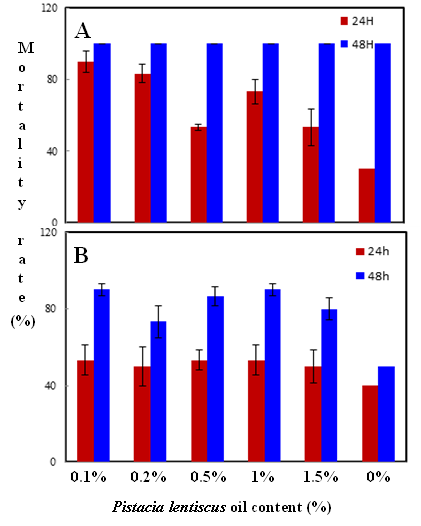
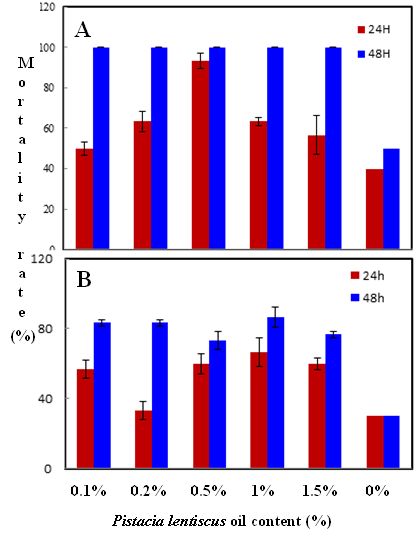
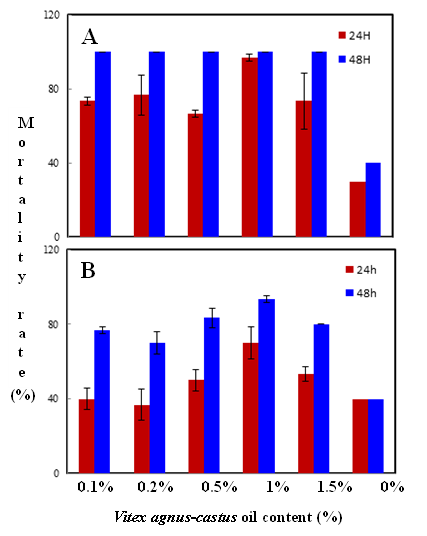
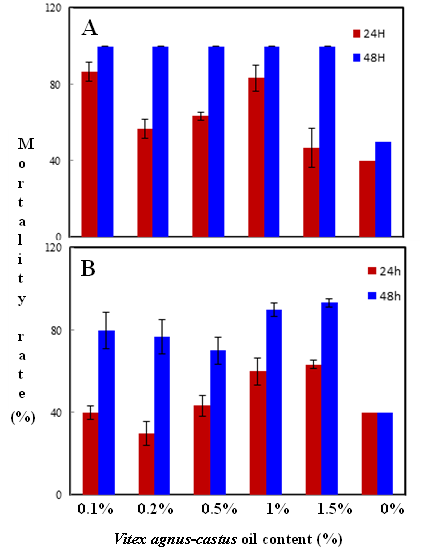
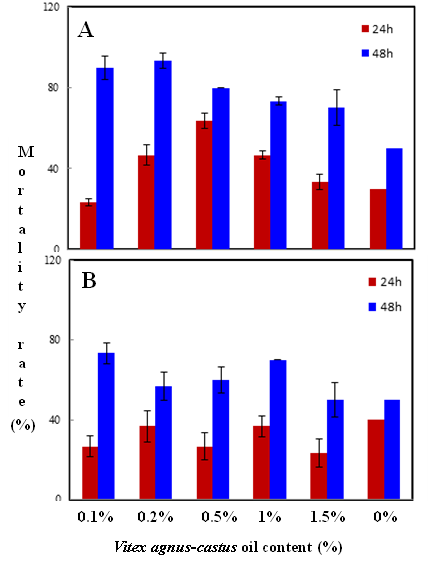
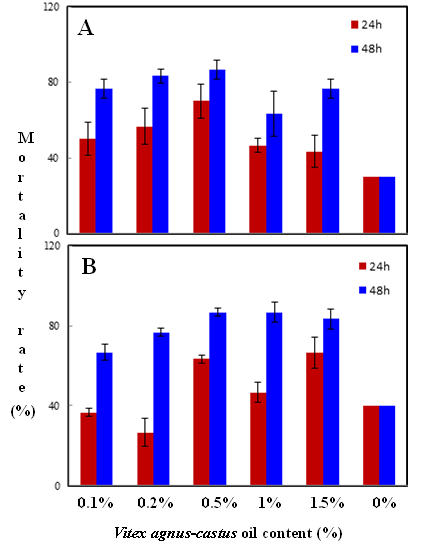
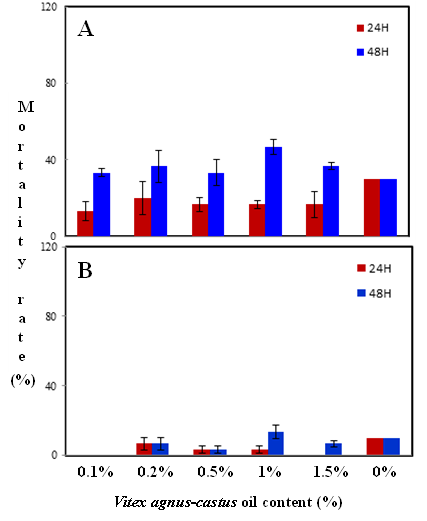
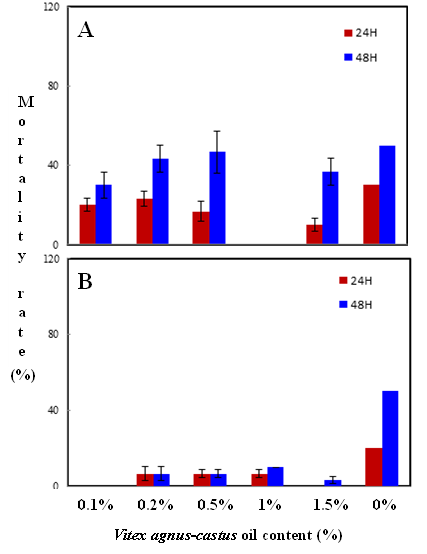
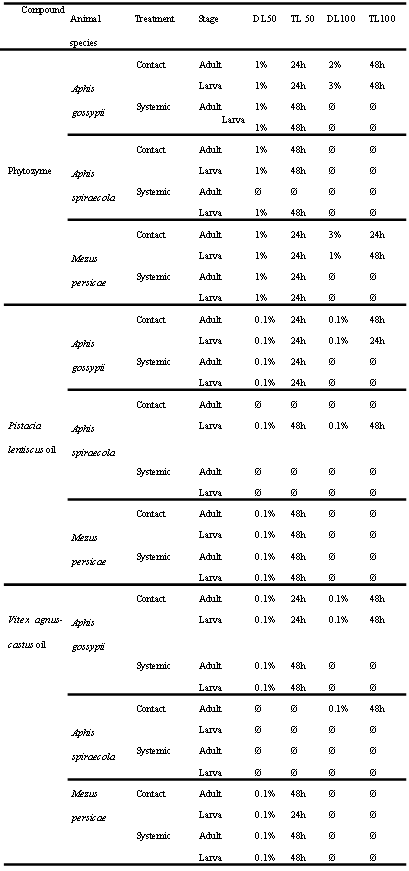
Habiba Ben Nacer2,4, Soumaya Oueslati2,4 , Dorsaf Yahyaoui4, Soumaya Elarbaoui1
1Shaqra University, College of Arts and Sciences Sager, Shaqraa, Riyadh Saudi Arabia
2ISSTE, University of Carthage, Technopole of Borj Cedria, Hammam-Lif 2050, Tunisia
3Laboratory of Water, Membranes and Environment Biotechnology (LEMBE), Technopole of Borj Cedria (CERTE), 2050 Hammam-Lif, Tunisia
4Citrus Technical Center (CTA), B.P. N ° 318, Zaoulett Jedidi 8099 - Tunisia