Anastasia Papadaki.
Tel. number: +302810379424, mobile: +306938721900, fax number: +302810318204,
E-mail: apapadaki@staff.teicrete.gr
© 2019 Sift Desk Journals. All Rights Reserved
Anastasia Papadaki.
Tel. number: +302810379424, mobile: +306938721900, fax number: +302810318204,
E-mail: apapadaki@staff.teicrete.gr
Anastasia A. Papadaki*, Kalliopi Ladomenou
Department of Agriculture, School of Agriculture Food and Nutrition, Technological Educational Institute of Crete, 71004, Heraklion, Greece.
Anastasia Papadaki, Interactive effects of leaf age and inoculum concentration on downy mildew of cucumber plants and the implication of nutrients. (2018)SDRP Journal of Plant Science 3(1)p:140-149
Background: Artificial fungal inoculation of plants is known to be affected by numerous factors as leaf age, inoculation method and inoculum density. Concentrations of nutrients in soil and plant tissues are also implicated in disease incidence. This study examined how inoculum concentration and leaf age levels affected response of cucumber plants to Pseudoperonospora cubensis, under natural conditions along with the potential impact of soil and leaf nutrients to downy mildew development.
Methodology: Two different aged cucumber leaves were inoculated with three concentrations of a sporangia suspension of P. cubensis in a pot experiment established in a factorial design with five replicates. Measurements of leaf and infection area at various intervals as well as determination of nutrients in both soil and cucumber tissues were conducted. Data were subjected to analysis of variance and covariance.
Results: Disease symptoms emerged on cucumber plants grown under greenhouse conditions regardless of leaf age. Moreover, no significant differences observed on both types of leaves, relative to P. cubensis growth in all the samplings. Main effects showed that leaf area developed independently of the amount of inoculum. However, an interaction between the development of the younger leaf and high inoculum load at the primary stage of the infection was observed. High inoculum loads increased the disease severity on both leaves, while the lowest concentration resulted in higher lesion area on the old leaves compared to the young. Leaf Fe, Zn and Ca content had statistically significant effect on disease emergence at p<0.05, after covariance analysis.
Conclusion: Downy mildew severity was similar on both type of leaves. The size of young leaves was inhibited by the high fungal load whereas their infection had lower progress under limited inoculum density. Leaf but not soil nutrient status was implicated in disease incidence. The findings of this study will be useful for artificial inoculations of cucumber leaves with P. cubensis under greenhouse conditions.
Key words: infection area; greenhouse conditions; leaf size; pot experiment; Pseudoperonospora cubensis; old plant tissue.
Downy mildew of cucumber, caused by P. cubensis, is considered one of the most damaging diseases of cucurbitaceous crops worldwide [1]. As obligate parasites cannot be cultivated on artificial nutritive media, investigations regarding artificial inoculation on living host, is of great scientific interest due to novel knowledge provided about pathogen cycle and management control of the disease. However, downy mildew of cucumber can be screened more effectively in natural epidemics rather than artificial screening [2]. Therefore, directly inoculation of plants grown in pots under greenhouse environment combines the above benefits. Defined inoculum load permits thoroughly comparable results concerning infection, moreover the study area allows pathogen development under natural conditions.
Many studies showed that inoculum concentration is an important factor for successful artificial inoculations [3,4,5]. Inoculum density may influence the period from penetration until visible external symptoms [1] or the accurate assessment of susceptibility to various plant pathogens [6]. Failed attempt for optimum infection due to low concentration of zoospore suspension or high spore content which did not permit the differentiation of environmental effects, reported by researchers [7]. It has been suggested that inoculum concentration for artificial inoculation of cucumber plants with P. cubensis should be adjusted to 12x104 sporangia ml-1 [8]. Similar fungal densities have been widely used since then [9]. However, differences relative to zoospore content depended on the inoculation method used were recorded. Cucumber leaves where sprayed with 1x105 sporangia ml-1 of P. cubensis though five times higher load was used when placing suspension droplets on the leaf surface [10]. Nonetheless, artificial inoculations with lower spore concentrations of P. cubensis sporangia ml-1 (2.5x104) [11], (5x103) [12] or even lower 1.5x102 [13] and 1.4-0.8x103 [2] resulted in successful plant inoculations.
Furthermore, leaf age is quite important for infection [14]. Artificial inoculation of cucumber parts, with other pathogens was hardly achieved [15] or susceptibility to disease increased with leaf age of cucumber cultivars [16]. Although, cucumber plants may be infected at all developmental stages, symptoms on young developing leaves are rather rare under natural infection conditions and cotyledons are actually more susceptible than true leaves [1].
Plant nutrition has also a considerable influence in disease development [17] due to relationship between nutrients availability and growth of the pathogens. Reduction of P. cubensis growth was observed under unbalanced N, P and K while balanced levels of nutrients resulted in disease development in Cucumis melo. Besides, nutrients supply from the surrounding healthy leaf tissue is considered to be responsible for the density of sporangiophores of P. cubensis on small-area lesions [1].
Unlike the effects of inoculation methods in relation to ideal environmental conditions on disease development, reproduction of various sporangia densities of obligate parasites on differed aged leaves of cucumber plants, have not been widely studied, under natural environment. This study attempts to achieve homogeneous P. cubensis attack on leaves with comparable and specific inoculum density under natural conditions. More specifically, the objectives of the current research were (a) to examine the main effects and interactions between inoculum concentration of P. cubensis and leaf age of cucumber plants on the disease spread under greenhouse conditions and (b) to investigate the content of nutrients in soil and cucumber leaves relative to downy mildew development.
The experiment consisted of a factorial arrangement of cucumber leaves (young or old), and spore concentrations of P. cubensis. Treatments were arranged according to a randomized block design and replicated five times with eight plants per plot. Three levels of spore concentrations were used (1.5 x102, 1x103 and 1x104 sporangia ml-1) in all possible combinations with the two cucumber leaves of different age.
Soil analysis was conducted prior to establishment of the experiment with the following results NO3-N 110 mg kg-1, Ρ 42 mg kg-1, K 175.8 mg kg-1, pH 8.02, E.C. 4.38 S m-1, total CaCO3 34.44%. Cucumber plants (Cucumis sativus L. cv. Knossos) were grown in 7 L plastic pots under greenhouse conditions. Balanced fertigation consisted of phosphorus (P) 50 mg L-1, nitrogen (N) 100 mg L-1 and potassium (K) 200 mg L-1 and supplied to crop a week after transplantation by adding 500 ml nutrient solution in each pot. Recommended cultivation practices were applied uniformly to all the treatments.
The third leaf (young) and the seventh (old) from the plant apex were inoculated with sporangium suspension of P. cubensis. The inoculum was obtained from a local greenhouse. The infected leaves were collected and placed for incubation on a wet filter paper in petri dishes for 2 days. Zoosporangia which formed on leaves were gently brushed in a container with distilled water, containing two drops of Tween-20. The concentrations of suspension were adjusted to 1.5 x102, 1x103 and 1x104 sporangia ml-1 with a hemocytometer. Four droplets (10μL each) of the above suspensions were applied on the upper surface of the leaves according to the experimental design. The cucumber plants were grown afterwards under natural greenhouse conditions with average minimum12 oC and maximum 34 oC air temperature.
Leaf and lesion area were measured digitally performing four series of photos of all the inoculated leaves. To study plant and soil nutritional status, samples were collected for nutrients analysis to determine concentrations of NH4-N, NO3-N, Ca, Mg, P, K, Fe, Mn, Cu and Zn on a leaf dry weight basis as well as NH4-N, NO3-N, P and K in soil. From each plant, most recent fully developed leaf was selected as suggested by Jones et al [18]. Six leaves were taken from each plot combined to form a mixed sample. Olsen method for used for soil P whereas K was ammonium acetate extracted from the soil. Leaf NH4-N and NO3-N were extracted with KCl and concentrations was determined using the Kjeldahl wet digestion method. Leaf P concentration was determined by spectrometry. For the rest macro- and micro- nutrients, the samples were dry-ashed in a muffle furnace at 515 ◦C for 5 h and dissolved in 3 mL of 6 N hydrochloric acid and the concentrations were determined by Atomic Absorption Spectrophotometry while K was determined using Flame Photometer [19].
Statistical analysis of data was done after checking normality of each variable using Kolmogorov-Smirnov and Shapiro-Wilk tests. To detect main effects and interactions between leaf age and inoculum concentrations, analysis of variance was performed. For comparison of the means Duncan’s multiple range test and the least significant difference, for P ≤ 0.05, was employed. The potential effect of nutrients on disease spread was examined after covariance analysis between leaf, lesion area and leaf, soil nutrient.
Effect of leaf age on infection area
The results of this research indicated that there were statistically significant differences relative to size between the young and the old leaf, in all the samplings (Figure 1A). The younger leaves obviously not fully developed, were smaller than the older ones which had reached their maximum size, regardless of the spore concentration. Disease incidence was noticed in all inoculated leaves. Figure 1B depicts that there were no significant differences between the two leaves with regard to infection area in all the samplings.
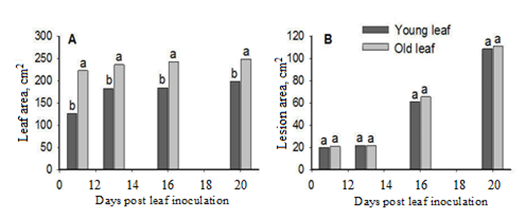
Figure 1. Leaf (A) and lesion (B) area of young and old age cucumber leaves, during the samplings (day 11, 13, 16, 20 post inoculation with P. cubensis).
Leaf size and downy mildew incidence under inoculum levels
The effect of inoculum content on leaf size of cucumber plants revealed no significant differences (Figure 2A). However, in day 13 post inoculation with P. cubensis, the highest sporangial load led to significantly larger leaf with regard to the rest inoculum levels.
Figure 2B shows the infection area caused by P. cubensis on leaves of cucumber as affected by the inoculum doses. All artificial inoculations resulted in disease incidence. Significantly elevated infection with increasing pathogen levels was recorded, at the day 11 and 13 post inoculation. Two higher spore levels had not statistical difference between them relative to infection, at day 16 and 20, only with regard to the lower inoculum concentration (day 20).
Downy mildew symptoms on cucumber leaves observed even at the lowest spore level (1.5x102 sporangia ml-1), 11 days after infection. Artificial inoculations with similar sporangia doses of P. cubensis on cucumber plants [13] or other vegetables [2] have been reported. Optimal rates of P. cubensis inoculum that were used in most researches seem to be around 103 to 104 sporangia mL-1 [9, 12, 20, 21] or much higher [11, 22, 23]. However, most of them performed inoculations by spraying a sporangium suspension on cucumber leaves although this method is in contrary to the natural properties of the fungal sporangia dispersal [20]. In the current investigation, where droplet method was used for accurate monitoring of downy mildew development, dominated environmental conditions possibly favoured even the few spores to germinate before the droplet dried.
It was apparent that disease severity significantly increased with the increasing fungal load at the initial infection stages. Nevertheless, when downy mildew developed, no significant difference observed between two higher inoculum loads, as reported for other pathogens [24,25].
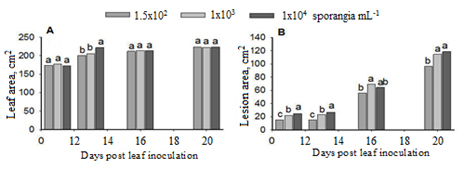
Figure 2. Effect of inoculum concentration (sporangia of P. cubensis ml-1) on leaf (A) and lesion (B) area, during the samplings (day 11, 13, 16, 20 post inoculation with P. cubensis).
Leaf age x Inoculum load interaction
Statistical analysis of the data obtained showed significant interaction between leaf age levels and inoculum doses for leaf area, at the first sampling (Figure 3A). The area of the young cucumber leaf was increased in the medial sporangium level with regard to the rest concentrations. In contrast, the same inoculum dose led to lower size of the old leaves in comparison with the others. Thus, the higher fungal load reduced the size of the young leaf whereas the corresponding area of the old leaf was not affected. This indicated that the development of the new leaves was prevented by the high inoculum load.
Although main effects of leaf age on infection area revealed no differences, an interaction of leaf age x inoculum level was obtained for this variable (Figure 3B). Therefore, the lower sporangial concentration led to significantly reduced infection area on the young leaves than the old ones. No statistical significant differences observed for the higher pathogen doses in relation to their affect to lesion area of both aged cucumber leaves. A possible explanation could be the unfavourable microclimate conditions for sporangia germination due to the small size of the young leaves and their higher position on the plant which might resulted in limited dew periods and good air circulation. Field resistance is known to occur with low inoculum levels and be remarkably dependent on environmental conditions rather than the pathogen itself [1].
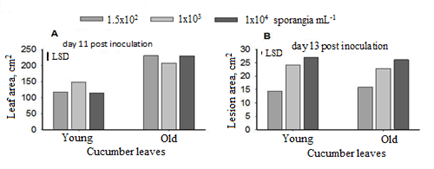
Figure 3. Interaction of leaf age x inoculum concentration with regard to the leaf and lesion area (day 11 and 13 post inoculation with P. cubensis). The least significant difference (P=0.05) among inoculum concentrations is presented with LSD bars.
Soil and leaf nutrients as covariates
Means of macro and micro nutrients are shown in Table 1. It can be concluded from the results obtained in this research all the nutrients were at sufficient to high levels in the cucumber leaf samples [18]. In general, there were not clear statistically significant differences in the nutrient concentration in cucumber tissue and soil. Although, the content of the primary nutrients and Mg tend to increase in young leaves probably due to their mobility. An opposite trend was observed for the rest immobile nutrients in new aged cucumber tissues. Nutrient soil content did not result in statistical significant differences (Table 1).
Table 1. Effect of treatments on macro and micro nutrients in cucumber leaves and soil.
|
Treatments |
Macronutrients in cucumber leaves |
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Leaf age |
Inoculum Concentration |
Ca |
Mg |
K |
P |
NH4-N |
|
NO3-N |
|
|||||||||||||||||||||||||||||||||||||||||||
|
|
Sporangia ml-1 |
% |
|
mg Kg-1 |
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
young |
1.5 x 102 |
6.5b* |
1.0 |
ab |
6.5 |
a |
0.9 |
ab |
82.8a |
129.2ab |
|
|||||||||||||||||||||||||||||||||||||||||
|
young |
1 x 103 |
6.0b |
0.9 |
bc |
5.2 |
b |
0.9 |
a |
84.7a |
146.4a |
|
|||||||||||||||||||||||||||||||||||||||||
|
young |
1 x 104 |
5.9b |
1.1 |
a |
4.9 |
b |
0.8 |
bc |
51.6b |
135.9ab |
|
|||||||||||||||||||||||||||||||||||||||||
|
old |
1.5 x 102 |
8.3a |
0.9 |
bc |
4.6 |
b |
0.8 |
c |
81.7a |
71.9c |
|
|||||||||||||||||||||||||||||||||||||||||
|
old |
1 x 103 |
6.0b |
0.9 |
bc |
4.7 |
b |
0.9 |
a |
33.7c |
104.5bc |
|
|||||||||||||||||||||||||||||||||||||||||
|
old |
1 x 104 |
6.5b |
0.8 |
c |
4.8 |
b |
0.7 |
c |
50.4b |
121.7ab |
|
|||||||||||||||||||||||||||||||||||||||||
|
|
Micronutrients in cucumber leaves (mg Kg-1) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
Fe |
Mn |
Cu |
Zn |
|||||||||||||||||||||||||||||||||||||||||||||||
|
young |
1.5 x 102 |
334.7 |
c |
140.0 |
bc |
118.8 |
cd |
39.7 |
d |
|
||||||||||||||||||||||||||||||||||||||||||
|
young |
1 x 103 |
339.1 |
c |
90.8 |
d |
121.0 |
cd |
43.6 |
cd |
|
||||||||||||||||||||||||||||||||||||||||||
|
young |
1 x 104 |
365.3 |
c |
108.6 |
cd |
105.3 |
d |
51.7 |
bc |
|
||||||||||||||||||||||||||||||||||||||||||
|
old |
1.5 x 102 |
450.7 |
b |
160.1 |
b |
153.2 |
b |
54.7 |
b |
|
||||||||||||||||||||||||||||||||||||||||||
|
old |
1 x 103 |
395.1 |
bc |
146.5 |
b |
138.4 |
bc |
58.7 |
b |
|
||||||||||||||||||||||||||||||||||||||||||
|
old |
1 x 104 |
535.2 |
a |
204.1 |
a |
187.5 |
a |
67.7 |
a |
|
||||||||||||||||||||||||||||||||||||||||||
|
|
Macronutrients in soil |
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
NH4-N |
NO3-N |
P |
K |
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
mg Kg-1 in soil |
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
young |
1.5 x 102 |
|
6.4c |
|
19.8a |
39.8a |
|
|
418.3ab |
|
|
|||||||||||||||||||||||||||||||||||||||||
|
young |
1 x 103 |
|
8.6b |
|
14.4a |
34.4a |
|
|
399.6b |
|
|
|||||||||||||||||||||||||||||||||||||||||
|
young |
1 x 104 |
|
6.4c |
|
19.2a |
39.2a |
|
|
438.0a |
|
|
|||||||||||||||||||||||||||||||||||||||||
|
old |
1.5 x 102 |
|
10.0a |
|
16.9a |
36.9a |
|
|
409.2ab |
|
|
|||||||||||||||||||||||||||||||||||||||||
|
old |
1 x 103 |
|
7.0bc |
|
16.1a |
36.1a |
|
|
424.7ab |
|
|
|||||||||||||||||||||||||||||||||||||||||
|
old |
1 x 104 |
|
6.8bc |
|
17.6a |
37.7a |
|
|
411.8ab |
|
|
|||||||||||||||||||||||||||||||||||||||||
*Means within columns followed by different letters are significantly different (Duncan, P=0.05).
Plant nutrient status is already known to affect the physiology of the plant host, which eventually influences the microclimate and reduces infection by pathogens [26]. Moreover, differences in nutrient availability could be responsible for observed differences in virulence [27]. Since the availability of nutrients to plants affects the inoculum potential of the pathogens, it could be concluded that under high nutrient status of the cucumber leaves, the infection success was favourable in all inoculums levels of this obligate foliar pathogen.
Covariance analysis of the obtained data showed that infection caused by P. cubensis was affected by nutrient content in cucumber leaves, only at the early stage of the disease development. Therefore, the nutrients Fe, Zn and Ca of the leaves significantly influenced the total lesion area at P<0.05 in the day 11 post inoculation with P. cubensis (Table 2). The important role that nutrients play with regard to inoculum concentration has also been reported for other diseases [24]. Besides, a study by Amand and Wehner about the effect of various factors such as leaf age, stomata opening on cucumber gummy stem blight stated that nutrients provided by guttation were more important in the infection process than other factors [16]. Moreover, the high leaf content of Fe, Zn and Ca found in this study has been associated with host response to fungal diseases [28,29,30].
Table 2. ANCOVA for lesion area of cucumber plants in the day 11 post inoculation with leaf nutrients content of Fe, Zn and Ca, as covariates.
|
Source |
Mean square |
F |
Level of Significance |
|
Inoculum Concentration |
0.530 |
0.20 |
0.821 |
|
Fe |
15.407 |
5.77 |
0.028 |
|
Leaf age |
3.329 |
1.25 |
0.280 |
|
Replicates |
1.321 |
0.50 |
0.739 |
|
Fe*Inoculum Concentration |
1.612 |
0.60 |
0.558 |
|
Leaf age * Inoculum Concentration |
17.433 |
6.53 |
0.008 |
|
Fe* Leaf age |
1.474 |
0.55 |
0.468 |
|
Inoculum Concentration |
8.990 |
6.07 |
0.010 |
|
Zn |
11.427 |
7.71 |
0.013 |
|
Leaf age |
0.972 |
0.66 |
0.429 |
|
Replicates |
1.861 |
1.26 |
0.327 |
|
Zn* Inoculum Concentration |
7.666 |
5.17 |
0.018 |
|
leaf* Inoculum Concentration |
5.294 |
3.57 |
0.052 |
|
Zn* Leaf age |
0.410 |
0.28 |
0.606 |
|
Inoculum Concentration |
7.213 |
3.02 |
0.077 |
|
Ca |
18.028 |
7.54 |
0.014 |
|
Leaf age |
0.399 |
0.17 |
0.688 |
|
Replicates |
1.010 |
0.42 |
0.790 |
|
Ca* Inoculum Concentration |
9.933 |
4.16 |
0.035 |
|
leaf* Inoculum Concentration |
6.434 |
2.69 |
0.098 |
|
Ca* Leaf age |
0.043 |
0.02 |
0.894 |
The current study concluded that all tested spore concentrations resulted in successful infection of both young and old cucumber leaves, after artificial inoculation with P. cubensis in greenhouse conditions. Moreover, the pathogen attacked plant leaves regardless of age, causing the same infection area. The high spore contents led to equable emergence of downy mildew, as the disease increased with time. Leaf nutrient content of Fe, Zn and Ca was implicated in the early stages of infection. High sporangial load inhibited the size development only of the younger leaves while disease progressed rapidly on the old aged leaves of cucumber plants even under low P. cubensis inoculum.
Lebeda A. and Cohen Y. 2011. Cucurbit downy mildew (Pseudoperonospora cubensis)-biology, ecology, epidemiology, host-pathogen interaction and control. European Journal of Plant Pathology, 129:157-192.
View ArticlePandey K.K., Ram D., Pandey P.K. and Rai M., 2005. Artificial screening technique and sources of resistance to downy mildew disease in bitter gourd. International journal of vegetable science, 32(1): 107-108.
Viruega J.R., Roca L.F., Moral J. and Trapero A., 2011. Factors affecting infection and disease development on olive leaves inoculated with Fusicladium oleagineum. Plant Disease 95:1139-1146.
View ArticleImathiu S.M., Edwards S.G., Ray R.V. and Back M., 2014. Artificial inoculum and inoculation techniques commonly used in the investigation of Fusarium head blight in cereals. Acta Phytopathologica et Entomologica Hungarica 49(2): 129-139.
View ArticleHong J.K., and Hwang B. K., 1998. Influence of Inoculum Density, Wetness Duration, Plant Age, Inoculation Method, and Cultivar Resistance on Infection of Pepper Plants by Colletotrichum coccodes. Plant disease, 82(10): 1079-1083.
View ArticleTooley P.W., Browning M. and Leighty R.M., 2013. Inoculum Density Relationships for Infection of Some Eastern US Forest Species by Phytophthora ramorum. Journal of Phytopathology, 161: 595-603.
View ArticleBiles, C. L., Bruton, B. D., Wall, M. M. and Rivas, M. (1995). Phytophthora capsici zoospore infection of pepper fruit in various physical environments. Proceedings of the Oklahoma Academy of Science, 75, p. 1-5.
Williams H.P. and Palmer M.J., 1982. NC State and USDA Cucumber Disease Handbook.
Charoenwattana P., Khanobdee C. and Udomyotin A., 2017. Screening techniques for downy mildew resistance in gherkin cucumbers. International Journal of GEOMATE, 13(40): 35-42.
View ArticleLindenthal, M., Steiner, U., Dehne, H. W. and Oerke, E. C. (2005). Effect of downy mildew development on transpiration of cucumber leaves visualized by digital infrared thermography. Phytopathology, 95(3), p. 233-240. PMid:18943115
View Article PubMed/NCBIReuveni R. and Ravin M., 1997. Control of downy mildew in greenhouse-grown cucumbers using blue photoselective polyethylene sheets. Plant Disease, 81(9): 999-1004.
View ArticlePortz, D., Koch, E. and Slusarenko, A. (2008). Effects of garlic (Allium sativum) juice containing allicin on Phytophthora infestans and downy mildew of cucumber caused by Pseudoperonospora cubensis. European Journal of Plant Pathology, 122, p. 197-206.
View ArticleGeorgopoulos S.G. and Grigoriu A.C., 1981. Metalaxyl- Resistant strains of Pseudoperonospora cubensis in cucumber greenhouses of southern Greece. Plant Disease, 65: 729-731.
View ArticleHong J.K., and Hwang B. K., 1998. Influence of Inoculum Density, Wetness Duration, Plant Age, Inoculation Method, and Cultivar Resistance on Infection of Pepper Plants by Colletotrichum coccodes. Plant disease, 82(10): 1079-1083.
View ArticleWyszogrodzka, A. J., Williams, P. H. and Peterson, C. E. (1987). Multiple pathogen inoculation of cucumber (Cucumis sativus) seedlings. Plant Disease, 71(3), p. 275-280.
View ArticleAmand P.C. and Wehner T.C., 1995. Greenhouse, detached-leaf and field testing methods to determine cucumber resistance to gummy stem blight. Journal of the American Society for Horticultural Science, 120 (4): 673-680.
Datnoff L.E., Elmer W.H. and Huber D.M., 2007. Mineral nutrition and plant disease, edited by American Phytopathological Society, St. Paul, Minnesota, USA.
Jones, J.B., Wolf B. and Mills A.H., 1991. Plant analysis handbook. Micro - Macro Publishing Inc., United States of America.
Page A.L., Miller R.H. and Keeney D.R., 1982. Chemical and microbiological properties. In: Methods of Soil Analysis, American Society of Agronomy Inc and Soil Science Society of America Inc (eds). Madison, Wisconsin, 1159.
Sun S., Lian S., Feng S., Dong X., Wang C., Li B. and Liang W., 2017. Effects of Temperature and Moisture on Sporulation and Infection by Pseudoperonospora cubensis. Plant Disease, 101(4): 562-567.
View ArticleBaider, A. and Cohen, Y. (2003). Synergistic interaction between BABA and Mancozed in controlling Phytophthora infestans in potato and tomato and Pseudoperonospora cubensis in cucumber. Phytoparasitica, 31(4), p. 399-409.
View ArticleAlavi S.V. and Dehpour A.A. 2010. Evaluation of the Nanosilver Colloidal Solution in Comparison with the Registered Fungicide to Control Greenhouse Cucumber Downy Mildew Disease in the North of Iran. Acta horticulturae, Proc. 6th International Postharvest Symposium 877(877).
View ArticleOerke E.C., Steiner U., Dehne H.W. and Lindenthal. M., 2006.Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. Journal of Experimental Botany, 57(9):2121-32. PMid:16714311
View Article PubMed/NCBIOchola D., Ocimati W., Tinzaara W., Blomme G. and Karamura K., 2014. Interactive effects of fertilizer and inoculum concentration on subsequent development of xanthomonas wilt in banana. African Journal of Agricultural Research, 9(35): 2727-2735.
View ArticleDoullah M.A.U, Meah M. B. and Okazaki K., 2006. Development of an effective screening method for partial resistance to Alternaria brassicicola (dark leaf spot) in Brassica rapa. European Journal of Plant Pathology, 116: 33-43.
View ArticleAgrios N.G., 2005. Plant Pathology. 5th ed., Elsevier, Amsterdam. p.635.
Degani O., Maor R., Hadar R., Sharon, A., Horwitz B. 2004. Host Physiology and Pathogenic Variation of Cochliobolus heterostrophus Strains with Mutations in the G Protein Alpha Subunit, CGA1, Applied and Environmental Microbiology 70(8):5005-9.
View ArticleSpann T.M. and Schumann A.W., 2017. Mineral nutrition contributes to plant disease and pest resistance Horticultural Sciences Department, UF/IFAS Extension.
Duffy B., 2007. Zinc and plant disease. In: Datnoff L.E., Elmer W.H. and Huber D.M., American Phytopathological Society (ed.), Mineral. Nutrition and. Plant Disease, Minnesota, USA, p. 155-175. PMid:17538896
PubMed/NCBIRahman M. and Punja Z.K., 2007. Calcium and plant disease. In: Datnoff L.E., Elmer W.H. and Huber D.M., American Phytopathological Society (ed.), Mineral. Nutrition and. Plant Disease, Minnesota, USA, p. 79-93.
