Anastasia Papadaki.
Tel. number: +302810379424, mobile: +306938721900, fax number: +302810318204,
E-mail: apapadaki@staff.teicrete.gr
© 2019 Sift Desk Journals. All Rights Reserved
Anastasia Papadaki.
Tel. number: +302810379424, mobile: +306938721900, fax number: +302810318204,
E-mail: apapadaki@staff.teicrete.gr
Anastasia A. Papadaki*, Konstantinos Nikoloudis, Kalliopi Ladomenou
Department of Agriculture, School of Agriculture Food and Nutrition, Technological Educational Institute of Crete, 71004, Heraklion, Greece.
Tayeb Muhammad(tayebmuhammad@nwsuaf.edu.cn)
Shuifang Zhu(zhusf@caiq.gov.cn)
Xue Hua-Li(xuehuali77@sina.com)
Anastasia Papadaki, N and K interactions in cucumber plants artificially inoculated with P. cubensis(2018)SDRP Journal of Plant Science 3(1)p:149-158
Background: The use of fungicides in order to manage plant diseases can potentially pose a risk to the environment which could lead to adverse impacts to human health. An alternative practice for efficient plant protection need to be found. Nevertheless, the positive effect of host nutrition to disease incidence is well documented for many crops. This study examined the efficacy of N and K fertilization compared to fungicide applications in controlling downy mildew of cucumber plants. The relationship between disease intensity, soil nutrients’ and cucumber leaves was also assessed.
Methods: Cucumber plants grown in pots were treated with two N levels (200 and 300 ppm) and three levels of K (200, 300 and 400 ppm) along with two applications of Aliette 80 WG (protective and curative action) in a randomized block design. Inoculation of cucumber leaves with Pseuperonospora cubensis was carried out. Downy mildew development assessed at various intervals along with soil and leaf nutrient status of the plants. Experimental data were submitted to ANOVA.
Results: The results demonstrated that downy mildew declined with increasing rates of K when N applied in low levels while at high N dose disease was elevated as K was increased. High disease severity was observed in cucumber plants grown under low levels of N and K, at early growth of the pathogen while at late disease stage, high rates of both N and K exhibited increased infection. Even though the preventative fungicide application gave best control of downy mildew, its effectiveness on inhibition of P. cubensis growth did not differ from the impact of fertilization with low N and high K doses, as disease was spreading.
Conclusion: This work concluded that the application of N and K through inorganic fertilizers to cucumber plants had a suppressing effect on downy mildew disease. The positive impact of the correct management of nutrients could make integrative plant nutrition an essential component in sustainable agriculture.
Key words: disease control; fertilization; fungicide; soil and leaf nutrients.
Downy mildew of cucurbits caused by P. cubensis is a destructive worldwide-distributed disease not only in under cover crops but also in the open fields [1]. This ‘high risk’ pathogen possesses high evolutionary potential, therefore rapidly overcomes the host resistance and fungicides efficacy and in consequence the disease management becomes difficult [2]. In addition, to improve resistance to downy mildew in cucumber is arduous due to environmental variability and a narrow genetic base in cucumbers [3,4]. Although breeding programs to increase the level of resistance to specific races of P. cubensis in Cucumis melo and Cucurbita spp., were efficient, resistant cultivars of cucumber plants has not been effective yet [5].
Consequently, the use of fungicides is considered the most effective control method of cucumber downy mildew. It is often carried out an aggressive program of repeatable fungicide applications [6]. Protectant fungicides applications are necessary especially during humid weather however, they are less effective when used on susceptible varieties or under high disease pressure [4]. Both protectant and systemic fungicides should be used in combination in order to decrease disease intensity [7].
Systemic fungicides are highly effective even at low concentration levels [8] and succeed even complete disease control [9]. However, they have a single-site mode of action and therefore at a greater risk, control failures due to the development of resistance in the pathogen population. P. cubensis resistance to specific fungicides after their continuous use under high disease pressure have been well documented [10]. Moreover, fungicides have negative impact to the environment, may be toxic for non-target microorganisms and cause water pollution [8,11] as well as influence the biochemical reactions and fertility of soil [12].
Considering the fungicide resistance in P. cubensis seems to be frequent and ubiquitous, environmentally acceptable alternative approaches become a key component for the management of downy mildew. On the other hand, the impact of host nutrition to disease incidence is recognized for many crops [13, 14, 15] but the knowledge for downy mildew of cucumber plants is limited. More specifically, fertigation with high N and P and low K resulted in less downy mildew development on muslmelon while its low nutritional status predisposed plant to infection by P. cubensis [13]. In addition, high levels of P decreased disease intensity whereas low P doses along with N and K elevated downy mildew severity. Moreover, micronutrients Cu and Zn had negative impact on disease incidence of cucurbits [16]. Interactions of nutrients with regard to disease impact on plants has been also reported [17]. In some cases, nutrients supply resulted in remarkably control of plant diseases which was slightly less effective when compared with the efficacy of chemical control [18]. Thus, in an integrated disease management program, host nutrition via inorganic fertilization might be quite important.
The objectives of this research were (a) to study the main effects and interaction of nitrogen and potassium fertigation levels with regard to downy mildew emergence (b) to investigate the potential impact of the nutrient content in the soil and cucumber leaves on P. cubensis development (c) to examine the disease progress (d) to assess the effectiveness of a systemic fungicide in controlling the disease (e) to compare fungicide application and N, K fertilization relative to P. cubensis response.
Experimental Design
A 2x3 factorial experiment with four replicates and eight pots per plot, was established under greenhouse conditions. Two N levels (200 and 300 ppm) and three levels of K (200, 300 and 400 ppm) plus two fungicide treatments (protective and curative application) were applied to cucumber plants (Cucumis sativus L.) grown in 7 L pots. These concentrations of N and K considered slightly below and above ordinary rates applied for cucumber cultivation. The cultivar Knossou, susceptible to P. cubensis was used. Soil was sandy loam with the following characteristics, pH=7.4, EC =2.5 mS/cm, total CaCO3 25%.
Nutrients were supplied via fertigation (0.5 L per plant) and the systemic fungicide Aliette 80 WG (fosetyl-Al) at the recommended dose (2g L-1). Cucumber plants treated with this fungicide 24 hours before inoculation with P. cubensis (preventative activity) and 24 hours after symptoms appearance (curative action). To accomplish the combinations of N and K doses, the fertilizers ammonium nitrate (33.5-0-0) and potassium nitrate (13.5-0-46.2) were used in appropriate proportions. P was added to the above nutrient solution at constant concentration (50ppm) via orthophosphoric acid. Cucumber plants treated with fungicide were fertilized with standard solution contained N 100 ppm, P 50 ppm and K 200 ppm. All agronomic practices for the experimental units were kept uniform.
cubensis inoculation
Cucumber leaves were collected from the open fields, rinsed with tap water to remove sporangia, sealed in plastic bags to maintain 100% RH and placed in an incubator at 20°C for 12 h. The newly produced sporangia were gently brushed into distilled water to make a sporangial suspension. The inoculum was adjusted to15x104 sporangia ml-1 with a hemocytometer. Cucumber plants grown in pots under greenhouse conditions, were artificially inoculated by placing five droplets of P. cubensis spore suspension on the upper surface of the fifth leaf from the plant apex.
Disease assessment and nutrient determination
Leaf and lesion area caused by P. cubensis on cucumber leaves were digitally assessed at daily basis after appearance of the disease symptoms. The results were reported in cm2 as measured area. To examine the effect of nutrient content to downy mildew incidence soil and leaf samples were received two days post inoculation of cucumber plants with P. cubensis. Ammonium acetate method was used for the soil K. NH4-N and NO3-N was extracted with KCl and determined through direct distillation method. P was determined photometrically, K by flame photometer and the rest leaf nutrients were analyzed after wet oxidation with atomic absorption spectrophotometer [19].
Statistical analysis
Data obtained were subjected to analysis of variance (ANOVA) according to randomized block design (eight treatments) as well as individually (six factorial treatments) to detect interactions between N and K levels. Means were compared by Duncan Multiple Range Test (DMRT) at the significant level p=0.05. To evaluate the development rate of leaf and lesion area regression analysis was carried out. Pearson’s Rank Correlation was performed in order to examine the relationships among the variables.
N and K main effects on cucumber leaf and lesion area
Figure 1 depicts the main effects of N on leaf and infection area caused by P. cubensis on cucumber leaves. Both N fertigation doses resulted in the largest leaf area in the second disease screening compared with the others (Figure 1A). N level of 300 ppm significantly increased the leaf size in almost all the assessments (day 11, 16, 20 post inoculation). Disease severity increased in both N doses with time. The downy mildew infection was significantly increased under the higher N concentration than the lower N level in the day 16 and 20 (Figure 1B).
K fertigation level of 200 ppm resulted in the greates leaf size of cucumber plants with statistical significant differences among K treatments in all the assessments (Figure 2A). The infection intense increased with time (Figure 2B). The results indicated that K application at 200 ppm resulted in the highest infection in the day 11 and 13 whereas significant reduction of lesion area was noticed in the last two assessments.
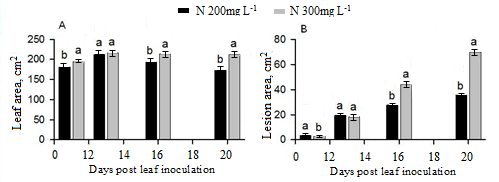
Figure 1. Main effects of N levels on leaf (A), lesion (B), caused by P. cubensis on cucumber plants grown in fertilization combinations of N and K.
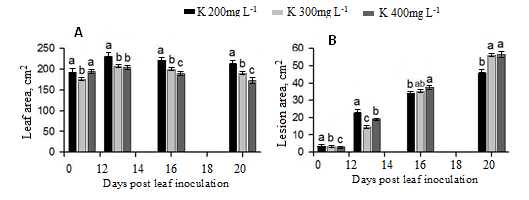
Figure 2. Main effects of K levels on leaf (A), lesion (B) area caused by P. cubensis on cucumber plants grown in fertilization combinations of N and K.
Interactions of N and K
Figure 3 shows the interactions of N x K fertilization regimes for the variables leaf and lesion area of cucumber plants during the days of assessment. K dose of 300 ppm decreased the size of leaves under both N concentrations, at day 11 and 13 with statistical significant differences (P=0.01) only at day 13. Leaf area was significantly reduced as K levels in soil were increasing when cucumber plants fertilized with 200 ppm N in comparison with 300 ppm N, at day 16 (P=0.05) and 20 (P=0.001).
Strong N x K interaction (P=0.001) was ascertained for lesion area caused by P. cubensis in all the days of screening (Figure 3). The infection intensity on cucumber plants grown under 200 ppm of N decreased with increasing K levels in the fertigation whereas higher N dose had adverse impact on downy mildew disease.
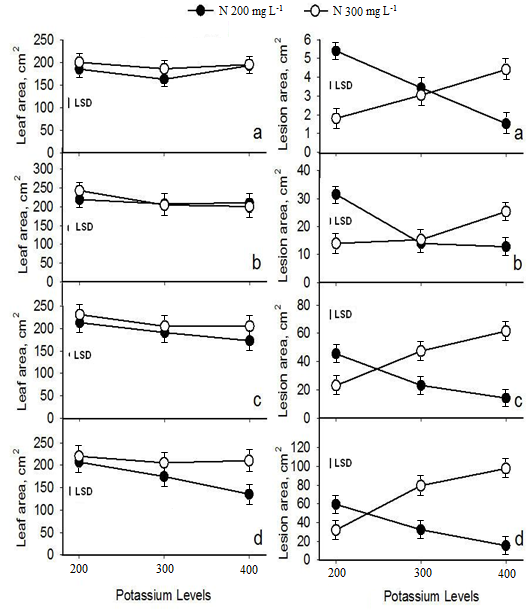
Figure 3. Interaction of N and K levels with regard to the leaf and lesion area caused by P. cubensis on cucumber plants at different stages of disease development (a-d: disease assessment at day 11, 13, 16, 20).
Impact of fungicide on leaf and lesion area
The area of cucumber leaves was not statistically significant different in both fungicide applications in all measurements (Figure 4). A slightly reduction tendency of leaf area observed in both treatments at the last assessments. Cubic model was best fitting the data obtained from leaf area development with time under fungicide treatments.
Preventative and curative activity of fungicide Aliette was tested against downy mildew of cucumber plants (Figure 5). Infection caused by P. cubensis was otherwise developed when fungicide applied protectively than after the symptoms appearance. The pre-inoculation addition resulted in a rather stable progress of disease severity, slightly increased in the last day of assessment (Figure 5). In contrast to this, downy mildew increased in cucumber plants treated with fungicide after symptoms appeared.
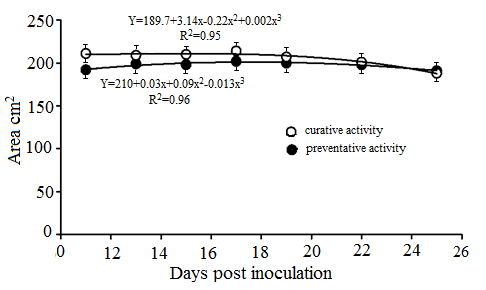
Figure 4. Development with time of leaf area of cucumber plants treated with fungicide pre and post inoculation with P. cubensis.
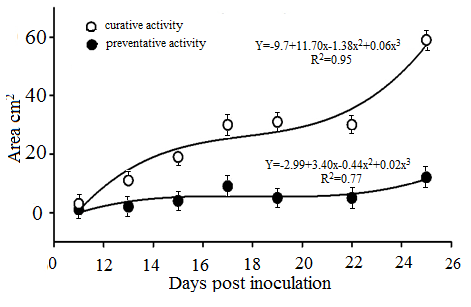
Figure 5. Downy mildew progress on cucumber leaves under preventative and curative fungicide application.
Effectiveness of fungicide and nutrient fertigation against downy mildew
ANOVA according to randomized block design was execute d and results obtained are presented in Table 1. Cucumber plants fertilized with N 300 ppm and K 200 ppm had the largest leaf area although clear significant differences were noticed only in the day13, when compared with the other fertigation regimes and the fungicide applications.
Findings also indicate that protective fungicide application to plants suppressed significantly the infection with regard to the rest treatments, in day 11 and 13 post inoculation. Nonetheless, fungicide used prior to infection with P. cubensis, gave the best disease control, though the differences compared to fertilization with N 200 ppm and K 400 ppm were not significant at the last two days of assessment (P=0.05) (Table 1). The curative fungicide application resulted in less efficient control of downy mildew relative to intermediate fertilization regime (low N plus high K dose and high N with low K dose), in day 11 and 13 (Table 1). In day 16 post inoculation with P. cubensis, lesion area of plants treated with fungicide after symptoms emergence was not significantly different when compared with that of cucumber plants grown under N 200 ppm - K 300 ppm and N 300 ppm - K 200 ppm. Moreover, in the last disease assessment, the above fertilization treatments had the lowest infection area than the curative fungicide supply. The results also revealed that the lowest rates of N and K significantly elevated downy mildew severity at day 11 and 13 whereas at day 16 and 20 the highest N and K doses resulted in significant increase of infection (Table 1).
Table 1. Effect of N and K fertigation and fungicide application on leaf and lesion area of cucumber plants. Means within columns followed by different letters are significantly different (Duncan, P=0.05).
|
Treatments |
Leaf area (cm2) |
|
|||||||||||||||||||||||||
|
mg L-1 |
|
|
Days post inoculation |
|
|||||||||||||||||||||||
|
N |
K |
|
11 |
13 |
16 |
20 |
|
||||||||||||||||||||
|
200 |
200 |
185.6 |
b |
219.3 |
b |
213.3 |
ab |
207.3 |
ab |
|
|||||||||||||||||
|
200 |
300 |
162.9 |
c |
207.7 |
bc |
191.3 |
bc |
175.0 |
d |
|
|||||||||||||||||
|
200 |
400 |
193.3 |
ab |
210.4 |
bc |
172.9 |
c |
135.5 |
e |
|
|||||||||||||||||
|
300 |
200 |
200.7 |
ab |
242.8 |
a |
231.4 |
a |
220.0 |
a |
|
|||||||||||||||||
|
300 |
300 |
186.3 |
b |
204.8 |
bc |
205.1 |
ab |
205.6 |
abc |
|
|||||||||||||||||
|
300 |
400 |
195.8 |
ab |
200.7 |
c |
205.4 |
ab |
210.3 |
a |
|
|||||||||||||||||
|
Preventative Fung. application |
192.4 |
ab |
198.2 |
c |
222.2 |
ab |
190.9 |
bcd |
|
||||||||||||||||||
|
Curative Fung. application |
210.7 |
a |
210.0 |
bc |
214.1 |
ab |
188.3 |
cd |
|
||||||||||||||||||
|
|
|
|
|
Lesion area (cm2) |
|
||||||||||||||||||||||
|
200 |
200 |
5.404 |
a |
31.5 |
a |
45.5 |
b |
59.5 |
c |
||||||||||||||||||
|
200 |
300 |
3.437 |
c |
13.9 |
d |
23.17 |
c |
32.3 |
d |
||||||||||||||||||
|
200 |
400 |
1.525 |
d |
12.8 |
d |
14.1 |
d |
15.3 |
e |
||||||||||||||||||
|
300 |
200 |
1.805 |
d |
13.9 |
d |
22.9 |
c |
32.0 |
d |
||||||||||||||||||
|
300 |
300 |
3.046 |
c |
15.3 |
d |
47.4 |
b |
79.5 |
b |
||||||||||||||||||
|
300 |
400 |
4.409 |
b |
25.4 |
b |
61.5 |
a |
97.8 |
a |
||||||||||||||||||
|
Preventative Fung. application |
0.663 |
e |
3.7 |
e |
8.8 |
d |
12.4 |
e |
|
||||||||||||||||||
|
Curative Fung. application |
2.815 |
c |
19.4 |
c |
29.8 |
c |
59.1 |
c |
|
||||||||||||||||||
Assessment of nutritional status of cucumber plants
No correlation observed among treatments and infection, as well as between leaf or soil nutrients and downy mildew incidence of cucumber plants (data not shown). Table 2 presents the main effects of N and K rates and the impact of fungicide applications on nutrient concentration of cucumber leaves and soil. N level of 200 ppm significantly increased P, Cu and NH4-N leaf content when compared with the higher N dose. The opposite noticed for leaf Ca and NO3-N in both leaves and soil. No significant differences observed in all the other cases. Leaf and soil K, leaf Zn and soil NH4-N were significantly higher in level of K 400 ppm when compared with the other K rates whereas NO3-N content in soil was the lowest in this K application dose. The concentration of the rest nutrients did not statistically differ among K levels applied to cucumber plants (Table 2).
In general, leaf and soil nutrient content had not statistically significant differences due to fungicide applications except Fe and NO3-N of leaves which were lower in plants treated with fungicide prior to their inoculation with P. cubensis when compared with fungicide applied curatively (Table 2).
Table 2. Main effects of N and K levels and fungicide applications on nutrient content in cucumber leaves and soil. Means within columns followed by different letters are significantly different (Duncan, P=0.05).
|
Levels |
Macronutriens in cucumber leaves
|
|
|||||||||||||||||||||||||||||||||||
|
|
% |
|
|||||||||||||||||||||||||||||||||||
|
ppm |
Ca
|
Mg
|
K
|
P
|
|
||||||||||||||||||||||||||||||||
|
N |
% |
|
|||||||||||||||||||||||||||||||||||
|
200 |
3.31 |
b |
0.60 |
a |
4.48 a |
a |
0.75 |
a |
|
||||||||||||||||||||||||||||
|
300 |
3.69 |
a |
0.58 |
a |
4.48 a |
a |
0.66 |
b |
|
||||||||||||||||||||||||||||
|
K |
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||
|
200 |
3.49 |
a |
0.58 |
a |
4.33 b |
b |
0.70 |
a |
|
||||||||||||||||||||||||||||
|
300 |
3.44 |
a |
0.58 |
a |
4.39 b |
b |
0.70 |
a |
|
||||||||||||||||||||||||||||
|
400 |
3.56 |
a |
0.61 |
a |
4.72 a |
a |
0.72 |
a |
|
||||||||||||||||||||||||||||
|
Fungicide |
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||
|
pre-inoculation |
3.98 |
a |
0.85 |
a |
4.63 a |
a |
0.81 |
a |
|
||||||||||||||||||||||||||||
|
post-inoculation |
3.80 |
a |
0.71 |
a |
4.79 a |
a |
0.83 |
a |
|
||||||||||||||||||||||||||||
|
|
Micronutrients in cucumber leaves |
|
|||||||||||||||||||||||||||||||||||
|
|
Fe
|
Mn
|
Zn
|
Cu
|
|
||||||||||||||||||||||||||||||||
|
N |
ppm |
|
|||||||||||||||||||||||||||||||||||
|
200 |
170.1 |
a |
70.7 |
a |
25.8 |
a |
52.0 |
a |
|||||||||||||||||||||||||||||
|
300 |
180.6 |
a |
74.7 |
a |
26.6 |
a |
31.3 |
b |
|||||||||||||||||||||||||||||
|
K |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
|
200 |
189.4 |
a |
69.0 |
a |
25.0 |
b |
48.7 |
a |
|||||||||||||||||||||||||||||
|
300 |
167.8 |
a |
75.1 |
a |
25.5 |
b |
30.9 |
b |
|||||||||||||||||||||||||||||
|
400 |
168.7 |
a |
74.0 |
a |
28.2 |
a |
45.4 |
a |
|||||||||||||||||||||||||||||
|
Fungicide |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
|
pre-inoculation |
207.8 |
b |
81.7 |
a |
23.7 |
a |
61.6 |
a |
|||||||||||||||||||||||||||||
|
post-inoculation |
257.2 |
a |
85.1 |
a |
26.7 |
a |
62.4 |
a |
|||||||||||||||||||||||||||||
|
|
N in leaves |
Nutrients in soil |
|
||||||||||||||||||||||||||||||||||
|
N |
NH4-N |
NO3-N |
NH4-N |
NO3-N |
K |
|
|||||||||||||||||||||||||||||||
|
|
ppm |
|
|||||||||||||||||||||||||||||||||||
|
200 |
42.8 |
a |
51.6 |
b |
14.2 |
a |
28.5 |
b |
430.9 a |
|
|||||||||||||||||||||||||||
|
300 |
33.1 |
b |
73.0 |
a |
12.5 |
a |
42.6 |
a |
423.3 a |
|
|||||||||||||||||||||||||||
|
K |
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
200 |
43.1 |
a |
61.0 |
ab |
11.0 |
b |
36.9 |
a |
372.1 c |
|
|||||||||||||||||||||||||||
|
300 |
33.6 |
a |
57.2 |
b |
14.1 |
a |
37.1 |
a |
432.9 b |
|
|||||||||||||||||||||||||||
|
400 |
37.1 |
a |
68.7 |
a |
15.0 |
a |
32.7 |
b |
476.3 a |
|
|||||||||||||||||||||||||||
|
Fungicide |
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
pre-inoculation |
16.4 |
a |
58.4 |
b |
12.8 |
a |
14.3 |
a |
393.6 a |
|
|||||||||||||||||||||||||||
|
post-inoculation |
19.9 |
a |
75.1 |
a |
13.3 |
a |
16.1 |
a |
409.0 a |
|
|||||||||||||||||||||||||||
Table 3 shows the effect of treatments analyzed as randomized block design on nutrients of cucumber leaves and soil. Leaf content of Ca and Fe was the lowest in treatments N 200-K 300, N 200-K 400 and N 300-K 200 ppm. K and NO3-N concentrations in leaves of cucumber plants significantly increased under both fungicide applications and higher doses of N and K. The combination of N 300 ppm with all K doses resulted in significantly the lowest P and Cu content in leaves. Mg in leaves increased and NO3-N in soil decreased in both fungicide applications when compared with the other treatments.
Table 3. Nutrients in soil and leaves of cucumber plants grown under different fertilization regimes and fungicide applications. Means within columns followed by different letters are significantly different (Duncan, P=0.05).
|
Treatments |
Macronutriens in cucumber leaves |
||||||||||||||||
|
N |
K |
Ca |
Mg |
K |
P |
||||||||||||
|
ppm |
% |
||||||||||||||||
|
200 |
200 |
3.90 |
ab |
0.61 |
c |
4.46 |
cde |
0.71 |
cd |
||||||||
|
200 |
300 |
3.16 |
c |
0.60 |
c |
4.50 |
cd |
0.75 |
bc |
||||||||
|
200 |
400 |
2.86 |
c |
0.60 |
c |
4.46 |
cde |
0.78 |
ab |
||||||||
|
300 |
200 |
3.09 |
c |
0.55 |
c |
4.19 |
e |
0.68 |
d |
||||||||
|
300 |
300 |
3.72 |
b |
0.56 |
c |
4.27 |
d |
0.65 |
d |
||||||||
|
300 |
400 |
4.25 |
ab |
0.63 |
c |
4.99 |
a |
0.66 |
d |
||||||||
|
Fungicide pre-inoculation |
3.98 |
a |
0.85 |
a |
4.63 |
bc |
0.81 |
ab |
|||||||||
|
Fungicide post-inoculation |
3.80 |
ab |
0.71 |
b |
4.79 |
ab |
0.83 |
a |
|||||||||
|
|
|
Micronutrients in cucumber leaves |
|||||||||||||||
|
N |
K |
Fe |
Mn |
Zn |
Cu |
||||||||||||
|
ppm |
ppm |
||||||||||||||||
|
200 |
200 |
234.3 |
ab |
71.9 |
bcd |
26.0 |
b |
68.6 |
a |
||||||||
|
200 |
300 |
134.8 |
d |
72.8 |
bcd |
25.7 |
b |
30.1 |
c |
||||||||
|
200 |
400 |
141.2 |
d |
67.6 |
cd |
25.7 |
b |
57.2 |
b |
||||||||
|
300 |
200 |
144.5 |
d |
66.1 |
d |
23.9 |
b |
28.8 |
c |
||||||||
|
300 |
300 |
200.9 |
c |
77.4 |
abc |
25.2 |
b |
31.7 |
c |
||||||||
|
300 |
400 |
196.3 |
c |
80.5 |
abc |
30.6 |
a |
33.5 |
c |
||||||||
|
Fungicide pre-inoculation |
207.8 |
bc |
81.7 |
abc |
23.7 |
b |
61.6 |
ab |
|||||||||
|
Fungicide post inoculation |
257.2 |
a |
85.1 |
a |
26.7 |
ab |
62.4 |
ab |
|||||||||
|
|
|
N in leaves |
Nutrients in soil |
||||||||||||||
|
N |
K |
NH4-N |
NO3-N |
NH4-N |
NO3-N |
K |
|||||||||||
|
ppm |
ppm |
||||||||||||||||
|
200 |
200 |
51.0 |
a |
56.6 |
c |
11.3 |
b |
30.6 |
c |
381.6 |
cd |
||||||
|
200 |
300 |
28.1 |
cde |
49.8 |
c |
14.4 |
ab |
28.7 |
c |
437.2 |
ab |
||||||
|
200 |
400 |
49.3 |
ab |
48.3 |
c |
16.9 |
a |
26.1 |
c |
474.0 |
a |
||||||
|
300 |
200 |
35.1 |
bcd |
65.4 |
bc |
10.7 |
b |
43.1 |
ab |
362.7 |
d |
||||||
|
300 |
300 |
39.2 |
abc |
64.5 |
bc |
13.8 |
ab |
45.4 |
a |
428.6 |
b |
||||||
|
300 |
400 |
24.9 |
cde |
89.2 |
a |
13.1 |
ab |
39.3 |
b |
478.5 |
a |
||||||
|
Fungicide pre-inoculation |
16.4 |
e |
58.4 |
bc |
12.8 |
b |
14.3 |
d |
393.6 |
bcd |
|||||||
|
Fungicide post inoculation |
19.9 |
de |
75.1 |
ab |
13.3 |
ab |
16.1 |
d |
409.0 |
bc |
|||||||
Findings revealed an interaction between N and K levels with regard to response of cucumber plants to P. cubensis attack and leaf area development. Therefore, the size of the leaves was lowered as K increased when N was applied at low dose, whereas it was not altered with increasing K level under high N. In general, leaf area growth is mainly influenced by nutrients and modified by N addition [20] however determination of leaf size is complicated and runs counter to prediction [21]. The results of the present study confirm that N and K combined at high levels was more beneficial in terms of leaf size, probably due to their implication in plant growth, as shown for other nutrient combination [22].
Downy mildew severity was reduced significantly under low N as K rates increased while high infection was noticed under elevated doses of N and K. Interaction between N and K application relative to plant response against pathogens has also been ascertained for other fungal infections. Prabhu et al concluded that fertilization with increased K had significantly linear negative response on rice blast only in the absence of additional N [23]. It is well documented that plant reaction against attack of pathogens is influenced by nutrients availability and balanced nutrition [24,25].
Principally, the role of essentials nutrients N and K is quite important relative to susceptibility of plants to diseases. Specifically, the growth of Phytophora infestans (Peronosporales), in potato plants was defined by N: K ratio [17]. In the current investigation, the high disease intensity observed in high K level was eliminated by low rates of N. Huber confirmed that the negative impact of high N levels to mildew diseases could be countervailed by balanced rates of P and K [26].
Although leaf area of cucumber plants was not influenced by any protective or curative application of fungicide, a decline of leaf size was noticed mainly for cucumber plants soil drenched with the chemical after the symptoms emerged. High disease pressure probably caused leaf shrinkage.
The preventative treatment of the fungicide inhibited the fungus growth whereas curative supply only slightly delayed the disease development which was greatly increased with time. In addition, Fosetyl-Al did not suppress lesion expansion caused by the obligate parasite Phytophthora citrophthora, when applied on curative basis [27]. The ineffectiveness of chemical control of foliar diseases when used after symptoms appearance compared to application at the latent infection period was reported [28]. However, Aliette was highly effective against P. viticola when applied in mixture with other chemicals [29].
Although, the protective use of fungicide controlled downy mildew markedly, its effectiveness was similar to that of combination rates N 200 ppm-K 400 ppm, when the disease was extended. Thus, fungicide applied prior to inoculation with P. cubensis significantly suppressed the infection intensity with regard to fertilization treatments only in low disease pressure. Moreover, low Ν and high K fertilization regime led to the greatest leaf size of cucumber plants and strongly inhibited P. cubensis development compared to curative chemical treatment. On the contrary, fertilizer application gave poor control of other foliar diseases with regard to chemical supply under greenhouse conditions [30]. Findings also indicated that low rates of N and K were ineffective in controlling downy mildew at the early stages of the disease progress. In contrast, at high disease pressure, increased infection was observed in cucumber plants grown under high levels of N and K.
Leaf and soil nutrient of cucumber plants had not significant differences in any fungicide application, except concentration of Fe and NO3-N in leaves. In contrast, accumulation of Ca, P, Cu, NH4-N and NO3-N in leaves and NO3-N in soil showed statistically significant differences due to N levels applied while K levels in the fertigation modified content of K, Zn, Cu, NO3-N in leaves and soil K along with both N forms. Given that no correlation was found between nutrients and disease progress, the above significant differences of nutrient content in leaf and soil among treatments implied an indirect influence on development of leaf size and infection progress on cucumber leaves.
The results came out of this study demonstrated the significant role of the fertilizers ammonium nitrate and potassium nitrate in controlling downy mildew of cucumber plants. Moreover, increased K fertilization dose from 200 to 400 ppm decreased the infection at low N rate (200 ppm). Low levels of N and K resulted in high disease intensity at the primary stage of P. cubensis reproduction whereas as the disease was extended, high rates of N and K were ineffective of decreasing the lesion area on cucumber leaves. Disease was better managed by preventative use of Aliette than curative application. However, at high disease pressure the impact of fungicide on downy mildew was comparable to that of low N plus high K fertilization regime. The inhibitory effectiveness of nutrients upon plant diseases makes inorganic fertilization a potential major component of an integrated pest management program.
Lebeda A. and Cohen Y., 2011. Cucurbit downy mildew (Pseudoperonospora cubensis)-biology, ecology, epidemiology, host-pathogen interaction and control. European journal of plant pathology, 129:157-192.
View ArticleMcDonald B.A. and Linde C., 2002. Pathogen population genetics, evolutionary potential and durable resistance. Annual review of phytopathology, 40: 349-379. PMid:12147764
View Article PubMed/NCBILebeda A., 1999. Pseudoperonospora cubensis on Cucumis spp. and Cucurbita spp. – resistance breeding aspects. Acta Horticulturae, 492: 363-370.
View ArticlePalti J. and Cohen Y., 1980. Downy mildew of cucurbits (Pseudoperonospora cubensis): The fungus and its hosts, distribution, epidemiology and control. Phytoparasitica 8: 109-147.
View ArticleLebeda A. and Widrlechner M.P., 2003. A set of Cucurbitaceae taxa for differentiation of Pseudoperonospora cubensis pathotypes. Journal of plant diseases and protection, 110(4): 337-349.
Granke L.L., Morrice J.J and Hausbeck M.K, 2014. Relationships Between Airborne Pseudoperonospora cubensis Sporangia, Environmental Conditions, and Cucumber Downy Mildew Severity. Plant disease, 98(5): 674-681.
View ArticleOjiambo P.S., Paul P.A. and Holmes G., 2010. A Quantitative Review of Fungicide Efficacy for Managing Downy Mildew in Cucurbits. Disease control and pest management, 100(10): 1066-1076.
Rani A., Singh R., Kumar P. and Shukla G., 2017. Pros and cons of fungicides: An overview. International journal of engineering sciences and research technology, 6(1): 112-117.
Wong F.P. and Wilcox W.F., 2001. Comparative and physical mode of action of azoxystrobin, mancozeb and metalaxyl against Plasmopara viticola (grapevine downy mildew). Plant disease 85: 649-656.
View ArticleUrban J. and Lebeda A., 2006. Fungicide resistance in cucurbit downy mildew – methodological, biological and population aspects. Annals of applied biology, 149: 63-75.
View ArticleWightwick A., Walters R., Allinson G., Reichman S. and Menzies N., 2010. Environmental Risks of Fungicides Used in Horticultural Production Systems, Fungicides, Odile Carisse (Ed.), InTech.
Wainwright M., 1978. A review of the effect of pesticides on microbial activity in soil. European journal of soil science, 29: 287-298.
View ArticleBains S.S. and Jhooty J.S., 1978. Relationships between mineral nutrition of muskmelon and development of downy mildew caused by Pseudoperonospora cubensis. Plant and soil, 49: 85-90.
View ArticleChaluvaraju, G., Basavaraju, P., Shetty N.P., Deepak S.A., Amruthesh K.N., Shetty H.S., 2004. Effect of some phosphorous-based compounds on control of pearl millet downy mildew disease. Crop Protection, 23(7): 595-600.
View ArticlePanicker S. and Gangadharan K., 1999. Controlling downy mildew of maize caused by Peronosclerospora sorghi by foliar sprays of phosphonic acid compounds. Crop Protection, 18(2): 115-118. 00101-X
View ArticleGupta S.K. and Thind TS., 2018. Disease Problems in Vegetable Production, 2nd Edition. Scientific publishers, India.
Prabhu A.S., Fageria N.K., Huber D.M. and Rodrigues F.A., 2007. Potassium and plant disease. In: Mineral nutrition and plant disease, edited by L.E. Datnoff, W.H. Elmer, D.M. Huber, American Phytopathological Society, St. Paul, Minnesota, USA, p. 57-78. PMid:21124653 PMCid:PMC2980722
PubMed/NCBIReuveni R., Dor G. and Reuveni M., 1998. Local and systemic control of powdery mildew (Leveillula taurica) on pepper plants by foliar spray of mono-potassium phosphate. Crop protection, 17(9): 703-709. 00077-5
View ArticlePage A.L., Miller R.H. and Keeney D.R., 1982. Chemical and microbiological properties. In: Methods of Soil Analysis, American Society of Agronomy Inc and Soil Science Society of America Inc (eds). Madison, Wisconsin, 1159.
Repkova J., Brestic M. and Olsovska K., 2009. Leaf growth under temperature and light control. Plant soil environment, 55(12): 551-557.
View ArticleJurik T.W., Chabot J.F. and Chabot B.F., 1982. Plant Physiology. () 70, 1044-1048 0032-0889/82/70/1044/05/$00.50/0 Effects of Light and Nutrients on Leaf Size, CO2 Exchange, and Anatomy in Wild Strawberry (Fragaria virginiana).
Shah A.I.Z. and Khan Khalil S., 2016. Phosphorus and Zinc Interaction Influence Leaf Area Index in Fine Vs. Coarse Rice (Oryza Sativa L.) Genotypes in Northwest Pakistan. Journal of plant stress physiology, 2: 1-8.
Prabhu A.S., Barbosa Filho M.P., Filippi M.C. and Zimmermann F.J.P., 1999. Relationship between potassium fertilization and panicle blast severity in upland rice. Pesquisa agropecuaria brasileira, 34(9): 1729-1732.
View ArticleDordas C., 2008. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agronomy for sustainable development, 28(1): 33-46.
View ArticleFatima U. and Senthil-Kumar M., 2015. Plant and pathogen nutrient acquisition strategies. Plant and pathogen nutrient acquisition strategies. Frontiers in plant Science 6:750. PMid:26442063 PMCid:PMC4585253
View Article PubMed/NCBIHuber D.M., 1980. The role of mineral nutrition in defense. In: Plant disease-An Advanced Treatise, edited by Academic press Inc., New York, p. 380-406.
View ArticleAlvarez L.A., Vicent A., Soler J.M., De la Roca E., Bascon J. and Garcia-Jimenez J., 2008. Comparison of application methods of systemic fungicides to suppress branch cankers in clementine trees caused by Phytophthora citrophthora. Plant disease, 92(9): 1357-1363.
View ArticleMueller D. and Robertson A., 2008. Preventative vs. Curative Fungicides. Integrated Crop Management News. Iowa State University Extension and Outreach.
Rekanovic E., Potocnik I., Stepanovic M., Milijasevic S. and Todorovic B., 2008. Field Efficacy of Fluopicolide and Fosetyl-Al Fungicide Combination (Profiler®) for Control of Plasmopara viticola (Berk. & Curt.) Berl. & Toni. in Grapevine, Journal of Pesticides and Phytomedicine (Belgrade), 23: 183-187.
View ArticleYorinori M.A., Klingelfuss L.H., Paccola-Meirelles L.D. and Yorinori J.T., 2004. Effect of time of spraying of fungicide and foliar nutrient on soybean powdery mildew. Phytopathology, 152: 129-132.
View Article