Zhenhong Wang
Email: w_zhenhong@126.com
Tel: 86+029+ 82339952
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 6
Page No: 749-762
Zhenhong Wang
Email: w_zhenhong@126.com
Tel: 86+029+ 82339952
Zhenhong Wang 1*, Chen Chen 2, Qing Wu2, Li Wan3, Amit Patil1, XiaoLe He1
1Key Laboratory of Subsurface Hydrology and Ecological Effects in Arid Region, Ministry of Education, School of Environmental Science and Engineering, Chang`an University, No.126, Yanta Road, 710064, Xian, China
2College of Life Science, Guizhou University, Huaxi District, 550025, Guiyang, China
3Laboratory of Riverine Ecological Conservation and Technology, Chinese Research Academy of Environmental Sciences, Beijing, China
Zhenhong Wang , Chen Chen , Qing Wu, Li Wan, Amit Patil, XiaoLe He, Microbial abundance and enzyme activity in sediments of the rapid-pool-benchland systems in the natural Duliu River of China(2019)Journal of Earth Sciences & Environmental Studies 4(6) p:749-762
River ecosystems have been greatly impaired by negative human activities. It is important to understand the relationships among the river structure, microbial abundance and enzyme activity of natural rivers for the restoration of impaired river ecosystems. The repeatedly occurring rapid-pool-bench land system (RPBS) is deemed a basic unit of natural river structure. Microbial abundance and enzyme activity in sediments of the RPBS are key processes that regulate a variety of ecological functions. However, detailed studies relating to a RPBS are scarce. We selected nine RPBSs in the Duliu River in China and studied the microbial abundance, enzyme activity, nutrients and heavy metals as influencing factors in the sediments of these RPBSs using the plate counting method, colorimetric methods, and atomic absorption spectrometry. The RPBSs were significantly different in microbial abundance, enzyme activity, nutrients and heavy metals. Bacteria, ammonifiers, actinomycetes, fungi, and denitrifying bacteria in the sediments of the pools of these RPBSs showed the greatest abundances. The number of bacteria was significantly larger than that of four other microbial groups. Total nitrogen had significant effects on microbial abundance. Catalase in bench lands, as well as phosphatase, urease and dehydrogenase in pools, exhibited high levels of activity and were significantly correlated with microbial abundances. Enzyme activity was substantially affected by N, Pb and As. The habitat heterogeneity represented by RPBSs altered the microbial abundance and enzyme activity, which regulated different ecological functions. The restoration of degraded rivers to include RPBSs is beneficial to diverse ecosystem functions.
Key words: The rapids-pool-bench land system, microbes, enzyme activity, nutrient, ecosystem function, natural river
Rivers are corridors that connect the terrestrial environment to the ocean realm. Rivers play important roles in shipping and water purification (Wu et al., 2008; Padmalal and Maya, 2014). Most ancient civilizations originated on the banks of rivers, such as the Indian civilization along the Ganges River, the Egyptian civilization along the Nile River, the Mesopotamian civilization along the Tigris and Euphrates Rivers, and the Chinese civilization along the Yellow River (Wang et al., 2013). Even today, most cities are built along rivers. Rivers are footstones for the development of human civilization. However, human activities have imposed tremendous pressure on rivers, thereby altering most of them, especially the small rivers, to degrees often beyond their natural resilience capacities, in terms of both structure and function (Dong et al., 2009; Padmalal and Maya, 2014). These alterations include occupation of riverways, transformation of meanders in riverways into straight forms, replacement of natural riverbanks by concrete, and changes in sediment resulting from soil erosion and nonpoint source pollution (Dong et al., 2009; Padmalal and Maya, 2014). These modifications result in significant alteration of river structure and, consequently, declines in river habitat heterogeneity, biodiversity, self-purification capacity, landscape value, and affinity between humans and waterbodies (Wang et al., 2013).
River structures are represented by spatial variations of river forms, riverbeds, waterflow, riverbanks, organisms and riverbed materials (Padmalal and Maya, 2005; Gregory et al., 2008). Previously, based on extensive investigations, we identified a regular structure called a rapid-pool-bench land system (RPBS) along the continuum of a natural river (Fig. 1, graphical abstract and Supplemental file 1) (Vannote et al., 1980; Ma et al., 2014; Wu et al., 2014; Chen et al., 2015).The RPBS recurs from the upper to the lower reaches of a river, even during flooding. A RPBS is successively linked to another RPBS along the continuum of a river. The RPBS consists of a relatively steep slope with a rapid water flow (rapid section), a bowl-like structure (pool) in the lower reaches of the rapid section, and a variably formed bench land formation beside the rapid and the pool (Fig. 1, graphical abstract and Supplemental file 1). The rapid section has a relatively large potential energy to form a rapid water flow and forms the pool by long-term scouting. The rapid section frequently occurs along the middle line of the river. The pool is a deep waterbody, in which there are fine sediments and sludge deposited with the slow water flow (Ma et al., 2014; Wu et al., 2014). Normally, the pool occurs close to the riverbank. The bench is formed by the sedimentation of a variety of riverbed materials carried by floods. Some benches form parts of riverbanks. A significant characteristic of the bench formation is the frequent fluctuation of wet and dry periods resulting from changes in water levels during a year. Accordingly, aquatic and terrestrial organisms can occur within the bench formation. In a natural river, the proportions of area or perimeter of rapids to those of pools or benches are almost constant. In the headstream of a natural river, the RPBS is simplified into the step-pool system (Whittaker and Jaeggi, 1982; Wang et al., 2006).
However, the responses of microbial abundance and enzyme activity and the influencing factors of nutrients and heavy metals in sediments to the RPBS structure that changes regularly in a natural river remain unclear. Microbial abundance and enzyme activity in sediments are important indicators of river health. Microorganisms control geochemical cycles of the river by absorbing, oxidizing, decomposing and precipitating organic matter (OM) and nutrients (Winiarski et al., 2006). Enzyme activity is a significant parameter for measuring the biological quality and ecological functions of sediments (Benitez et al., 2004). Enzyme activity is also used as one of the most sensitive indicators for evaluating the toxicity of heavy metals and the potential degradation of organic matter in sediments from different origins (Ellis et al., 2001; Irha et al., 2003; Freixa et al., 2016). If it is found that the structures of the RPBS may result in differences in microbial abundance and enzyme activity in the sediment of a river which are beneficial to maintenance of river health, the RPBS can be used as a reference model for restoration of degraded rivers to healthy rivers.
Previously, there have been many studies of microbial abundance in soils and sediments based on laboratory cultivation of isolates from natural environments and identification by classical techniques (Prosser, 2002). Later, the techniques of advanced polymerase chain reaction and catalyzed reporter deposition-fluorescence in situ hybridization were developed to identify species of microbes for a variety of investigative purposes (Inagaki et al., 2006; Wang et al., 2012; Freixa et al., 2016). Some studies have demonstrated the importance of enzymes in matter transformation within sediments of wetlands, lakes, seas, rivers, and forest soil based on tests of enzyme activity by titration, colorimetric methods, and fluorescent substrates methods (Reboreda and Cacado, 2008; Sinsabaugh et al., 2010). These studies indicate that a positive correlation exists between dehydrogenase activity and CO2 emission in soils, and dehydrogenase activity is known to be sensitive to heavy metal pollutants (Neto et al., 2007). Enzyme activity in sediment is closely related to anthropogenic nutrients and inputs of different types of organic matter, such as cellulose and hemicellulose and recalcitrant compounds from terrestrial origins (Millar et al., 2015; Freixa et al., 2016). Ecologists have also identified significant impacts of the removal of total nitrogen (TN) on the numbers of soil microbes and the activities of enzymes in wetlands (Huang et al., 2012). In a lake, there is obviously spatial heterogeneity in microbial abundance and enzyme activity in sediments (Deng et al., 2009).
In the present study, three successively connected RPBSs in the upper, middle, and lower reaches of the Duliu River, a typical natural river in China, were selected. Sediment samples from each RPBS were collected. Microbial abundance; the activities of four important extracellular enzymes; levels of nutrients, including TN, total phosphorous (TP) and OM; and levels of heavy metals (Pb, Cr, Cd and As) in these sediment samples were then tested by traditional methods that could meet the requirements of the study. We assumed that the RPBS recurred continually to create habitat heterogeneity along the river. This structure would result in differences in microbial abundance, corresponding enzymatic activity, levels of nutrients affecting microbial abundance, and levels of heavy metals affecting enzyme activity in sediments of rapids, pools and benches along the river. The present study aims to answer the following questions: i) Are there differences in microbial abundance, enzymatic activity, and levels of nutrients and heavy metals in sediments among rapids, pools and benches from the upper to lower reaches of the river? ii) In the presence of any such differences, what relationships exist among levels of nutrients and heavy metals, microbial abundance and enzyme activity in sediments in the RPBS of the river? iii) What ecological processes or functions are represented by discovered enzymatic activities?
2.1. Site description
The Duliu River is a primary tributary of the Pearl River, the third largest river in China. The Duliu River originates in Dushan County and flows through four counties in Guizhou Province before joining the upper reach of the Pearl River. The total length of the Duliu River is 310 km, and the drop of the mainstream is 84.5 m. The catchment area is 11,326 km2. Annual mean rainfall ranges from 1350-1500 mm. The average annual flow is 145 m3/s. The forest coverage is 74.1% in the Duliu River watershed. There are no dams or artificial riverbanks along the river. For the present study, an upper reach in Puan town (26°04′ N, 107°48′ E), a middle reach in Sandu County (25°58′N, 107°53′E) and a lower reach in Rongjiang County (25°89′N, 108°50′E), were selected as research sites. The values of river parameters varied among different reaches of the river (Table 1).
Table 1. Parameters of the river
|
River reaches |
Mean river width (m) |
Ranges of water depth (m) |
Longitudinal gradient |
Bending coefficient |
Rapid area (m2) |
Pool area (m2) |
Benchland area (m2) |
|
UR* |
51.5±6.2 |
0.3-3.1 |
0.05±0.04 |
1.16±0.00 |
791.16±262.61 |
872.12±294 |
796.58±273.63 |
|
MR |
148.2+9.7 |
0.5-5.3 |
0.02±0.01 |
1.05±0.00 |
1991.16±462.41 |
1322.15±441.96 |
1492.06±386.82 |
|
LR |
257.3+12.3 |
0.8-7.4 |
0.01±0.00 |
1.20±0.00 |
3128.52±714.4 |
3691.54±794.8 |
12261.48±1307.98 |
In each of the three reaches, three sets of RPBSs successively connected with each other were selected as the river reaches for sampling (Fig. 1 and 2)

Fig 1 An occurrence of RPBS in the middle reach of the Duliu River. The square symbol: pool; the triangular symbol: rapid; the circle symbol: bench. The widths of pool, bench and rapid are approximately 75 m, 75 m and 30 m, respectively, in the photo. We can only see one RPBS in the photo, but the middle reach of the river for sampling includes three RPBSs connected with each other.
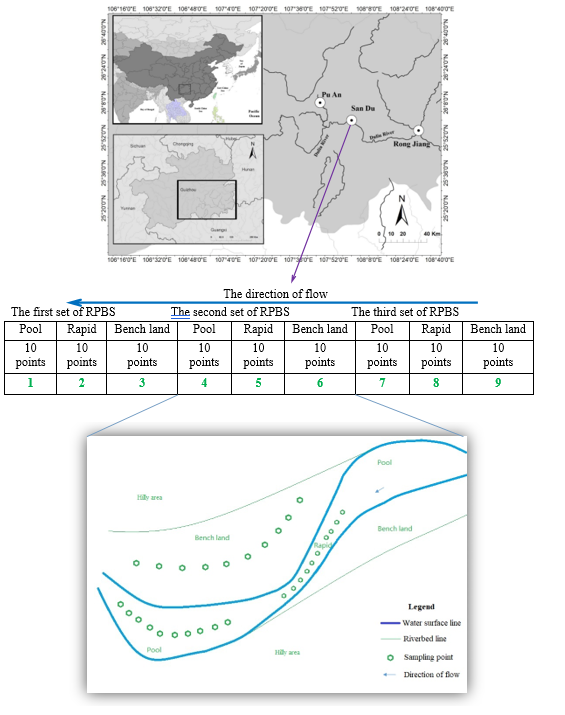
Fig 2 Sampling method. This figure shows the numbers of sampling points in one sampling reach of the river. (Altogether three sampling reaches; i.e., upper, middle and lower reaches were delineated along the river in the study.) One sampling reach includes three occurrences of RPBS. Ten points were sampled in each of the rapids, pools and benches in each RPBS. Then, all ten samples from each rapid, pool or bench were mixed into one sample. In each sampling reach, we collected 9 mixed samples from 90 points of three sets of RPBSs. In total, we collected 27 mixed samples from 270 points along the whole river. The picture below the table shows the distribution of sampling points in the RPBS that is illustrated in Fig. 1.
2.2. Field sampling
Sediment samples were collected from the rapid, pool and bench in each RPBS (Fig. 2). Specifically, ten sampling points were initially established along a central axis of the rapid, pool or bench in each RPBS. A sediment sampler was used to collect approximately 300 g of the surface sediments from each point. Sediments collected from all ten points in the rapid, pool or bench were thoroughly mixed into one sample, and then approximately 3 kg of the mixed sediment was sampled from the mixed sample and stored at 4°C. Sediments in the rapid and pool were collected from the bottom of the river, but bench sediments were collected from the surface. The number of mixed samples in each reach of the river (upper, middle or lower reach) were 9, including 3 from three rapids, 3 from three pools and 3 from three benches (Fig. 2). The total number of mixed samples was 27.
2.3. Tests of microbial abundance
2.3.1. Bacteria, fungi, and actinomycetes
The plate counting method was used to determine the number of bacteria, fungi and actinomycetes. First, beef protein medium, Martin medium and actinomycetes culture medium were prepared per the manufacturer’s instructions. After sterilization, these media were poured into three sterilized Petri dishes. In a triangular flask, 10 g of fresh sediment was mixed with 100 ml of sterile water. This soil solution was diluted to 10-6, 10-7 and 10-8 for bacteria, and 10-4, 10-5 and 10-6 for fungi and actinomycetes, in a sterile working chamber. The diluted soil solutions were transferred to other Petri dishes using pipettes. The Petri dishes were incubated at 28°C. Each dilution series was replicated three times.
2.3.2. Ammonifying and denitrifying bacteria
The most-probable-number (MPN) method was used to determine numbers of ammonifying and denitrifying bacteria. First, peptone agar medium and denitrifying bacterial medium were prepared, and 5 mL of the each medium was subpacked into cuvettes. A reversed Durham's fermentation tube was inserted into each of the filled cuvettes to analyze collected gas. Each cuvette was plugged with a silica gel stopple and sterilized. In a triangular flask, 10 g of fresh sediment was suspended in 100 ml of sterilized water by oscillating for 30 min. Subsequently, the suspension was diluted to four different concentrations, namely, 10-4, 10-5, 10-6 and 10-7 times of the suspension. These diluted solutions were transferred to cuvettes filled with medium using sterile pipettes. The cuvettes were then incubated at 30°C. Experiments with each dilution were replicated three times. After three and five days, the turbidity of media were measured to test for the growth of ammonifying bacteria. After seven days of incubation, Nessler’s reagent was used to test for the presence of ammonia. For identifying denitrifying bacteria, the following observations were made: presence of bubbles in the cuvettes, turbidity of the solution in the cuvettes after 14 days, and presence of ammonia as tested with Nessler’s reagent. Based on the bacterial growth, the number of live bacteria per ml of the suspension before dilution was determined using an MPN table.
2.4. Tests of enzyme activity
2.4.1. Catalase
Permanganate titration was used to determine the catalase activity (Liu et al., 2013). In a triangular flask, 5 g of fresh sediment was mixed with 25 mL of hydrogen peroxide (H2O2) solution and a little distilled water. The triangular flask was oscillated continuously for 30 min. The chemical reactions occurring in the triangular flask were terminated by the addition of sulfuric acid (H2SO4). The mixtures in the triangular flasks were filtrated, and the filtrates were titrated against potassium permanganate (KMnO4) solution until the color changed to red. The volume (mL/g) of KMnO4 solution consumed in the test represented the catalase activity.
2.4.2. Alkaline phosphatase
The phenyldisodium phosphate colorimetric method was used to determine the activity of alkaline phosphatase (Liu et al., 2013). In a triangular flask, 5 g of fresh sediment was dissolved in toluene-disodium phenyl phosphate-borate buffer. The flask was incubated for 24 h, and the contents were filtrated. The chromogenic agent was added to the filtrate, the filtrate was transferred to a volumetric flask and diluted to a constant volume of 750 mL, and the absorbance was measured at a wavelength of 660 nm. The amount (mg) of phenol released from 1 g of sediment represented the enzyme activity.
2.4.3. Urease
Urease activity was determined using the phenol sodium hypochlorite colorimetric method (Liu et al., 2013). In a 50 mL volumetric flask, 5 g of fresh sediment was added to 1 mL of toluene. The volumetric flask was tightly plugged and gently shaken for approximately 15 min. To the soil solution in the volumetric flask, 5 mL of 10% urease solution and 10 mL of citrate buffer (pH=7.6) were added, mixed completely by shaking and incubated at 37°C for 24 h. Distilled water (at 38°C) was used to dilute the solutions to constant volume. In this state, toluene floated on the solutions. Each solution was further mixed evenly, and the solid was filtered. Subsequently, 1 mL of the filtrate was transferred to a 50 mL volumetric flask, and 4 mL of sodium phenate solution and 3 mL of chlorine were added. After the development of color, the solution was distilled to a constant volume, and the absorbance was measured after 1 h at a wavelength of 578 nm. The amount (mg) of phenol released from 1 g of sediment represented the enzyme activity.
2.4.4. Dehydrogenase
The chloride three-phenyl tetrazole colorimetric method was used to determine the dehydrogenase activity (Liu et al., 2013). In a triangular flask, 5 g of fresh sediments, 2 mL of 1% triphenyltetrazolium chloride (TTC) solution, glucose solution, Tris-HCl buffer, and Na2S solution were successively added. The triangular flask was oscillated for 2 h, followed by the addition of oxygen-free water and sodium sulfite solution. After mixing the solution thoroughly, the absorbance was measured at a wavelength of 485 nm. Enzyme activity was calculated based on the quantity of 3-phenyl formazan yielded per unit soil and time.
2.5. Tests of nutrients and heavy metals
We used the potassium dichromate heating oxidation-volumetric method (GB9834, 1988), Kjeldahl distillation (GB7173, 1987) and molybdenum-antimony anti-spectrophotometric method (HJ632, 2011) to detect OM, TN and TP in sediments, respectively. Both Pb and Cr in sediments were tested by KI-MIBK extraction flame atomic absorption spectrophotometry (GB/T17140, 1997). Cd, Hg and As were detected by flame atomic absorption spectrometry (HJ491, 2009), cold atomic absorption spectrophotometry (GB/T17136, 1997), and the potassium borohydride silver nitrate spectrophotometric method (GB/T17135, 1997) (National Standards of these test methods were listed in Supplemental file 2).
2.6. Data analysis
We used analysis of variance (ANOVA, one-way) to identify the differences in microbial abundances, enzyme activities, and levels of nutrients and heavy metals in sediments among rapids, pools and benches (i.e., three components of the RPBS), from the upper to lower reaches of the Duliu River. Previous studies have indicated that there are great differences in water depth, riverbed slope, particle size and sediment nutrient levels among rapids, pools and benches, suggesting that they are three different habitats (Ma et al., 2014; Wu et al., 2014). Therefore, we here considered the rapids, pools and benches in the RPBS occurring from the upper to lower reaches of the Duliu River as the three levels under a single factor; i.e., the type of river habitat. If there were significant differences in examined ecological processes among the three habitats based on ANOVA, Tukey`s honestly significant difference (HSD) test was further applied to determine significant differences between any two. Then, Pearson`s correlations among microbial abundances, enzyme activities, and levels of nutrients and heavy metals in sediments of these RPBSs were analyzed to identify the impacts on each other.
3.1. Abundance of microorganisms
The abundances of bacteria, fungi, actinomycetes, ammonifying and denitrifying bacteria in the sediment of pools clearly exceeded those in rapids and benches; these types of microbes were also more abundant in benches than in rapids (Table 2). The results of ANOVA and Tukey`s HSD test showed that there were very significant differences in microbial abundance for five types of microbes among rapids, pools and benches (p=0.001-0.01). Based on observations and tests of nutrients, the accumulation of rich nutrients in the sediments of pools, resulting from the slower water flow than in the rapids, was beneficial for the growth and reproduction of all microorganisms, leading to high abundance of microbes in the sediment of pools. In benches, flooding usually resulted in the frequent occurrence of alternate wet and dry environments. Nutrient delivery by floods in this habitat consequently promoted the flourishing of aerobic microorganisms. However, in the sediment of rapids, high nutrient loss from rapid water flow restricted the growth of microorganisms, resulting in the lowest microbial abundance. Overall, the abundances of bacteria, ammonifying bacteria, actinomycetes, fungi, and denitrifying bacteria present in all RPBSs accounted for 60%−99.07%, 5%−31.89%, 0.28%−23.21%, 0.012%−0.172% and 0.009%−0.027%, respectively, of total numbers of microorganisms. Moreover, the results also indicated that microbial abundance was higher in the upper reaches than that in the lower reaches of the river because of the increased input of nutrients and organic pollutants from croplands and towns along the two banks of the Duliu River.
Table 2. Number of microorganisms in sediments of the RPBS
|
River reaches |
Systems
|
Bacteria (×106·g-1) |
Actinomycetes (×104·g-1) |
Fungi (×104·g-1) |
Ammonifying bacteria (×105·g-1) |
Denitrifying bacteria (×102·g-1) |
Total (×106·g-1) |
|
Upper reaches |
Pool |
5.50±0.32a |
18.20±1.39a |
11.00±0.71a |
21.44±1.72a |
14.47±1.05a |
7.84±0.53a |
|
Rapid |
2.22±0.15b |
0.71±0.06b |
1.68±0.11b |
3.21±0.15bd |
5.32±0.23b |
2.55±0.12b |
|
|
Bench land |
2.77±0.12b |
15.60±1.22c |
1.76±0.15b |
5.17±0.18b |
9.29±0.38c |
3.45±0.16b |
|
|
Middle reaches |
Pool |
2.14±0.17b |
10.01±1.07de |
4.60±0.21cd |
5.21±0.20b |
4.31±0.17b |
2.68±0.13b |
|
Rapid |
0.32±0.02c |
0.61±0.03b |
1.08±0.05b |
0.33±0.01c |
1.22±0.01d |
0.66±0.02c |
|
|
Bench land |
0.96±0.08d |
8.55±0.57de |
1.12±0.04b |
1.46±0.08bd |
1.79±0.01d |
1.20±0.04b |
|
|
Lower reaches |
Pool |
3.79±0.21e |
12.30±0.82df |
6.10±0.34cd |
18.34±1.20a |
8.89±0.49c |
5.75±0.24d |
|
Rapid |
0.14±0.08f |
9.12±0.47de |
0.18±0.01e |
2.45±0.09bd |
1.35±0.01d |
1.50±0.04b |
|
|
Bench land |
1.26±0.09g |
10.40±0.66de |
0.21±0.01e |
3.23±0.10bd |
3.79±0.22b |
1.57±0.04b |
3.2. Enzyme activity
Catalase activity ranged from 1.74-5.465 mL/g with an average of 3.638 mL/g (Fig. 3A). The activity of the enzyme was higher in the lower than in the upper and middle reaches. Among the rapids, pools and benches in the upper, middle or lower reaches, there were significant differences in the activity of the enzyme based on ANOVA and Tukey`s HSD test. This result was due to good soil aeration in benches, enhancing enzyme activity. However, the enzyme activity was highest in the rapids of the lower reaches. Sediments in benches were coarser and dryer than in rapids, probably causing inactivation or low enzyme activity.
Phosphatase activity ranged from 0.357-8.889 mg/g, with an average of 3.066 mg/g. ANOVA and Tukey’s HSD test also showed significant differences in phosphatase activity among the three sections of the river (P<0.01, Fig. 3B). In contrast to catalase activity, phosphatase activity was highest in upper reaches and lowest in the lower reach. Phosphatase activity in pools and rapids of the upper reaches was approximately 2 times and 2-5 times, respectively, as high as in the middle and lower reaches. Generally, phosphatase activity in pools was 5 times as high as in benches. These interesting results might be due to the relatively rich nutrients present in the sediments of pools and rapids.
Urease activity in sediments ranged from 0.243-2.511 mg/g, averaging 1.245 mg/g. Urease activity differed significantly among rapids, pools and benches (ANOVA), but differences based on the Tukey`s HSD test comparisons only occurred between pools and rapids and between pools and benches (P<0.01, Fig. 3C). Overall, the activity of the enzyme in rapids, pools and benches in the lower reaches was higher than in the upper and middle reaches. A regular increase or decrease was not found in urease activity from the upper to lower reaches, contrasting with results for catalase and phosphatase activity.
Dehydrogenase activity ranged from 0.61-1.65 μg/g·h, averaging 1.071 μg/g·h. The average dehydrogenase activity was the lowest of the four enzymes studied. Dehydrogenase activity did not exhibit a regular change from the upper to lower reaches (Fig. 3D). Significant differences in dehydrogenase activity were not observed among rapids, pools and benches in the upper reach with ANOVA and Tukey’s HSD test (P>0.05) but were observed in the middle and lower reaches. Dehydrogenase in sediments in the lower reaches showed similar activity in pools and benches, which was far greater than that detected in rapids.
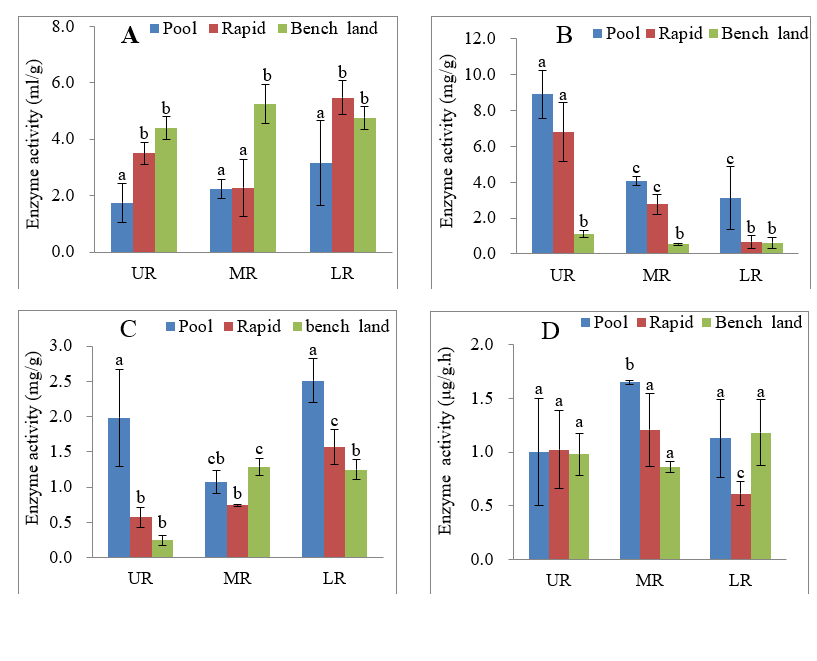
Fig 3. Enzyme activities in the sediments of the RPBS from the upper, middle and lower reaches in the Duliu River. A: catalase; B: phosphatase; C: urease; D: dehydrogenase. Lowercase letters at the tops of error lines in each figure show the results of Tukey`s HSD test. When there are completely different lowercase letters at the tops of error lines corresponding to any two habitats (rapid, pool or bench), the mean values of the two habitats are significantly different.
3.3. Nutrient levels
Numerically, the concentration of OM (8.79-32.76 g/kg) was far greater than concentrations of TN (0.41-2.93 g/kg) and TP (0.06-0.57 g/kg) in sediments (Fig. 4). ANOVA and Tukey`s HSD test showed that there were significant differences in concentrations of TN and TP among rapids, pools and benches (p<0.05, Fig. 4), but not in concentrations of OM (p>0.05). The average level of OM in sediments of rapids, pools and benches only reached the fourth grade according to Standards of Soil Nutrients for Assessment of Soil Fertility in China (there are six grades of nutrients), which was less favorable than the second grade of TN level and more favorable than the fifth grade of TP level.
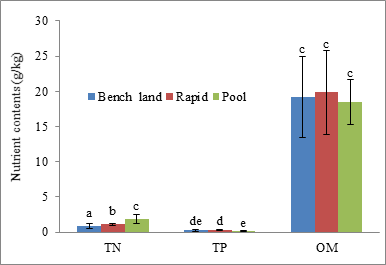
Fig 4. Average concentrations of nutrients in sediments of the RPBS along the river. Lowercase letters at the tops of error lines indicate the same meaning as in Fig. 3.
3.4. Heavy metal contents
There were significant differences in the concentrations of Pb, Cr and As in the sediments among the rapids, pools and benches of RPBS based on ANOVA and Tukey’s HSD test (p=0.00007-0.05, Table 3) but not in the concentrations of Hg and Cd (p>0.05). The means, minimums and maximums of Pb concentrations in sediments of pools were far larger than those in benches and rapids. According to Environmental Quality Standards for Soils in China, Pb, Cr and As contents only reached the first grade, which represents natural background levels. However, Hg and Cd concentrations reached the second grade, indicating the sediments were lightly polluted by Hg and Cd.
Table 3. Heavy metal contents in sediments of rapids, pools and bench lands
|
|
Heavy metal |
Means (mg/kg) |
Minimums (mg/kg) |
Maximums (mg/kg) |
Standard deviation |
Coefficients of variation |
|
Rapid |
Pb |
17.44a |
12.47 |
20.45 |
2.68 |
0.15 |
|
Cr |
25.86a |
16.15 |
40.67 |
7.94 |
0.31 |
|
|
Cd |
0.40a |
0.20 |
0.58 |
0.113 |
0.28 |
|
|
Hg |
0.32c |
0.09 |
0.62 |
0.197 |
0.61 |
|
|
As |
29.27d |
17.07 |
40.35 |
6.94 |
0.24 |
|
|
|
|
|
||||
|
Pool |
Pb |
39.66b |
23.12 |
63.36 |
14.65 |
0.37 |
|
Cr |
37.66b |
27.73 |
54.55 |
7.94 |
0.21 |
|
|
Cd |
0.32a |
0.15 |
0.59 |
0.161 |
0.5 |
|
|
Hg |
0.28c |
0.09 |
0.60 |
0.189 |
0.68 |
|
|
As |
39.09f |
22.48 |
58.53 |
12.93 |
0.33 |
|
|
|
|
|
||||
|
Bench land |
Pb |
14.71a |
7.27 |
22.79 |
5.48 |
0.37 |
|
Cr |
46.65c |
29.82 |
67.95 |
11.77 |
0.25 |
|
|
Cd |
0.30a |
0.21 |
0.45 |
0.08 |
0.27 |
|
|
Hg |
0.23c |
0.02 |
0.69 |
0.252 |
1.08 |
|
|
As |
26.67d |
14.49 |
51.76 |
12.82 |
0.48 |
|
3.5. Relationships among microbial abundance, nutrients, heavy metals and enzyme activity
There were significantly positive correlations between microbial abundance and TN levels in sediments of rapids, pools and benches, but no significant correlations between microbial abundance and TP or OM contents (Table 4). This result may be due to low concentrations of TP and OM in sediments and small differences among rapids, pools and benches (Fig. 4). However, we found that microbial abundance showed highly significant relationships with Pb and As levels in sediment. This result may be because the two heavy metals stimulated the growth of microbes in a habitat with low concentrations of heavy metals. All correlation coefficients between abundances of five types of microorganisms and the activities of four enzymes were positive (Table 4). More significant relationships were found between abundance and the activities of urease and phosphatase than for other enzymes. The source of enzymes in sediments was microorganisms. The higher the microbial abundance was, the larger was the release amount of enzymes necessary to catalyze reactants. These positive relationships suggest that microorganisms indirectly regulated catalytic processes in sediments. In addition, the activities of catalase, phosphatase and urease showed significant positive and negative correlations with the concentrations of Pb, Hg and As (Table 4). Positive correlations may have resulted from enzyme activation by heavy metals, but the negative correlations might indicate certain effects of enzyme limitation, such as the deactivation of enzymes. There were also both positive and negative correlations between nutrient levels and the activities of four enzymes (Table 4). However, only TN concentration was significantly correlated with the activities of catalase, phosphatase and dehydrogenase.
Table 4. Pearson`s correlation coefficients among abundance of microbes, contents of nutrients and heavy metals, and enzyme activity in sediments of RPBS from the upper to lower reaches
|
|
TN |
TP |
Organic matter |
Pb
|
Cr
|
Cd
|
Hg
|
As
|
Catalase |
Phosphatase |
Urease |
Dehydrogenase |
|
Bacteria |
0.59** |
-0.2 |
-0.11 |
0.83*** |
0.10 |
0.07 |
0.25 |
0.57** |
0.42* |
0.84*** |
0.65*** |
0.62*** |
|
Fungi |
0.44* |
-0.1 |
-0.08 |
0.92*** |
0.16 |
0.20 |
0.19 |
0.61*** |
0.31 |
0.76*** |
0.50** |
0.09 |
|
Actinomycetes |
0.73*** |
-0.2 |
-0.1 |
0.63*** |
0.36* |
-0.03 |
-0.13 |
0.19 |
0.61*** |
0.71*** |
0.68*** |
0.13 |
|
Amonifying bacteria |
0.61*** |
-0.2 |
-0.12 |
0.90*** |
0.07 |
0.10 |
0.15 |
0.59** |
0.23 |
0.65*** |
0.81*** |
0.37* |
|
Denitrifying bacteria |
0.56** |
-0 |
-0.1 |
0.82*** |
0.07 |
0.16 |
0.24 |
0.51** |
0.08 |
0.67*** |
0.79*** |
0.11 |
|
TN |
|
|
|
0.77*** |
-0.06 |
0.23 |
0.24 |
0.34 |
-0.52** |
0.60*** |
0.24 |
0.35* |
|
TP |
|
|
|
-0.11 |
-0.23 |
0.31 |
0.11 |
-0.08 |
0.01 |
-0.08 |
-0.27 |
-0.23 |
|
Organic matter |
|
|
|
-0.10 |
0.01 |
-0.11 |
-0.08 |
-0.29 |
0.14 |
-0.12 |
-0.14 |
0.04 |
|
Pb |
|
|
|
|
|
|
|
|
-0.59** |
0.66*** |
0.54** |
0.03 |
|
Cr |
|
|
|
|
|
|
|
|
0.1 |
-0.17 |
0.17 |
0.03 |
|
Cd |
|
|
|
|
|
|
|
|
-0.24 |
0.33 |
-0.02 |
-0.15 |
|
Hg |
|
|
|
|
|
|
|
|
-0.27 |
0.55** |
-0.01 |
0.12 |
|
As |
|
|
|
|
|
|
|
|
-0.41* |
0.66*** |
0.40* |
0.05 |
Sign***, ** and * represents significance at the confidence levels of 0.95, 0.99 and 0.999, respectively; N=27.
Previously, some studies have focused on microbial abundance and enzyme activity in sediments present in wetlands, lakes and polluted or severely disturbed riverways with an aim of addressing the challenges of water pollution (Prosser, 2002; Reboreda and Cacado, 2008). In contrast, relatively few investigations have focused on natural or less disturbed rivers (Winiarski et al., 2006; Neto et al., 2007; Huang et al., 2012; Freixa et al., 2016). In a natural river, the RPBS is seen recurrently and is defined as the basic unit of river structure that makes a river complete and heterogeneous in habitat (Ma et al., 2014; Wu et al., 2014; Chen et al., 2015; Wang et al., 2015; Fig. 1 and Supplemental file 1). A complete river structure is able to maintain the good functions of an ecosystem (Padmalal and Maya, 2014). Therefore, we selected nine RPBSs along the Duliu River and measured microbial abundance, enzyme activity, and levels of nutrients and heavy metals in sediments to clarify the effects of habitat heterogeneity and the relationships among microbial abundance, enzyme activity and their influencing factors nutrients and heavy metals. We used traditional methods to test these indices because these methods have been able to meet the goal of understanding ecological impacts of habitat heterogeneity in a river. Freixa et al. (2016) conducted a comparable study of bacterial community composition, enzyme activities and environmental variables and their relationships along the Tordera river (865 km2 watershed area), with no reservoir along its main course, in Catalonia, at the northeastern corner of the Iberian Peninsula. That study focused on the effects of environmental heterogeneity (different environmental factors) at 13 sites along the river on ecological processes, but our study was concerned with the effects of habitat heterogeneity in nine RPBSs at a relatively broad scale along the Duliu River, consisting of 27 sites of rapids, deep pools and bench formations. Each site included 10 sampling points for collecting mixed samples.
Overall, the abundance of microbes (bacteria, fungi, actinomycetes, ammonifying bacteria and denitrifying bacteria), the activities of enzymes, and the concentrations of nutrients and heavy metals in sediments were significantly different among rapids, pools and bench lands from the upper to lower reaches along the continuum of the Duliu River. These results confirm our hypothesis that the repeated occurrence of the RPBS, creating a kind of habitat heterogeneity along the river continuum, can cause variation in riverine ecological processes. Freixa et al.’s (2016) investigation showed that environmental variation in sediment characteristics resulted in far more abundant bacterial communities, as identified by catalyzed reporter deposition-fluorescence in situ hybridization, than did our work, which also argues for application of molecular techniques to environmental studies of microbes. Of the five types of microorganisms in the study, bacteria were always more abundant than the other four types of microorganisms; the abundance of ammonifying bacteria, denitrifying bacteria, actinomycetes and fungi were ranked from second to fifth, respectively. The order of microbial abundance in different habitats of the RPBS was similar to that present in artificial wetland (Du et al., 2013) and calcareous purple paddy soil (Gu et al., 2008). Thus, we suggest that relative abundances of particular groups of microorganisms are stable in different habitats. In a river, pools are sections that accumulate pollutants and nutrients, and four types of microorganisms in the sediments of pools were far more abundant than those in rapids and benches. Water in rapids can absorb more oxygen and flow into deep pools for oxidation of pollutants and nutrients; benches often cause deposited pollutants and nutrients to shift between dry and wet conditions and consequently promote their rapid degradation (Chen et al., 2015). Thus, the different habitats within an RPBS along the continuum of a river are beneficial to self-purification of the waterbody and maintenance of diverse ecological functions.
There were different correlations among microbial abundances, the activities of enzymes, and levels of nutrients and heavy metals in the sediments of the rapids, pools and benches along the continuum of the river. First, the significantly positive correlations of microbial abundance with TN concentrations in the sediments of these RPBS sections indicated that TN level had key impacts on microbial abundances. However, TP and OM concentrations showed no significant correlations with five types of microbial abundance. Second, microbial abundance exhibited identically positive correlations with the activities of specific enzymes in three sections of the RPBS because enzymes are secreted by microorganisms (Gardner and White, 2010). However, the levels of significance of the relationships differed. This observation may be because the effects of other factors, such as sediment depths and levels of nutrients and heavy metals regulated by habitat heterogeneity of the RPBS, on enzymes changed the relationships to differing degrees (Zhang et al., 2011). Similar studies also support this point. For example, there was a poor correlation between microbial colonization rate and phosphatase activity in croplands with conventional tillage, but a significant correlation in cropland with the no-tillage management (Hu et al., 2015). Additionally, no correlations between microbial abundance and enzyme activity were found in the soils of forests because of intense human disturbances (Hu et al., 2002). Negative relationships between microbial abundance and the activities of catalase, urease and dehydrogenase were even found in artificial wetlands because of interactions among several factors such as Cl ions and toluene (Duddridge and Wainwright, 1980; Filimon, 2007; Qin et al., 2010; Huang et al., 2012). These results suggest that microbial abundance is a main factor, but not the only factor, controlling enzyme activity.
Enzyme activity in soils may reflect various ecological processes and functions, such as soil fertility and health, soil biological activities, vitality of soil microbial populations and the potential degradation of organic matter (Ellis et al., 2001; Margesin et al., 2000; Neto et al., 2007; Reboreda and Cacador, 2008). Catalase can play an important role in catalyzing the decomposition of hydrogen peroxide, thereby protecting soil and microorganisms from its harmful effects (Yang et al., 1987; Huang et al., 2012). Catalase activity in sediments of benches and rapids was higher than that in pools. Therefore, benches and rapids have the potential to provide protection against the harmful effects of hydrogen peroxide and to maintain river health. Dehydrogenase activity is a sensitive marker for certain ecological processes and toxicity of pollutants with respect to microflora (Kelly et al., 1999; Irha et al., 2003). Positive correlations exist between dehydrogenase activity and CO2 emissions or soil biomass linked to respiration and the carbon cycle (Kelly et al., 1999; Benitez et al., 2004). High dehydrogenase activity denotes healthy soil with active microbial populations, reduced soil pollution, and relatively high emission of CO2 (Irha et al., 2003). In sediments of pools in the middle reaches of the Duliu River, dehydrogenase activity was higher than in those of rapids and benches. These findings indicate the presence of relatively favorable sediments for these ecological functions in pools. Alkaline phosphatase and soil urease catalyze dephosphorylation and hydrolysis of urea to accelerate the release of phosphate ions and ammonia from OM, as well as the cycling of phosphorus and nitrogen (Deng et al., 2009; Millar et al., 2015). These processes also result in the synthesis of small molecules, such as adenosine triphosphate, to regulate biochemical functions (Hu et al., 2015). Alkaline phosphatase and urease activities in sediment from pools were significantly higher than those in rapids and benches. This result indicates the ability of pools to efficiently release phosphate ions and ammonia and to enhance cycling of phosphorus and nitrogen.
The RPBS reflects the habitat heterogeneity of a river and directly regulates microbial abundance and enzyme activity in sediments. The RPBS can result in differences in the concentrations of nutrients and heavy metals in sediments and further affect microbial abundance and enzyme activity. In sediments of the pools in RPBS, there were higher abundances of bacteria, fungi, actinomycetes, ammonifying bacteria, and denitrifying bacteria than in rapids and benches. High activity of catalase in sediments of benches and rapids is beneficial for preventing the harmful effects of hydrogen peroxide. The activities of phosphatase, urease and dehydrogenase in sediments of pools indicate potential for efficient release of phosphate ions and ammonia. High dehydrogenase activity in pool sediments reflects relatively healthy conditions for growth of microbes, reduction of pollution and increase of CO2 emission. These beneficial ecological processes are mainly performed by the RPBS. The restoration of river structures altered by humans towards the RPBS is recommended for river health.
Supplemental file 1: https://www.siftdesk.org/articles/images/602/s1.pdf
Supplemental file 2: https://www.siftdesk.org/articles/images/602/s2.pdf
This work was supported by grants from the Fundamental Research Funds for the Central Universities (No.300102298303) and the National Natural Science Foundation of China (No. 40861015).
Benitez,E., Melgar,R., Nogales, R.,2004. Estimating soil resilience to a toxic organic waste by measuring enzyme activities. Soil Biol. Biochem. 36, 1615-1623.
View ArticleChen,C.,Wang, Z.H.,Ma, Z. 2015. Evaluations on the difference of water quality of the riffle-deep pool-benchland systems of watercourses in Duliujiang River. Environ. Sci. Tech.38: 182-188.
Deng, J.J.,Huang,X.F., Hu, J.W.,Li, C.X.,Yi, Y., Long, J. 2009. Distribution of several microorganisms and activity of alkaline phosphatase in sediments from Baihua Lake. Asia-Pacif J. Chem. Eng. 4, 711-716.
View ArticleDong, Z.R., Sun, D.Y., Peng, J. 2009. Theories and practices of river eco-restoration. Wat. Res. Hydropr. Eng. 40, 4-9.
Du, G., Huang, L., Gao, X.,Lu, Y.Y., Liu, M., Guo, J.S.2013. Number of microbe and relationship between it and removal of pollutants in constructed wetlands. Wetland Sci. 11, 13-17.
Duddridge, J.E.,Wainwright, M., 1980. Effect of sodium chloride on enzyme activity and synthesis in river sediments. Environ. Tech. 1, 319-326
View ArticleEllis, R.J., Neish, B., Trett, M.W., Best, J.G., Weightman, A.J., Morgan, P., Fry, J.C.2001. Comparison of microbial and meiofaunal community analyses for determining impact of heavy metal contamination. J. Microbiol. Methods 45, 171-185. 00245-7
View ArticleFilimon, M.N.2007. Enzymological studies regarding the present of the sediments in Bega channel. Anal. Univ. Oradea. Fasc. Biol. Tom. XIV, 43-46.
Freixa, A., Ejarque, E.,Crognale, S. Amalfitano, S.,Fazi, S., Butturini, A., Romaní, A. M., 2016. Sediment microbial communities rely on different dissolved organic matter sources along a Mediterranean river continuum. Limn.Ocean. 61,1389-1405.
View ArticleGardner, L.M., White, J.R., 2010. Denitrifcation enzyme activity as an indicator of nitrate movement through a diversion wetland. Wetl. Soils 74, 1037-1047.
View ArticleGregory, K.J., Benito, G., Downs, P.W., 2008. Applying fluvial geomorphology to river channel management: background for progress towards a palaeohydrology protocol. Geo-morphology 98: 153-172.
View ArticleGu, Y.F., Yun, X., Zhang, X.P., Tu, S.H., Sun, X.F., Kristina, L.2008. Effect of different fertilizer treatments on soil microbes and ammonium oxidizing bacterial community in a calcareous purple paddy Soil. Sci. Agric. Sin. 41, 4119-4126.
Hu,H.,Zhang, J.,Gao, Z.,Chen, S.,Zang, T., 2002. Study on quantitative distribution of soil microorganism and relationship with enzyme activity and physical, chemical property of shelter-forest in rocky coastal area. For. Res. 15,88-95.
Hu, J.L., Yang, A., Zhu, A.N., Wang, J.H., Dai, J., Wong, M.H., Lin, X.G., 2015.Arbuscular mycorrhizal fungal diversity, root colonization, and soil alkaline phosphatase activity in response to maize-wheat rotation and no-tillage in North China. J. Microbiol. 53, 454-461. PMid:26115994
View Article PubMed/NCBIHuang, L.,Gao, X.,Liu, M.,Du, G.,Guo, J., 2012. Correlation among soil microorganisms, soil enzyme activities, and removal rates of pollutants in three constructed wetlands purifying micro-polluted river water. Ecol. Eng. 46, 98-06.
View ArticleInagaki, F. Nunoura, T., Nakagawa, S., Teske, A., Lever. M., Lauer, A., Suzuki, M., Takai, K., Delwiche, M., Colwell, F.S., Nealson, K.H., Horikoshi, K., D'Hondt, S., Jørgensen, B.B., 2006. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc. Nat. Acad. Sci.103, 2815-20. PMid:16477011
View Article PubMed/NCBIIrha, N., Slet, J., Petersell,V.,2003. Effect of heavy metals and PAH on soil assessed via dehydrogenase assay. Environ. Intern. 28, 779-782. 00124-1
View ArticleKelly. J.J., Häggblom, M., Tate, III.R., 1999. Changes in soil microbial communities over time resulting from one time application of zinc: A laboratory microcosm study. Soil. Biol. Biochem. 31,1455-1465. 00059-0
View ArticleLiu, X.W., Xie, D.P., Li, K.M., Zhou, W.J., Wang, H.J., Jia, Y., 2013. Effects of variation of DO on the enzyme activity and microbial diversity in sediments. Environ. Sci. Tech. 36, 6-10.
Ma, Z., Wang, Z.H., Chen, C.,2014. Investigation in river channel parameters of riffle-deep pool-sand bank system in Duliujiang River. J. Mount. Agr. Biol. 33, 049-052.
Margesin, R., Zimmerbauer, A., Schinner, F.,2000. Monitoring of bioremediation by soil biological activities. Chemosphere 40, 339-346. 00218-0
View ArticleMillar, J.J., Payne, J.T., Ochs, C.A., Jackson, C.R., 2015. Particle-associated and cell-free extracellular enzyme activity in relation to nutrient status of large tributaries of the Lower Mississippi River. Biogeochemistry 124, 255-271.
View ArticleNeto, M., Ohannessian, A., Delolme, C., Bedell, J.P.,2007. Towards an optimized protocol for measuring global dehydrogenase activity in storm-water sediments. J. Soils Sedim. 7, 101-110.
View ArticlePadmalal, D.,Maya, K.,2014. Rivers-structure and functions. In: Padmalal, D.,Maya, K.(ed.) Sand Mining. Springer Verlag, Berlin, pp. 9-22.
View ArticleQin, S.P.,Dong, W.X.,Hu, C.X., 2010. Tillage affecting the turnover rate of soil urease: implications for enzyme assays and ecological modeling. Fresen. Environ. Bull. 19, 717-720.
Prosser, J.I., 2002. Molecular and functional diversity in soil micro-organisms. Plant Soil, 244, 9-17.
View ArticleReboreda, R., Cacador, I.,2008. Enzymatic activity in the rhizosphere of Spartina maritima: potential contribution for phytoremediation of metals. Mar. Environ. Res, 65, 77-84. PMid:17935772
View Article PubMed/NCBISinsabaugh, R L., Lauber, C.L., Weintraub,M.N., Ahmed, B, Allison, S.D., Crenshaw,C., 2008. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 11, 1252-1264. PMid:18823393
View Article PubMed/NCBIVannote, R.L., Minshall, G.W., Cummins, K.W., Sedell, J.R., Cushing, C.E., 1980. The river continuum concept. Can.J. Fish. Aquat. Sci.37, 129-137.
View ArticleWang, F.Q., Hou, S.F.,Xie, H., Yu, L.J.,Song, A.D., 2012. Research on seasonal dynamic change of microbial population structure in Jialu River. J. Henan Agric. Univ. 46, 438-441.
Wang, Q.H., Wang, Z.H., Liu, L.B., Guo, C.X.,2015. Influence of typical structures of a natural river on self-purification of water. J. Mount. Agr. Biol. 34, 63-67.
Wang, Z.H., Wu, Y. G., Zhang, C.Y., Liu, H.Y., Zhou, Y.C., Ying, X.L., Xu, C.M., Zhang, M.J., 2013. Typical terrestrial ecosystems in Yungui plateau (II) Typical watershed ecosystems, water ecological processes and non-point source pollution control. Science Press, Beijng.
Wang, Z.Y.,Cheng, D.S.,He, Y.P., Wang, H.Z., 2006. A Study of the Ecological Functions of Step-pool System in Southwest Mountain Streams. Adv. Earth. Sci. 21, 409-416.
Whittaker, J.G.,Jaeggi, M.N.R.,1982. Origin of step-pool systems in mountain streams. J. Hydr. Div. 108, 758-773.
Winiarski, T., Bedell, J.P., Delolme, C., Perrodin, Y., 2006. The impact of stormwater on a soil profile in an infiltration basin.Hydrogeol. J. 14, 1244-1251.
View ArticleWu, E.N., Chen, Y., Zhang, H.W., Yang, K.,2008. Urban river regulation: past, present and prospects. Chin.Wat.Wastewat, 24, 13-18.
Wu, Q., Wang, Z.H., Cheng, C., 2014. Evaluation of heavy metal pollution on riffle-deep pools-sand bank systems in different section Duliu River. J. Mount.Agr. Biol. 33, 066-071.
Yang, T., Du, Y.G., Li, Y.Y., Jiang, W.B., Sun, X.C.,1987. Study on soil calatase activities in alpine meadow. Chin. J. Ecol. 6, 52-54.
Zhang, Y.,Wu, E.,Yin, X.L., Li, G.C., Liu, K., Zhong,Y.J., 2012. Progress of urban stream transformation of critical forms and stability relationships. Progr. Geogr. 31, 837-845.
