Anastasia Papadaki.
Tel. number: +302810379424, mobile: +306938721900, fax number: +302810318204,
E-mail: apapadaki@staff.teicrete.gr
© 2019 Sift Desk Journals. All Rights Reserved
Anastasia Papadaki.
Tel. number: +302810379424, mobile: +306938721900, fax number: +302810318204,
E-mail: apapadaki@staff.teicrete.gr
Anastasia A. Papadaki*, Eirini Papazoglou, Kalliopi Ladomenou
Department of Agriculture, School of Agriculture Food and Nutrition, Technological Educational Institute of Crete, 71004, Heraklion, Greece.
Anastasia Papadaki, The impact of potassium on downy mildew of cucumber and its leaf/soil nutritional status.(2018)SDRP Journal of Plant Science 2(3)p:133-139
Background: The interaction of plant nutrition and disease development has been well documented. Potassium (K) application has been widely recognized to decrease disease incidence in many host plants. However, contradictory reports about its impact on plant diseases have been ascertained therefore further research is needed for each host-pathogen system. The objective of the present study was to examine the effect of K levels on downy mildew severity and assess their impact on soil and nutritional status of cucumber plants.
Methods: K (200-700 mg L-1) was applied to plants grown in pots under greenhouse conditions through fertigation, according to a randomized block design with four replicates. Nitrogen (N) and phosphorus (P) were given to plants at stable concentrations. The cucumber leaves were inoculated with a zoospore suspension of Pseudoperonospora cubensis, the causal agent of downy mildew. Leaf and lesion area as well as nutrients in plant tissues and soil were determined.
Results: Plants treated with 300 and 400 mg L-1 of K demonstrated the lowest downy mildew severity. Above these rates, K supply led to downy mildew increase in cucumber leaves. The cubic regression curve can be fitted to the disease progress for downy mildew, irrespective of K doses. Leaf and soil nutrient content appeared to affect infection and leaf size. Reduced concentration of soil NH4-N was noticed in fertigation levels of 300 and 400 mg L-1 K and the lowest Mn content appeared in leaves of cucumber plants grown in 400 mg L-1 K.
Conclusion: The suppression effect of K on downy mildew had an optimum limit and beyond this, disease spread was observed. K enrichment of soil via potassium nitrate fertilizer could be considered as an efficient method for the control of downy mildew of cucumber plants.
Key words: disease control; lesion area; macro- micro- elements; Pseudoperonospora cubensis.
Downy mildew is known to be one of the most destructive diseases of cucumber crop, threatening its production and causing significantly economic losses. The obligate biotrophic oomycete P. cubensis is responsible for this disease and it has been reported in over 70 countries [1]. According to researches, 100% decrease of production yield can occur without the use of fungicides in young cucumber plants, while if fungicides are applied one week after the symptoms appearance, yield is reduced at about 50% [2].
In order to control P. cubensis spread, a lot of fungicides are used worldwide, however, the oomycetes develop resistance to them quite quickly [1]. Therefore, to reduce the use of chemicals, research interest has been focused on alternatives such as enhancement of the plant immunity [3]. Also, priority is given to methods that decrease plant disease spread and preserve safety for the human health [4]. Nutrients can affect the development of a disease by affecting plant physiology or pathogens, or both [5]. K has a vital role to the plant’s metabolic process as it possesses a regulator of many different physiological pathways [6] and it is correlated to the development of the epidermal cells’ thickness. The crop responses to K are generally not as clear as those of N and P [7].
Even though the function of K in the plant’s system seems to be well understood, there are many contradictory results of the way it affects the development of a disease. K is generally reported to reduce plant infection, although in other investigations it seems to intensify the disease symptoms. For example, K limited the growth of P. cubensis on cucumber [8] and increased the resistance of grapevine against P. viticola [3]. Sweeney et al. demonstrated that K fertilizer suppressed leaf rust severity of wheat [9]. A beneficial impact of NPK fertilizer against both a facultative and an obligate parasite on maize plants was also observed [10]. Furthermore, downy mildew of soybean decreased linearly with increasing K rate [11]. Similar conclusions have been obtained in the case of isabgol, where K found to reduce downy mildew severity [12]. Wang also outlined that the apple tree had a higher resistance against Valsa ceratosperma with the K supply [13].
In contrast, experiments about downy mildew of onion showed that K in different application rates was not effective in controlling the oomycetes Peronospora destructor [14]. Downy mildew in muskmelon was more severe on the plants grown in elevated K concentrations [15]. Furthermore, K fertilization of creeping bentrgrass crop enhanced the symptoms severity of Microdochium nivale [16]. Additionally, there are numerous host-pathogen cases in which the unfavourable impact of K on plant diseases have been documented [7]. Nevertheless, the relationship between P. cubensis and K nutrition of cucumber has not been examined extensively.
The present research aims (a) to investigate the influence of various K rates in the fertigation on downy mildew incidence of cucumber plants (b) to evaluate the temporal disease progress curves and (c) to examine the impact of K on soil and leaf nutrient content.
Experimental Design
Cucumber (Cucumis sativus L. cv. Knossos) plants were grown under average minimum12 oC and maximum 34 oC air temperature during spring in a greenhouse. Plants were grown in 7 L plastic pots containing a surface layer (0-20 cm) of soil [sandy loam, pH=7.4, electric conductivity (EC) =2.6 mS/cm, total calcium carbonate (CaCO3) 24%] sieved at 4 mm particle size.
Six K treatments (200, 300, 400, 500, 600 and 700 mg L-1) were established in a randomized block design with four replicates. Each plot consisted of 12 pots, which were kept 50 cm apart, resulting in 288 pots in total. The distance between the plots was 80 cm and the replicates were 100 cm from one another. The experimental concentrations applied to plants following transplanting, through fertigation (500 mL) according to the plants’ water requirements. N and P were supplied to the above solutions in a stable concentration (250 and 50 mg L-1, respectively), so the plants received a balanced nutrition. Ammonium nitrate (33.5-0-0), orthophosphoric acid (H3PO4) and potassium nitrate (13.5-0-46.2) fertilizers were used in relevant proportions to achieve the desirable concentrations. Standard cultivation practices were used without any insecticide/pesticide application.
Pathogen Inoculation
All the plants were artificially inoculated with the isolate P. cubensis. The inoculum (which was obtained from freshly sporulated lesions on leaves, two weeks after their inoculation) was adjusted to a concentration of 15´104 zoospores/mL with a hemocytometer. The inoculation was performed by placing five droplets (10μL each) of the above suspension on the upper surface of the fully expanded leaf- fifth leaf from the top of each plant (properly marked). The plants were then kept in a humidity chamber (average RH=95% and temperature at 25 oC) for 24 h in the dark for successive inoculation. The cucumber plants were transferred to the greenhouse in their final positions and a week later, the first symptoms were appeared.
Measurements
Digital images of all the infected leaves [n=4 independent leaves (in different plants) per treatment] were taken 10 days after inoculation, considered as the initial infection. Five series of images (samplings) were made at 11, 12, 13, 15, 17 and 21 days post inoculation. Both total leaf and lesion area were determined in each sampling using special software (Bersoft Image Measurement) and the results reported as measured area (in cm2).
In order to conduct plant tissue and soil analysis, leaf and soil samples (n=4, whereas each replication consisted of a bulk of 10-12 leaves/soil from different plants/pots respectively) were received two days before the plants’ inoculation. Wet oxidation was used to determine the nutrients in the leaf samples. Both ammonium and nitrate nitrogen of leaves were extracted with KCl while K was extracted from soil with ammonium acetate. The determination of ammonium and nitrate nitrogen was made by using the direct distillation method. Ca, Mg, Fe, Mn, Cu and Zn were determined with atomic absorption, P by the vanado-molybdo-phosphate yellow colour method and K by flame [17].
Experimental design and statistical analyses
Analysis of variance (ANOVA) was performed to detect differences in disease incidence as well as leaf and soil nutritional status between treatments. Duncan Multiple Range Test (DMRT) was used for comparison between means at the significant level p=0.05. Cubic regression analysis was used to model the leaf and lesion area (including the 21th day).
Downy mildew and leaf development under potassium fertilization
The infection area of cucumber leaves was significantly different due to K levels in the fertigation (Figure 1), however no correlation observed among K treatments and the infection. The application of 300 and 400 mg L-1 K resulted in the most statistically significant decrease of lesion area with regard to the rest treatments. The infection was enhanced in the lower and higher K levels (200, 500-700 mg L-1 respectively). The highest K concentration (700 mg L-1 K) provided the most severe disease spread, although significant differences observed only at sampling 3 (Figure 1C), with regard to the rest of the treatments. Statistically significant differences in lesion area at 21 days post inoculation, where the entire infected leaf, did not occurred (Figure 1F).
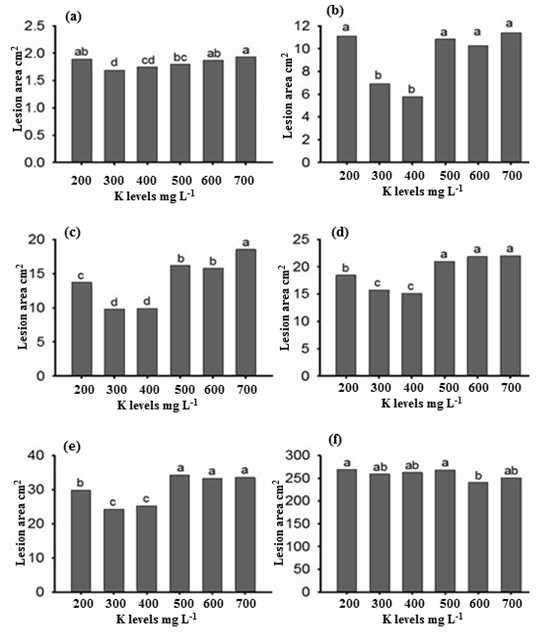
Figure 1. Effect of potassium levels on lesion area of cucumber leaves at the six samplings (a-f; days 11, 12, 13, 15, 17 and 21).
The beneficial effect of K on the development of plant diseases has been also ascertained for many other crops [18]. Furthermore, Amtmann et al. concluded that K supply lowered the severity of plant disease, in most cases [19], although many researchers [9, 20] indicated that the application of K led to disease reduction due to Cl‑ in the KCl fertilizer. In the present investigation, K significantly suppressed the infection caused by P. cubensis on cucumber plants when grown up to 400 mg L-1 K but beyond this level no further decrease suppression was noticed. Likewise, this threshold effect of K applications was stated by Dordas probably due to promotion of thicker outer walls of epidermal cells [5]. The significant increase of the downy mildew disease at the higher K doses (500 to 700 mg L-1) could be explained by possible alterations in nutrient balance or the N: K ratio, as N and P were given to cucumber plants at fixed concentrations of 250 and 50 mg L-1 respectively. The N: K ratio was implicated in severity of many diseases caused by various plant pathogens [20, 21].
Moreover, the importance of the K fertilizer type used on plant disease development has been discussed [20]. Therefore, KCl reported to decrease infection while similar rates of KNO3 enhanced severity of maize stalk rot and K2SO4 supply had little effect on it. The increase of downy mildew infection, found in treatments >400 mg L-1 K, which considered above recommended rates for cucumber cultivation, could be due to the KNO3 applied although cucumber plants belong to a different family group than maize.
Leaf area of cucumber plants did not show pronounced significant differences due to K fertigation treatments (Table 1). However, the application of 400 mg L-1 K formed the largest leaves in all the samplings although statistically significant differences were found only with the elevated K doses at the 3rd and 5th sampling (Table 1). Inorganic nutrient application has been broadly considered to have a direct, apparent influence in extending the leaf area of the plants grown in soil [22]. Although an increasing leaf area trend as the K dose was increased from 200 to 400 mg L-1 was noticed, higher K applications to soil, above the former threshold, tend to decrease the leaf size of cucumber plants possibly due to the significant extension of lesion area observed in these treatments. This could be interpreted by the leaf shrinkage as a consequence of high infected leaves.
Table 1. Leaf area of cucumber plants grown in different potassium levels, post inoculation with P. cubensis.
|
K (mg L-1) |
Leaf area (cm2) |
|
|
||||
|
Days post leaf inoculation |
|
|
|||||
|
11 |
12 |
13 |
17 |
21 |
|||
|
200 |
205.29 b1 |
247.07 a |
294.82 ab |
320.68 a |
339.24 b |
||
|
300 |
205.48 b |
247.71 a |
298.18 ab |
316.09 a |
339.98 b |
||
|
400 |
226.88 a |
265.57 a |
309.99 a |
316.61 a |
351.35 ab |
||
|
500 |
215.10 ab |
248.19 a |
291.62 ab |
298.76 ab |
342.84 ab |
||
|
600 |
210.88 ab |
244.48 a |
286.84 b |
283.49 b |
364.77 a |
||
|
700 |
215.34 ab |
252.45 a |
283.54 b |
287.14 b |
332.21 b |
||
1 Means within columns followed by different letters are significantly different (Duncan, P<0.05).
Overall, the leaf size increased with time in all K dose treatments. For this variable, the polynomial curve showed the best fit in all the cases. The disease progress curves (cubic model) are described (R2=0.99) as a consequence of different K doses and in more detail their equations were: Y=-24.7+33.3x-8.9x2+0.8x3 for the 200 mg L-1 K; Y=-23.2+30.2x-8.3x2+0.7x3 for the 300 mg L-1 K; Y=-22.5+29.3x-8.1x2+0.7x3 for the 400 mg L-1 K; Y=-25.3+33.5x-8.6x2+0.7x3 for the 500 mg L-1 K; Y=-23.4+30.8x-7.7x2+0.6x3 for the 600 mg L-1 K; and Y=-26.3+34.7x-8.7x2+0.7x3 for the 700 mg L-1 K treatment. Thus, both leaf and lesion area of cucumber plants, increased with time, regardless of soil K level. The greatest difference (up to 80.7 and 88.5 cm2) on day 21 in terms of leaf area produced and lesion area covered by infection was found in the 300 and 400 mg L-1 K application which highlights the slower lesion growth when comparing with leaf development (Table 1 and Figure 1).
Soil and leaf nutrients accumulation
All nutrient concentrations in cucumber leaves were in the sufficient range except Mn and Zn which were between the low levels [23]. The results indicated that K supply rates did not markedly influence the content of macronutrients Ca, Mg and P (Table 2) as well as the micronutrients Fe, Cu and Zn (Table 2) in plant tissues. However, elevated K doses in soil (500, 600 and 700 mg L-1) slightly increased K concentration in plant tissues [18]. K levels of 300 and 400 mg L-1 (concentrations that reduced downy mildew severity) exhibited the lowest NH4-N and ΝΟ3-Ν content in leaves, respectively, though no significant differences were found with regard to the rest K application doses (Table 2). Although N generally is associated with increase of crop susceptibility to pathogens, inverse relations with plant disease incidence has been reported [24]. Leaf Mn content was statistically decreased in the 400 mg L-1 K. Mn is among the most studied micronutrients with regard to its positive impact on the reduction of plant diseases [25] however here it had adverse effect.
Table 2. Nutrient concentration in leaves of cucumber plants, two days before inoculation with P. cubensis, grown in different potassium levels.
|
K level |
Nutrients in dry weight of leaves |
|
||||||||||||||||||||||||||||||||||||
|
Ca |
Mg |
K |
P |
NH4N NO3N |
|
|||||||||||||||||||||||||||||||||
|
mg L-1 |
% |
|
mg L-1 |
|
||||||||||||||||||||||||||||||||||
|
200 |
2.48 |
a1 |
0.95 |
a |
4.67 |
a |
0.47 |
a |
115.44 |
a |
218.50 |
c |
|
|||||||||||||||||||||||||
|
300 |
2.08 |
a |
0.85 |
a |
4.80 |
a |
0.46 |
a |
93.17 |
b |
259.38 |
abc |
|
|||||||||||||||||||||||||
|
400 |
2.45 |
a |
0.84 |
a |
4.84 |
a |
0.47 |
a |
107.53 |
ab |
210.96 |
c |
|
|||||||||||||||||||||||||
|
500 |
2.71 |
a |
0.95 |
a |
5.15 |
a |
0.50 |
a |
107.48 |
ab |
292.95 |
ab |
|
|||||||||||||||||||||||||
|
600 |
2.85 |
a |
0.90 |
a |
5.15 |
a |
0.51 |
a |
117.26 |
a |
298.42 |
a |
|
|||||||||||||||||||||||||
|
700 |
2.14 |
a |
0.85 |
a |
5.12 |
a |
0.50 |
a |
110.72 |
ab |
239.37 |
bc |
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||
|
K level |
Fe |
Mn |
Zn |
Cu |
|
|||||||||||||||||||||||||||||||||
|
mg L-1 |
mg L-1 |
|
|
|||||||||||||||||||||||||||||||||||
|
200 |
75.80 |
a |
40.99 |
a |
14.85 |
a |
8.72 |
ab |
|
|||||||||||||||||||||||||||||
|
300 |
84.06 |
a |
38.50 |
ab |
14.97 |
a |
9.30 |
ab |
|
|||||||||||||||||||||||||||||
|
400 |
74.77 |
a |
34.76 |
c |
14.07 |
a |
9.35 |
ab |
|
|||||||||||||||||||||||||||||
|
500 |
73.52 |
a |
37.13 |
bc |
15.16 |
a |
9.86 |
a |
|
|||||||||||||||||||||||||||||
|
600 |
76.06 |
a |
39.79 |
ab |
13.97 |
a |
9.50 |
ab |
|
|||||||||||||||||||||||||||||
|
700 |
73.92 |
a |
38.03 |
ab |
15.42 |
a |
8.22 |
b |
|
|||||||||||||||||||||||||||||
1 Means (n=4) within columns followed by different letters are significantly different (Duncan, P<0.05).
Findings revealed that K concentration gradually increased in soil, with statistically significant differences for almost all the treatments, as a consequence of the K fertigation levels (Table 3). Moreover, addition of K affected the NH4-N content in soil but not that of soil ΝΟ3-Ν. The concentration of soil NH4-N in the application with 300 and 400 mg L-1 K was significantly decreased when compared with the other K doses (Table 3).
Table 3. Macronutrients in soil fertilized with different potassium levels in cucumber plants, two days before P. cubensis inoculation.
|
K level mg L-1 |
K |
NH4N |
NO3N |
|
||||
|
|
mg L-1 |
|
|
|||||
|
200 |
190.86 |
d1 |
36.32 |
a |
36.83 |
a |
||
|
300 |
236.55 |
d |
24.91 |
b |
32.87 |
a |
||
|
400 |
312.30 |
c |
22.86 |
b |
34.26 |
a |
||
|
500 |
390.72 |
b |
37.06 |
a |
36.64 |
a |
||
|
600 |
464.98 |
a |
40.30 |
a |
38.43 |
a |
||
|
700 |
516.94 |
a |
39.16 |
a |
35.34 |
a |
||
Overall, the K content in the fertigation found to play an important role with regard to downy mildew infection, leaf size and nutrients content in cucumber leaves and soil. K levels of 300 and 400 mg L-1 in the fertigation reduced downy mildew and lowered the concentration of NH4-N in soil. Moreover, the use of 400 mg L-1 K decreased Mn content in plant tissues and formed the largest cucumber leaves. Hence, K fertilization with 300 and 400 mg L-1 via potassium nitrate is suggested for its suppressing impact on cucumber downy mildew caused by P. cubensis.
Miao J., Dong X., Chi Y., Lin D., Chen F., Du Y. and Liu P., 2018. Pseudoperonospora cubensis in China: Its sensitivity to oxathiapiprolin. Pesticide Pestic. Biochem. Physiol., 147: 96-101 PMid:29933999
View Article PubMed/NCBIHolmes G., Ojiambo P.S., Hausbeck M., Quesada-Ocampo L.M. and Keinath A.P., 2015. Resurgence of cucurbit downy mildew in the United States: a watershed event for research and ecxtension. Plant Dis., 99: 428-441
View ArticleEl-Sharkawy H.H.A., Abo-El-Wafa A.S.T. and Ibrahum A.A.S., 2018. Biological control agents improve the productivity and induce the resistance against downy mildew of grapevine. J. Plant Pathol., 100: 33-42
View ArticleFarouk S., Bebal B.E.A. and El-Sharkawy H.H.A., 2017. The role of some elicitors on the management of Roumy Ahmar grapevines downy mildew disease and it's related to inducing growth and yield characters. Sci. Hortic., 225: 646-658
View ArticleDordas C., 2008. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustainable Dev., 28: 33-46.
View ArticleGuest D. I. and Grant B.R., 1991. The complex action of phosphonates as antifungal agents. Biol. Rev. Cambridge Philos. Soc., 66: 159-187
View ArticlePrabhu A.S., Fageria N.K., Huber D.M. and Rodrigues F.A., 2007. Potassium and plant disease. In: Datnoff L.E., Elmer W.H. and Huber D.M., American Phytopathological Society (ed.), Miner. Nutr. Plant Dis., Minnesota, USA, p. 57-78. PMid:21124653 PMCid:PMC2980722
PubMed/NCBIRamezani M., Ramezani F., Rahmani F. and Dehestani A., 2018. Exogenous potassium phosphate application improved PR-protein expression and associated physio-biochemical events in cucumber challenged by Pseudoperonospora cubensis. Sci. Hortic., 243: 335-343
View ArticleSweeney D.W., Granade G.V., Eversmeyer M.G. and Whitney D.A., 2000. Phosphorus, potassium, chloride and fungicide effects on wheat yield and leaf rust severity. J. Plant Nutr., 23: 1267-1281
View ArticleReuveni M., Agapov V. and Reuveni R., 1996. Controlling powdery mildew caused by Sphaerotheca fuliginea in cucumber by foliar sprays of phosphate and potassium salts. Crop Protection, 15 (1), p. 49-53. 00109-3
View ArticleSilva O.C., Santos H.A.A., Dalla Pria M. and May-De Mio L.L., 2011. Potassium phosphite for control of downy mildew of soybean. Crop Prot., 30: 598-604
View ArticleMandal K., Saravanan R. and Maiti S., 2008. Effect of different levels of N, P and K on downy mildew (Peronospora plantaginis) and seed yield of isabgol (Plantago ovata). Crop Prot., 27: 988-995
View ArticleWang J., Li M., Zhang P. and Qiao Z., 1998. Correlation between potassium content in apple bark in the spring and the occurrence of Valsa canker. Acta Phytophylacica- Sin., 25(1), p. 61-64.
Araujo E., Goncalves P.A.S. and Alves D.P., 2017. Acibenzolar-S-methyl, and potassium and calcium phosphites are not effective to control downy mildew of onion in Brazil. Australas. Plant Dis. Notes, 12: 30
View ArticleBains S.S. and Jhooty J.S., 1976. Relationship between mineral nutrition of muskmelon of downy mildew caused by Pseudoperonospora cubensis. Plant Soil, 29:105.
Bier V.P., Persche M., Koch P. and Soldat J.D., 2018. A long term evaluation of differential potassium fertilization of a creeping bentgrass putting green. Plant Soil, 431: 303-316
View ArticlePage A.L., Miller R.H. and Keeney D.R., 1982. Chemical and microbiological properties. In: Methods of Soil Analysis, American Society of Agronomy Inc and Soil Science Society of America Inc (eds). Madison, Wisconsin, 1159.
Grewal H.S. and Williams R., 2002. Influence of potassium fertilization on leaf to stem ratio, nodulation, herbage yield, leaf drop and common leaf spot disease of alfalfa. J. Plant Nutr., 25: 781-795
View ArticleAmtmann A., Troufflard S. and Armengaud P., 2008. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant., 133: 682-691 PMid:18331404
View Article PubMed/NCBIHuber D. M., 1980. The role of mineral nitrition in defence. In: Plant disease-An Advanced Treatise, edited by Academic press Inc., New York
Prabhu A.S., Barbosa Filho M.P., Filippi M.C. and Zimmermann F.J.P., 1999. Relationship between potassium fertilization and panicle blast severity in upland rice. Pesqui. Agropecu. Bras., 34: 1729-1732
View ArticleReuveni R. and Reuveni M., 1998. Foliar fertilizer therapy-a concept in integrated pest management. Crop Prot., 17: 111-118 00108-7
View ArticleJones, J.B., Wolf B. and Mills A.H., 1991. Plant analysis handbook. Micro - Macro Publishing Inc., United States of America.
Huber D.M. and Thompson I.A., 2007. Nitrogen and plant disease. In: Mineral nutrition and plant disease, edited by Datnoff L.E., Elmer W.H. and Huber D.M., American Phytopathological Society, St. Paul, Minnesota, USA, p. 31- 44. PMid:17190481
PubMed/NCBIHuber D.M. and McCay-Buis T.S., 1993. A multiple component analysis of the take-all disease of cereals. Plant Dis., 77: 437-447
View Article