Weidong Yan
Tel.: +86 571 8795 1430, Fax: +86 571 87951895;
E-mail: yanweidong@zju.edu.cn
ORCID: 0000-0003-4209-006X (B. Liu), 0000-0002-5125-310X (W. Yan).
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 6
Page No: 830-839
Weidong Yan
Tel.: +86 571 8795 1430, Fax: +86 571 87951895;
E-mail: yanweidong@zju.edu.cn
ORCID: 0000-0003-4209-006X (B. Liu), 0000-0002-5125-310X (W. Yan).
Yundong Shao2, Bingbing Liu 1, Weidong Yan 1, *
1 Department of Chemistry, Zhejiang University, Hangzhou, 310027, China
2 Zhejiang Skyherb Biotechnologies Co., Ltd., Anji, 313300, China
Guan-Hu Bao(baoguanhu@ahau.edu.cn)
Xiuting Li(lixt@th.btbu.edu.cn)
Sumio Hayakawa(hayakawa_sci@icloud.com)
Junqiang Qiu(qjq000000@163.com)
Weidong Yan, Yundong Shao, Bingbing Liu, A NOVEL SYNTHESIS METHOD OF LIPOPHILIC EGCG PALMITATE AND EVALUATION FOR ITS ALPHA AMYLASE AND ALPHA GLUCOSIDASE INHIBTORY POTETIAL (2019) Journal of Food Science & Technology 4(6), 830-839
This work describes a green and novel approach to synthesize a lipophilic EGCG palmitate by chemical acylation of EGCG with palmitoyl chloride, and thus the significant elevated stability and bioavailability of EGCG are achieved. Various parameters affecting the acylation reaction process, such as the base, solvent, as well as the mole ratio of palmitoyl chloride have been studied, in order to optimize the acylation procedure. The compound 1 in the reaction mixture was separated by silica column chromatography. The compound 1 was confirmed by HPLC-MS and NMR and was identified as the EGCG palmitate (PEGCG). The stability of the EGCG palmitates and EGCG in different conditions were studies. Compared with EGCG, PEGCG showed better inhibition on the activities of α-amylase and α-glucosidase, with IC50 values of 1.64 and 0.22 µM, respectively. These observations open a novel and effective synthetic pathway for derivation of EGCG and suggest that the lipophilic PEGCG may act as an antidiabetic agent.
Keywords: EGCG; Acylation; α-Glucosidase inhibition; α-Amylase inhibition; Antidiabetic activities
(-)-Epigallocatechin gallate (EGCG), a compound with versatile bioactivities, is the most abundant tea polyphenol in green tea leaves. EGCG is reported to have specific health-promoting activities, including antioxidative, antibacterial, anticancer, cardioprotective and cholesterol-lowering effects (Amarowicz, Pegg, Dykes, Troszynska & Shahidi, 2005; Kurahashi, Sasazuki, Iwasaki, Inoue & Shoichiro, 2008; Kuriyama, Shimazu, Ohmori & Et, 2006; Raederstorff, Schlachter, Elste & Weber, 2003; Takechi et al., 2016). In addition, EGCG is found to possess antidiabetic activity. Wolfram et al. (2006) found that EGCG beneficially modified glucose and lipid metabolism in H4IIE cells and markedly enhanced glucose tolerance in diabetic rodents. Hepatic glucose production was repressed by EGCG through PI3K-dependent manner (Waltner-Law et al., 2002). Kamiyama et al. (2010) found that green tea supplementation increased insulin sensitivity and had anti-diabetic effects in animal models of insulin resistance.
Oral administration of EGCG has been reported to be first absorbed in the intestine (Kale et al., 2010). However, EGCG is unsuitable as an oral agent, since it is unstable in the alkaline intestine system. For example, studies have shown that EGCG is unstable in sodium phosphate buffer (pH 7.4), where 90% of it is lost in only 3 h (Sang, Lee, Hou, Ho & Yang, 2005). Moreover, EGCG is hydrophilic, which means it cannot easily pass through the lipid bi-layer cell membranes by passive diffusion, and will be easily inactivated or irreversibly oxidized to diverse reductions, thus leading to low availability by human body (Chow et al., 2005; Hong et al., 2002). Previous studies showed that only 0.01% of EGCG could be absorbed in rats given 56 mg of EGCG orally, and 0.32% of EGCG absorbed in human after a single oral intake of 97 mg (Nakagawa & Miyazawa, 1997).
In order to increase the metabolic stability and bioavailability of EGCG, various efforts have been made on the structural modification of EGCG, especially acylation of EGCG with fatty acyl chloride or fatty acid anhydride. Previous reports have shown the excellent bioactivities of EGCG fatty acyl esters, such as antioxidant (Matsumura et al., 2008), antitumor, and antiviral properties. Matsumura et al. (2008) reported EGCG-C16 suppressed tumor growth in vivo in colorectal tumor bearing mice compared to control, vector control (DMSO) and EGCG. EGCG palmitate was found to act as a topical antiviral agent for herpes simplex virus (HSV-1) (Oliveira et al., 2013). EGCG-PUFA esters showed good inhibitory effect against hepatitis C virus (HCV) (Zhong, Ma & Shahidi, 2012).
Acylation of EGCG can be attained by chemical synthesis (Mori et al., 2008; Zhong & Shahidi, 2011). or lipase catalysis (Zhu et al., 2013). Although lipase catalysis is regioselective, it has the disadvantage of being expensive, time-consuming and limited to mild conditions. Contrarily, chemical synthesis is fast and cheap, and with higher yields. However, it often uses toxic reagents such as pyridine (Zhong & Shahidi, 2011). Since Mori et al. (2008) indicated that the positions of the acyl groups of EGCG derivatives do not affect their antiviral activities, we determined to find a chemical synthesis method to obtain EGCG fatty acid esters by chemical synthesis.
In this work, the objectives were to better understand the relationships between various reaction variables (i.e. base (act as acid-binding agent to react with the hydrogen chloride released in the reaction), solvent, and mole ratio of the reagent) and the responses (conversion, product yield and composition) and to obtain the optimum condition for lipophilic EGCG derivatives. In addition, the pH, long term and thermal stability of the EGCG palmitates mixture were studied. Finally, the antidiabetic activity of the EGCG palmitate (PEGCG) was evaluated and compared with standard drug acarbose.
2.1. Materials
EGCG (purity 92%) was supplied by Pulimeidi Inc. (Hangzhou, China). Palmitoyl chloride, α-amylase and acetonitrile of HPLC grade were purchased from Sigma-Aldrich Co. (St. Louis, USA). α-Glucosidase was purchased from Sinopharm Group Chemical Reagent Co. (Shanghai, China). All of the other solvents and chemicals used were obtained from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China) and were of analytical grade.
2.2. Chemical acylation procedure
The method of acylation of EGCG was nearly the same as the litterateur (Zhong & Shahidi, 2011). But it is important modification that toxic pyridine was replaced by environment friendly bases, sodium acetate, sodium carbonate, and sodium bicarbonate. For an example, in a typical one-step synthesis protocol, 10 mmol EGCG (4.98 g) was added to 100 ml of solvents and heated in a water bath at 40 ºC. After complete dissolution, different bases were added to the solution. Subsequently, different mole ratios of palmitoyl chloride (1, 2, 3, and 4 mole ratio) were dropwise added to the solution, respectively, with stirring. The reaction was carried out in a stoppered flask in a water bath at 313 K . After 6 h, the reaction mixture was filtered and washed with 100 ml deionized water. Then 100 ml ethyl acetate was added to extract the mixtureand the organic phase was washed 2 times with deionized water thereafter. After that, the upper organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and then lyophilized to give yellow powdery products.
2.3. HPLC-MS analytical procedure
The composition of the acylated EGCG derivatives was determined by reversed phase HPLC-MS, which used an Agilent 1290 HPLC unit (Agilent Technologies, Palo Alto, USA) with a UV detector and a Cosmosil ODS C18 column (4.6 mm×250 mm, 5 µm; Nacilai Tesque Inc., Japan). Eluent A and eluent B were acetonitrile/water=10:90 (v/v), and acetonitrile/water=80:20 (v/v), each contained 0.2% formic acid (v/v). A gradient elution program was as follows: 0-20 min, linear gradient 0-10% B; 21-60 min, 88% B isocratic. The flow rate was 1.0 mL/min and fractions were detected at 275 nm. Mass spectroscopic (MS) analysis was performed using a 6460 Triple Quad MS detector system (Agilent Technologies, Palo Alto, USA). The eluent was introduced into an electrospray source at negative mode (desolvation temperature 325 ºC, capillary voltage 3.5 kV, nebulizer 45 psi). Argon was used as collision gas (collision energy 16 eV), and nitrogen was the desolvation gas (dry gas flow, 5 L/min).
The conversion of EGCG was calculated using the following equation:
Conversion (%) = (1 - contentEGCG ) x 100 (1)
Where contentEGCG is the content of EGCG, determined using the normalization of peak area of EGCG.
2.4. Purification and identification of EGCG palmitates
Flash column chromatography was used to separate individual EGCG palmitates. 10 g of EGCG derivatives were eluted on a silica column with a mixture of petroleum ether/ethyl acetate/acetic acid (3:1:0.05 v/v/v). The fractions were monitored by TLC (petroleum ether /ethyl acetate/acetic acid =1:1:0.05, v/v/v), and the predominant fraction was collected, washed three times with deionized water. Solvents were removed using a rotary evaporator. The structure of EGCG palmitates (referred to as compound 1, PEGCG) was identified by NMR analyses. The 1H and 13C spectra were recorded on a Bruker Avance III 400 MHz NMR spectrometer.
2.5. Stability of EGCG and EGCG palmitates in alkaline media
The stabilities of EGCG and EGCG palmitates in alkali condition were assessed since the intestine system is alkali (pH~7.2). Equivalent mole concentrations (1.3mM) of EGCG in ethanol/sodium phosphate buffer (PBS, 10 mM ) =80:20, and EGCG palmitates in ethanol/ sodium phosphate buffer (PBS, 10 mM) =80:20, were incubated in Pyrex test tubes (20×1.6 cm, i.d.) in open air without any agitation at 37 ºC, respectively; pH of each solution is 7.2. Aliquots (20 ml) of the incubation solution was periodically (1, 2, 5 h) sampled and subjected to HPLC analysis, respectively.
2.6. Comparison of long-term stability
To study the long term stability of EGCG and EGCG palmitates powder, 1 mole of the two powders were kept in open air at room temperature respectively. After 60 days, aliquots of the two powders were sampled and subjected to HPLC analysis, respectively.
2.7. Comparison of thermal stability
The thermal stability behaviors of EGCG and EGCG palmitates were investigated by a TG apparatus (SDT Q600 V20.9 Build 20). About 3.0 mg of each sample was used to perform the analysis by putting the powder on a TG pan. The sample was scanned from 30 to 400 ºC with the heating rate of 10 ºC/min under a nitrogen atmosphere.
2.8. α-Amylase inhibition assay
α-Amylase activity was measured using method described by Nampoothiri et al. (2011) with slight modifications. Briefly, equal volumes (100 μl) of sample (EGGC and acarbose dissolved in aqueous solution, and PEGCG in ethanol) and 1% starch solution in 10 mM sodium phosphate buffer (pH 6.9) were incubated in microtubes at 25 ºC for 10 min. A volume of 100 μl of porcine pancreatic α-amylase (0.5 mg/ml) was added to each tube and samples were incubated at 25 ºC for a further 10 min. The reaction was stopped with 200 μl of dinitrosalicyclic acid color reagent and tubes were incubated in boiling water for 5 min. Once samples were cooled to room temperature, 50 μl was removed from each tube and transferred to the wells of 96-well microplate. The reaction mixture was diluted by adding 200 μl of water to each well and absorbance was measured at 540 nm. Blank readings (no enzyme) were subtracted from each well and results were compared to the control. The pharmacological inhibitor, acarbose, was included as a positive control. Two controls were used, which is, control 1 for EGCG and acarbose (20 μl of PBS was added instead of the sample), and control 2 for PEGCG (20 μl of ethanol was added instead of the sample). The inhibition of α-amylase was calculated as follows:
%Inhibition = (1 - Asample/Acontrol ) × 100 (2)
Where Asample is the absorbance of sample and Acontrol is the absorbance of control sample.
2.9. α-Glucosidase inhibition assay
In vitro activity of α-glucosidase was determined in a 96-well plate using PNPG (4-Nitrophenyl β-D-glucopyranoside) as a substrate (Nampoothiri, Prathapan, Cherian, Raghu, Venugopalan & Sundaresan, 2011). A mixture of 100 μl of enzyme solution (1 U/ml α-glucosidase in 10 mM phosphate buffer) and 50 μl of different concentrations of samples (EGCG and acarbose dissolved in aqueous solution, and PEGCG in ethanol) was pre-incubated at 37 ºC for 10 min; then 50 μl of substrate solution (5 mM PNPG in 0.1 M phosphate buffer) was added to each well and incubated for 15 min at 37 ºC and the reaction was stopped by adding 80 μl of 0.2 M Na2CO3. The amount of PNP released was measured at 405 nm. The controls (no enzyme) were made by adding PBS instead of sample solutions. Two controls were used, which were, control 1 for EGCG and acarbose (20 μl of PBS was added instead of the sample), and control 2 for PEGCG (20 μl of ethanol was added instead of the sample). The inhibition of α-glucosidase was calculated as follows:
%Inhibition = (1 - Asample/Acontrol ) × 100 (3)
Where Asample is the absorbance of sample and Acontrol is the absorbance of control sample.
3.1. Chemical synthesis of EGCG palmitates
3.1.1. Effect of base on the conversions
In theory, every hydroxyl group of the EGCG molecule can react with palmitoyl chloride, thus the reaction product may be a complex mixture. Under the conditions employed, three EGCG monopalmitates were formed, which were confirmed by HPLC-MS analysis. This may be caused by the nonlinear structure of the long chain fatty acid, which rendered steric hindrance in the reaction, and poor reactivity due to the aromatic rings by electron withdrawing acyl groups (Zhu et al., 2014).
The yield of the product was expressed as weight of esters obtained/ weight of esters (g/g) expected if EGCG are fully reacted.
Different bases were screened and their conversion yields and the yields of compound 1 were recorded in Table 1. After reacting for 6 h at 40 ºC in acetone, the HPLC chromatogram of the products under four bases are shown in Fig. 1(1). Under the conditions that adding 2 mole ratio of palmitoyl chloride and 2 mole ratio of different bases, conversion yields of EGCG were almost the same. But the yields of compound 1 when using sodium acetate and tirethylamine were higher than using sodium carbonate and sodium bicarbonate. This might be that the former two are organic bases which have good solubility in acetone, and the latter two are inorganic bases which have poor solubility in acetone. Thus, there are different acid-binding effect.
Interestingly, the relative contents of three EGGC monopalmitates are different, as shown in Table 1. That is, with different bases used, the ratios of the three monopalmitates are different. Sodium acetate had the highest compound 1 content (retention time 44.05 min), with other bases showing lower compound 1 contents. And since retention times of the three monopalmitates are very similar, it is hard to achieve baseline separation. Therefore, for the convenience of isolation of the predominant compound 1 (PEGCG), sodium acetate was considered as the suitable base and was used in the following experiments.
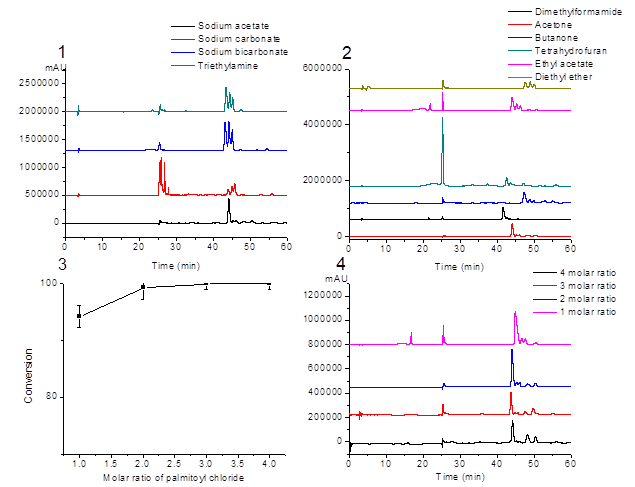
Figure 1. Effect of the conditions on the esterification reaction. 1) bases; 2) solvents; 3) and 4) mole ratio of palmitoyl chloride.
Table 1. Effect of bases on the esterification reaction.
|
Base |
EGCG conversion yield% |
Ratio of the three monopalmitates EGCG |
Total content of the three monopalmitates EGCG % |
Yield% of compound 1
|
|
Sodium acetate |
100 |
62.0:7.7:7.2 |
76.9 |
63.3 |
|
Sodium carbonate |
100 |
8.1:9.2:14.3 |
31.7 |
37.3 |
|
Sodium bicarbonate |
100 |
33.4:28.3:20.0 |
81.7 |
37.3 |
|
Triethylamine |
100 |
41.1:24.2:18.6 |
83.9 |
74.6 |
3.1.2. Effect of solvents on the conversions
The 1-octanol/water partition coefficient, log P, is recognized as one of the principal parameters to evaluate the lipophilicity of chemical compounds. Compound of higher lipophilicity has a higher log P value. The lipophilicity of the EGCG, palmitoyl EGCG and different solvents was computationally obtained by ALOGPS 2.1 (Tetko & Bruneau, 2004), listed in Table 2.
Under the conditions that 2 mole ratio of palmitoyl chloride and sodium acetate as base, the conversions yield of EGCG were almost the same in different solvents. However, the yields of EGCG esters varied depending on the solvents, showing the highest yield (63%) of compound 1 (PEGCG) in acetone (Table 2). The HPLC chromatograms of the reaction products in different solvents are shown in Fig. 1(2). From the log P values of solvents listed in Table 2, the relationship between log P with the yields of PEGCG cannot be found.
Table 2. Effect of solvent on the esterification reaction.
|
Solvent |
Log P |
EGCG conversion yield% |
Ratio of the three monopalmitates EGCG |
Total content of the three monopalmitates EGCG % |
Yield% of compound 1
|
|
|
Dimethylformamide |
-0.77 |
95.6 |
54.0:6.4:6.0 |
66.4 |
43.6 |
|
|
Acetone |
-0.29 |
100 |
62.0:7.7:7.2 |
76.9 |
63.3 |
|
|
Butanone |
0.41 |
100 |
41.3:9.3:7.2 |
57.8 |
35.9 |
|
|
Tetrahydrofuran |
0.35 |
92.5 |
9.4:4.2:1.7 |
15.3 |
12.7 |
|
|
Ethyl acetate |
0.74 |
92.3 |
26.5:11.6:8.8 |
46.9 |
50.2 |
|
|
Diethyl ether |
1.12 |
100 |
30.1:25.6:17.5 |
73.2 |
30.1 |
|
3.1.3. Effect of mole ratio of palmitoyl chloride on the conversions
In order to investigate the effect of mole ratio of palmitoyl chloride on the reaction process, the reaction temperature (40 ºC), reaction time (6 h), the quantity of EGCG (10 mM) and sodium acetate (40 mM) were held constantly, and only the mole ratio of palmitoyl chloride was changed. As shown in Fig. 1(3), the conversion of EGCG was 94.3% when the mole ratio of EGCG to palmitoyl chloride was 1:1. When the mole ratio of EGCG to palmitoyl chloride increased to 1:2, 1:3 and 1:4, the conversion of EGCG was enhanced to 99.3%, 100% and 100%. However, as shown in Fig. 1(4), the product compositions did not change, that is, the ratios of three EGGC monopalmitates were the same. It means that even when the palmitoyl chloride was in excess, the product was still a mixture of the three EGCG monopalmitates.
3.2. Identification of acylated EGCG derivatives
According to our previous study, the crude products of EGCG derivatives were separated into different fractions by silica column chromatography, and the predominant one was collected (compound 1). The three monopalmitates were identified by HPLC-MS (Fig. 2(1), peak 1-3). Theoretically, the molecular weight of EGCG momopalmitate is 696.8. As shown in Fig. 2(2), the m/z ion peaks at 695.3 detected at 36.4-37.8 min represented [M-H]- molecular ion of the EGCG palmitate. The peak at m/z 457.1 showed a mass loss of 238 from the molecular ion, which was the evidence of the [M-H-palmitoyl]- fragment.
Compound 1 yield: 51%; white crystals; m.p. 64.6 ºC. The 1H and 13C NMR analysis of compound 1 were as follows: 1H NMR (400 MHz, DMSO). δ 6.81 (s, 2H, H-2’’, H-6’’), 6.41 (s, 2H, H-2’, H-6’), 5.93 (d, J = 2.2 Hz, 1H, H-8), 5.82 (d, J = 2.2 Hz, 1H, H-6), 5.33 (s, 1H, H-3), 4.94 (s, 1H, H-2), 2.90 (m, 1H, H-4a), 2.65 (m, J = 16.0 Hz, 1H, H-4b), 2.27 (t, J = 7.4 Hz, 2H, H-p-2), 1.50 (m, 2H, H-p-2), 1.09-1.23 (m, J = 53.6 Hz, 24H, H-p-4 to 15), 0.85 (t, J = 6.8 Hz, 3H, H-p-16).
13C NMR (101 MHz, DMSO). δ 173.44 (C-p-1), 165.31 (C-11), 156.58 (C-7), 156.53 (C-9), 155.66 (C-5), 145.69 (C-3’’, C-5’’), 145.48 (C-3’, C-5’), 138.83 (C-4’’), 132.40 (C-4’), 128.65 (C-1’), 119.10 (C-1’’), 108.67 (C-2’’,C-6’’), 105.51 (C-2’, C-6’), 99.59 (C-10), 97.38 (C-8), 94.35 (C-6), 76.54 (C-2), 68.08 (C-3), 33.31 (C-p-2), 31.36 (C-p-3), 28.50-29.09 (C-p-4 to 13), 25.76 (C-4), 24.48 (C-p-4), 22.16 (C-p-15), 14.01 (C-p-16). P: palmitoyl.
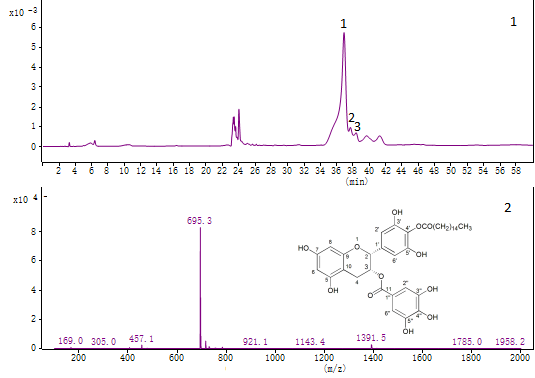
Figure 2. Analysis of EGCG derivatives by LC–MS. (1) The LC chromatogram of EGCG derivatives; (2) Mass spectra of PEGCG.
3.3. Comparison of stability in alkali condition
According to previous studies (Liu, 2019), EGCG was extremely vulnerable to degradation in alkaline solutions. If EGCG were not well absorbed, part of the mechanism may include selective degradation of EGCG in the intestine where pH becomes neutral or alkaline.
As shown in Fig. 3(1) and (2), under pH 7.2, EGCG turned into its dimers after 1 h, and completely degraded after 5 h. This is in accordance with a previous study that EGCG is completely destroyed at the end of 6 h incubation at pH 7.4 (Lun Su, Leung, Huang & Chen, 2003). While 93% of the EGCG palmitates was hydrolyzed to EGCG and its dimers after 1 h, and after 5 h 100% of the EGCG palmitates was hydrolyzed to EGCG and EGCG dimers. The above results indicate that the EGCG palmitates were significantly more stable than EGCG in alkali conditions, which means that the bioavailability of EGCG palmitates was elevated greatly.
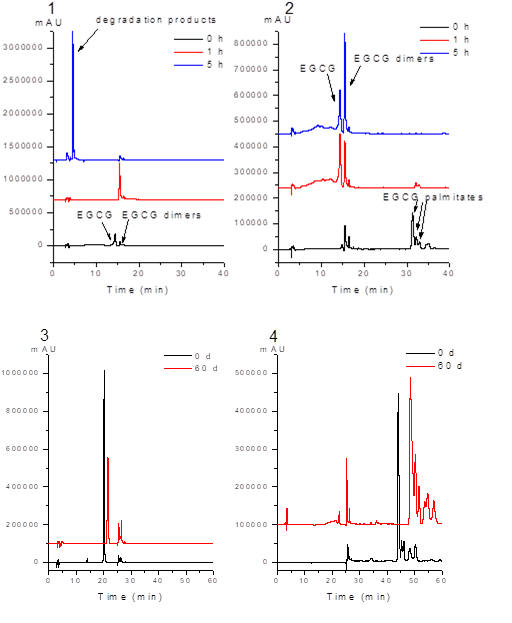
Figure 3. pH stability of (1) EGCG and (2) PEGCG in alkali condition; long- term stability of (3) EGCG and (4) PEGCG.
3.4. Comparison of long-term stability
After 60 days in the open air, the white-colored powder of EGCG turned to reddish-brown powder, while color of the EGCG palmitates was not changed. As shown in Fig. 3 (3), 69% of EGCG was remained and Fig. 3 (4), 78% of EGCG palmitate was remained after stored for 60 d at room temperature. The result indicated that EGCG palmitates was more stability than the EGCG.
3.5. Comparison of thermal stability
As is shown in Fig. 4, the thermal decomposition process of EGCG can be divided into two stages. The first stage at 63 ºC with a weight loss of 3.9% is attributed to the loss of water, and the second stage at 225 ºC with fast mass loss is attributed to decomposition. As for EGCG palmitates, the thermal decomposition process is divided into three stages. The first stage at 40 ºC with a weight loss of 4.8% is attributed to the loss of water, the second stage at 228.4 ºC with fast weight loss is attributed to partly thermal degradation, and the third stage at 301 ºC is the decomposition stage. The results of the TG analysis demonstrated that EGCG palmitates has no better thermal stability compared with EGCG.
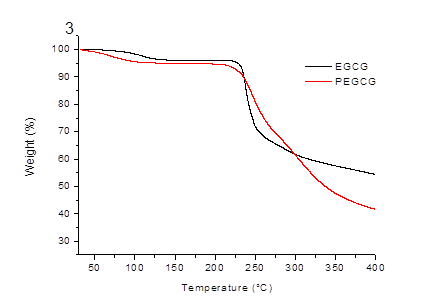
Figure 4. TG curves of EGCG and PEGCG.
3.6. Inhibition of α-amylase
According to various in vivo studies, inhibition of α-amylase and α-glucosidase is assumed to be one of the most effective approaches for diabetes care (Etxeberria, de la Garza, Campión, Martínez & Milagro, 2012). Several commercial anti-diabetic drugs, such as Acarbose, Miglitol, Voglibose, and Sitagliptin produce positive effects on glycemic values after food intake, but they have some gastrointestinal side effects, like abdominal cramping, flatulence and diarrhea (Hsieh, Shih, Chou & Chu, 2011; Li et al., 2011). Natural α-glucosidase and α-amylase inhibitors are therefore being investigated as new candidates to control hyperglycemia in diabetic patients, because they do not cause severe side effects and may also be beneficial in weight reduction in people consuming large amounts of starch (Bedekar, Shah & Koffas, 2010).
For α-amylase inhibition, as shown in Table 3, the inhibitory activities in descending order were acarbose > PEGCG > EGCG. It is notable that α-amylase inhibitory activity of EGCG palmitates is 4.5 times higher than EGCG and is comparable to acarbose.
Table 3. Inhibition of α-amylase and α-glucosidase.
|
Compound |
IC50 (µM) for α-Amylase |
IC50 (µM) for α-Glucosidase |
|
EGCG |
7.44 ± 0.05 |
11.50 ± 0.02 |
|
PEGCG |
1.64 ± 0.04 |
0.22 ± 0.03 |
|
Acarbose |
1.10 ± 0.03 |
0.15 ± 0.02 |
3.7. Inhibition of α-glucosidase
α-Glucosidase plays important roles in the digestion of carbohydrates and biosynthesis of viral envelope glycoproteins. α-Glucosidase inhibitors are promising candidates for their therapeutic effect against some diseases such as diabetes, cancer, hepatitis B and human immunodeficiency virus (HIV) (Sanae & Ali, 2016).
Similarly, the effect of α-glucosidase inhibition of PEGCG was greatly improved compared to EGGC. It was found that IC50 values of EGCG, PEGCG and acarbose were 11.50, 0.22 and 0.15 µM, respectively. α-Glucosidase inhibitory activity of PEGCG was 52 higher than EGCG, and 0.68 times than acarbose, indicating that the PEGCG strongly suppressed the α-glucosidase activity and have the possibility of controlling postprandial hyperglycemia.
Our results suggest a possibility of a green and efficient synthesis of lipophilic EGCG palmitate as an antidiabetic prodrug. In this study, the solubility of the base in the solvent, the polarity of the solvent, as well as the mole ratio of palmitoyl chloride on effect of the product yield and composition were elucidated. Furthermore, the pH stability in alkali condition, long term stability as well as thermal stability of EGCG and EGCG palmitates were studied, which showed that EGCG palmitates was significantly more stable than EGCG under these conditions, thus contributed to the convenience in storage. Moreover, the EGCG palmitate is capable of inhibiting α-amylase and α-glucosidase, suggesting its potential as an antidiabetic prodrug, and the oral toxicity tests of it needs to be further studied.
This work was supported by the National Key Research and Development Program of China (2017YFF0211000).
Amarowicz, R., Pegg, R. B., Dykes, G. A., Troszynska, A., & Shahidi, F. (2005). Antioxidant and Antibacterial Properties of Extracts of Green Tea Polyphenols. In F. Shahidi & C. Ho (Eds.), Phenolic Compounds in Foods and Natural Health Products (Vol. 909, pp. 94-106): American Chemical Society.
View ArticleBedekar, A., Shah, K., & Koffas, M. (2010). Chapter 2 - Natural Products for Type II Diabetes Treatment Advances in Applied Microbiology (Vol. 71, pp. 21-73): Academic Press. 71002-9
View ArticleChow, H. S., Hakim, I. A., Vining, D. R., Crowell, J. A., Ranger-Moore, J., Chew, W. M., Celaya, C. A., Rodney, S. R., Hara, Y., & Alberts, D. S. (2005). Effects of Dosing Condition on the Oral Bioavailability of Green Tea Catechins after Single-Dose Administration of Polyphenon E in Healthy Individuals. CLINICAL CANCER RESEARCH, 11 (12), 4627. PMid:15958649
View Article PubMed/NCBIEtxeberria, U., de la Garza, A. L., Campión, J., Martínez, J. A., & Milagro, F. I. (2012). Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. EXPERT OPINION ON THERAPEUTIC TARGETS, 16 (3), 269-297. PMid:22360606
View Article PubMed/NCBIHong, J., Lu, H., Meng, X., Ryu, J. H., Hara, Y., & Yang, C. S. (2002). Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. CANCER RESEARCH, 62 (24), 7241-7246. PMid:12819184
View Article PubMed/NCBIHsieh, S., Shih, K., Chou, C., & Chu, C. (2011). Evaluation of the efficacy and tolerability of miglitol in Chinese patients with type 2 diabetes mellitus inadequately controlled by diet and sulfonylureas. ACTA DIABETOLOGICA, 48 (1), 71-77. PMid:20963449
View Article PubMed/NCBIKale, A., Gawande, S., Kotwal, S., Netke, S., Roomi, W., Ivanov, V., Niedzwiecki, A., & Rath, M. (2010). Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallate from green tea extract. PHYTOTHERAPY RESEARCH, 24 (S1), S48-S55. PMid:19585479
View Article PubMed/NCBIKamiyama, O., Sanae, F., Ikeda, K., Higashi, Y., Minami, Y., Asano, N., Adachi, I., & Kato, A. (2010). In vitro inhibition of α-glucosidases and glycogen phosphorylase by catechin gallates in green tea. FOOD CHEMISTRY, 122 (4), 1061-1066.
View ArticleKurahashi, N., Sasazuki, S., Iwasaki, M., Inoue, M., & Shoichiro, T. F. T. J. (2008). Green Tea Consumption and Prostate Cancer Risk in Japanese Men: A Prospective Study. AMERICAN JOURNAL OF EPIDEMIOLOGY, 167 (1), 71-77. PMid:17906295
View Article PubMed/NCBIKuriyama, S., Shimazu, T., Ohmori, K., & Et, A. (2006). Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in japan: The ohsaki study. JAMA, 296 (10), 1255-1265. PMid:16968850
View Article PubMed/NCBILi, C., Hung, Y., Qamruddin, K., Aziz, M. F. A., Stein, H., & Schmidt, B. (2011). International noninterventional study of acarbose treatment in patients with type 2 diabetes mellitus. DIABETES RESEARCH AND CLINICAL PRACTICE, 92 (1), 57-64. PMid:21251726
View Article PubMed/NCBILiu, B.B. (2019). Preparation, characterization of lipophilic EGCG derivatives and study of the bioactivities and thermodynamic properties of the lipophilic EGCG derivatives. Dissertation, Zhejiang University.
Lun Su, Y., Leung, L. K., Huang, Y., & Chen, Z. (2003). Stability of tea theaflavins and catechins. FOOD CHEMISTRY, 83 (2), 189-195. 00062-1
View ArticleMatsumura, K., Kaihatsu, K., Mori, S., Cho, H. H., Kato, N., & Hyon, S. H. (2008). Enhanced antitumor activities of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives in vitro and in vivo. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS, 377 (4), 1118-1122. PMid:18983978
View Article PubMed/NCBIMori, S., Miyake, S., Kobe, T., Nakaya, T., Fuller, S. D., Kato, N., & Kaihatsu, K. (2008). Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: Effect of alkyl chain length. BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, 18 (14), 4249-4252. PMid:18547804
View Article PubMed/NCBINakagawa, K., & Miyazawa, T. (1997). Chemiluminescence-High-Performance Liquid Chromatographic Determination of Tea Catechin, (−)-Epigallocatechin 3-Gallate, at Picomole Levels in Rat and Human Plasma. ANALYTICAL BIOCHEMISTRY, 248 (1), 41-49. PMid:9177723
View Article PubMed/NCBINampoothiri, S. V., Prathapan, A., Cherian, O. L., Raghu, K. G., Venugopalan, V. V., & Sundaresan, A. (2011). In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. FOOD AND CHEMICAL TOXICOLOGY, 49 (1), 125-131. PMid:20951180
View Article PubMed/NCBIOliveira, D. A., Adams, S. D., Lee, L. H., Murray, S. R., Hsu, S. D., Hammond, J. R., Dickinson, D., Chen, P., & Chu, T. (2013). Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. FOOD AND CHEMICAL TOXICOLOGY, 52 (Supplement C), 207-215. PMid:23182741
View Article PubMed/NCBIRaederstorff, D. G., Schlachter, M. F., Elste, V., & Weber, P. (2003). Effect of EGCG on lipid absorption and plasma lipid levels in rats. The Journal of Nutritional Biochemistry, 14 (6), 326-332. 00054-8
View ArticleSanae, A., & Ali, B. A. M. B. (2016). Natural Alpha-Glucosidase Inhibitors: Therapeutic Implication and Structure- Activity Relation Ship. In (Vol.13, pp. 605-637).
View ArticleSang, S., Lee, M., Hou, Z., Ho, C., & Yang, C. S. (2005). Stability of Tea Polyphenol (−)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions. JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY, 53 (24), 9478-9484. PMid:16302765
View Article PubMed/NCBITakechi, R., Alfonso, H., Hiramatsu, N., Ishisaka, A., Tanaka, A., Tan, L. B., & Lee, A. H. (2016). Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. NUTRITION RESEARCH, 36 (3), 220-226. PMid:26923508
View Article PubMed/NCBITetko, I. V., & Bruneau, P. (2004). Application of ALOGPS to predict 1‐octanol/water distribution coefficients, logP, and logD, of AstraZeneca in‐house database. JOURNAL OF PHARMACEUTICAL SCIENCES, 93 (12), 3103-3110. PMid:15514985
View Article PubMed/NCBIWaltner-Law, M. E., Wang, X. L., Law, B. K., Hall, R. K., Nawano, M., & Granner, D. K. (2002). Epigallocatechin Gallate, a Constituent of Green Tea, Represses Hepatic Glucose Production. JOURNAL OF BIOLOGICAL CHEMISTRY, 277 (38), 34933-34940. PMid:12118006
View Article PubMed/NCBIWolfram, S., Raederstorff, D., Preller, M., Wang, Y., Teixeira, S. R., Riegger, C., & Weber, P. (2006). Epigallocatechin Gallate Supplementation Alleviates Diabetes in Rodents. The Journal of Nutrition, 136 (10), 2512-2518. PMid:16988119
View Article PubMed/NCBIZhong, Y., Ma, C., & Shahidi, F. (2012). Antioxidant and antiviral activities of lipophilic epigallocatechin gallate (EGCG) derivatives. Journal of Functional Foods, 4 (1), 87-93.
View ArticleZhong, Y., & Shahidi, F. (2011). Lipophilized Epigallocatechin Gallate (EGCG) Derivatives as Novel Antioxidants. JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY, 59 (12), 6526-6533. PMid:21526762
View Article PubMed/NCBIZhu, S., Li, Y., Ma, C., Lou, Z., Chen, S., Dai, J., & Wang, H. (2013). Optimization of lipasecatalyzed synthesis of acetylated EGCG by response surface methodology. Journal of Molecular Catalysis B: Enzymatic, 97, 87-94.
View ArticleZhu, S., Li, Y., Li, Z., Ma, C., Lou, Z., Yokoyama, W., & Wang, H. (2014). Lipase-catalyzed synthesis of acetylated EGCG and antioxidant properties of the acetylated derivatives. FOOD RESEARCH INTERNATIONAL, 56, 279-286.
View Article