Chenyan Lv
Email: 2019023@cau.edu.cn
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 7 ISSUE: 2
Page No: 456-467
Chenyan Lv
Email: 2019023@cau.edu.cn
Shuhan Dai, Jiachen Zang, Chenyan Lv*
College of Food Science & Nutritional Engineering, China Agricultural University, Beijing Key Laboratory of Functional Food from Plant Resources, Beijing 100083, China
Li J(lijouc@ouc.edu.cn)
Shuhan Dai, Jiachen Zang, Chenyan Lv, Distribution, purification, and delivery of astaxanthin in food system (2022) Journal of Food Science & Technology 7(2) :456-467
Highlights
A red-colored carotenoid pigment called astaxanthin possess high antioxidant capability, which is valued in biochemical research. Beside the pharmaceutical and cosmetic industries, it has a potential application in food, especially in foods that are beneficial to human health. Natural astaxanthin is abundantly distributed in food, especially in aquatic products, which is the secret of high-quality aquatic products. While the general extraction of natural astaxanthin has low purity and high cost, it is crucial to search a more economical, efficient and environmental-friendly purification method further. Meanwhile, due to the instability and low biological utilization of astaxanthin, the encapsulation of astaxanthin through delivery system plays a vital role in overcoming the challenges mentioned above and breaking the limitation of astaxanthin in food production and application.
Keywords: Astaxanthin, Distribution, Purification, Encapsulation, Application
Astaxanthin (AST), known as a kind of carotenoid presenting red color, was isolate from lobsters for the first time in 1937 [1]. The most outstanding characteristic of astaxanthin is excellent oxidation resistance [2], which typically owes to its unique structure. The key to achieving antioxidant capability is a carbon-carbon double chain conjugated olefin structure of astaxanthin, which is effective in eliminating reactive oxygen species and scavenging free radicals. On the other hand, the special structure of it also leads to its exceptionally sensitivity and instability to environment. Any factor included in light, high temperature and oxidative conditions can make astaxanthin degrade at a faster speed and weaken its bioactivity [3].
Astaxanthin is mostly employed in aquaculture and the food sector as a source of pigment supplements Adding astaxanthin to aquaculture feed, for example, can make the flesh of fish and shrimp appear pink, improving the quality of aquatic products and boosting sales [4]. Furthermore, astaxanthin is also applied in health supplements and pharmaceuticals. It provides anti-aging, anti-cancer, anti-inflammatory, and anti-diabetic benefits, all of which are improved by astaxanthin's antioxidant activity. Therefore, in the food industry, astaxanthin is used as both antioxidant and colorant.
From the viewpoints of sources, astaxanthin can be divided into natural astaxanthin and chemically synthesized astaxanthin. So far, natural astaxanthin was usually obtained through complex process, resulting in high cost, while effects of chemically synthesized astaxanthin on human health cannot be determined. At the same time, astaxanthin is deficient in stability, bioavailability and solubility, and its bioavailability can be improved by encapsulation. Therefore, research into the purification techniques and delivery systems in food would be more beneficial for the further development and utilization of astaxanthin in the food industry. This review article will describe the distribution of astaxanthin content in food, purification methods, encapsulation and applications to suggest ideas for further applications of astaxanthin in food.
2. Distributions of astaxanthin in food
Astaxanthin can be found in algae, bacteria, and phytoplankton in nature. Moreover, aquatic products contain a variety of astaxanthin. As a result of their long-term consumption of these algae, bacteria, and phytoplankton, some aquatic crustaceans, such as shrimp and crabs, have a red appearance and contain astaxanthin. Similarly, astaxanthin accumulates in fish that consumes crustaceans and algae. The accumulation and metabolism of carotenoids in marine species as they progress through the food chain [5] may be served as a reference for astaxanthin categorized as carotenoids (Figure 1).
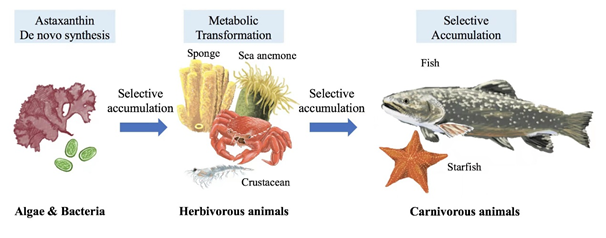
Figure 1. Accumulation & metabolism of astaxanthin in aquatic creatures through food chain [5].
Table 1. Astaxanthin content of wild and farmed salmonids [6]
|
Species |
Astaxanthin (mg·kg-1 flesh) |
|
|
Wild |
Farmed |
|
|
Arctic charr (Salvelinus alpinus) |
8.6 |
1–8 |
|
Atlantic salmon (Salmo salar) |
3-10 |
1-9 |
|
Chinook salmon (Oncorhynchus tshawytscha) |
5.4 |
- |
|
Chum salmon (Oncorhynchus keta) |
3-5 |
- |
|
Coho salmon (Oncorhynchus kisutch) |
10-21 |
- |
|
Masu salmon (Oncorhynchus masou) |
4.6 |
- |
|
Pink salmon (Oncorhynchus gorbuscha) |
4-7 |
- |
|
Rainbow trout (Oncorhynchus mykiss) |
24 |
12-25 |
|
Sockeye salmon (Oncorhynchus nerka) |
26-38 |
- |
Table 2. Astaxanthin content of other aquatic creatures
|
Species |
Astaxanthin |
Reference |
|
Black mussels from the Black Sea, Bulgaria |
1.42 ± 0.25 mg·kg-1 |
[58] |
|
Tiger prawn |
132.79 ± 1.5 mg·kg-1 |
[59] |
|
Farmed sea urchin Arbacia lixula egg |
27.0 ± 7.5 μg/mg |
[17] |
|
Snow crab |
119.6 mg·kg-1 |
[7] |
|
Crayfish |
153 mg·kg-1 |
[8] |
|
Shrimp |
147.7 mg·kg-1 |
[7] |
Ideal source of astaxanthin currently is a representational creature named Haematococcus pluvialis, which is considered to provide astaxanthin of high quality [6]. Salmonid, as well as algae, is another aquatic creature contained quite amount of astaxanthin. According to previous study, the amount of astaxanthin in the flesh of wild salmonid species varies considerably. Sockeye salmon Oncorhynchus nerka has the highest content about 38 mg/ kg, while chum salmon Oncorhynchus keta just has the lowest content about 3 mg/ kg [6]. Of course, differences existed in astaxanthin content between wild and farmed salmonid. In the light of EFSA (2005), astaxanthin content of wild Atlantic salmon Salmo salar (3-10 mg/kg flesh) is higher than farmed Atlantic salmon Salmo salar (1-9 mg/kg flesh). However, farmed salmonid can also have rich content of astaxanthin. For instance, the data shows that farmed rainbow trout has 12-25 mg/kg flesh astaxanthin [6]. In a word, salmonid fillets, either farmed or wild, may provide a rich dietary source of natural astaxanthin, according to the researchers [4].
Apart from that, astaxanthin is also distributed in shrimps, crabs and other crustaceans. It is found that shrimp contain 147.7mg/kg astaxanthin including 3.95% free form, 74.29% diester form and 19.72% monoester form [7]. Snow crab is reported to contain 119.6 mg/kg astaxanthin including 21.16% free form, 5.11% monoester form and 56.57% diester form [7]. When it comes to crayfish, it has even higher content of astaxanthin about 153 mg/kg astaxanthin including 40.3% free form and 49.4% ester form [8].
Taking shrimp as an illustration, the content of astaxanthin varies in different body components of shrimp. According to a study about carotenoid distribution in Indian shrimp [9], the distribution of astaxanthin and its esters in four distinct species of shrimp was summarized herein. The total content of astaxanthin and its esters in shrimp ranged from 7.42 to 14.01 µg·g-1 in meat, 25.81 to 124.47 µg·g-1 in head and 45.57 to 75.08 µg·g-1 in carapace (Table 3). From the comparison, it can be derived that astaxanthin is more concentrated in the head and carapace, and there are also diversities in the distribution of astaxanthin in distinct species of shrimp.
Table 3. Total astaxanthin and its esters (astaxanthin monoester & astaxanthin diester) content (μg·g-1) of different body components from four species of shrimp on average [9]
|
Species |
Meat (μg·g-1) |
Head (μg·g-1) |
Carapace(μg·g-1) |
|
Penaeus monodon |
14.01 |
39.24 |
75.08 |
|
Penaeus indicus |
7.42 |
25.81 |
45.57 |
|
Metapenaeus dobsonii |
7.61 |
34.83 |
63.97 |
|
Parapenaeopsis stylifera |
12.56 |
124.47 |
74.55 |
The forms of astaxanthin varied among different aquatic species too. For instance, it has been pointed out that the equal quantity of shrimp astaxanthin may have a stronger oxidation resistance than trout astaxanthin [10]. Researchers found that cis-astaxanthin has stronger oxidation resistance than trans-astaxanthin [11-12]. While wild rainbow trout contains about 94.6%-95% astaxanthin in all-trans isomer, shrimp species have higher cis-astaxanthin content [13]. For extraction of natural astaxanthin, most of them are currently made from Haematococcus pluvialis. Besides, it is also a fine choice to extract astaxanthin from shrimp waste. Reusing the discarded shrimp shells can make a considerable resource saving. Many studies have described that shrimp by-products like head and body bones can be reused for the extraction of astaxanthin [14-15]. It is also stressed by De Holanda and Netto [16] that astaxanthin as a valuable co-product.
In aquaculture industry, astaxanthin is often used as feed for aquaculture, which contributes to a better color appearance, high cumulative astaxanthin content in the body, more nutritional value and improved quality. In 2017, Cirino et al. found higher bioactivity in individuals harvested from sea urchin Arbacia lixula egg fed a special diet contained astaxanthin. High concentrations of astaxanthin (27 μg/mg) were showed by the result evaluated by HPLC analysis, which is around 15 times about astaxanthin content of wild sea urchins [17]. Researchers held the opinion that it is potential to consider farmed sea urchin (Arbacia lixula) as a new source of astaxanthin [17].
3. Purification of astaxanthin from food
The methods available for the purification of astaxanthin include solvent extraction, chromatography, supercritical CO2 extraction and other types of extraction methods (Figure 2)[2].
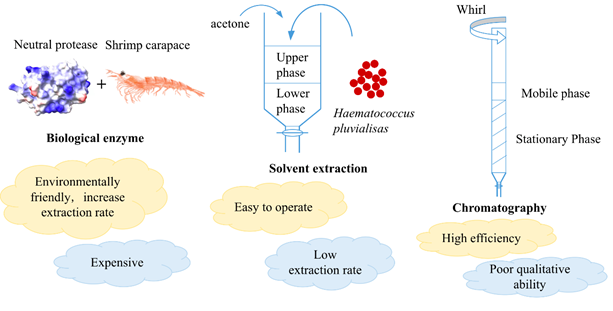
Figure 2. Methods for the purification of astaxanthin and the related advantages and disadvantages.
3.1. Solvent extraction
The most popular extraction solvents include acetone, methanol, isopropanol, petroleum ether, and n-hexane. Varying solvents have different extraction capabilities in general. Because of the variety of raw materials, the best extractant varies [9]. Ethanol, acetone, acid, sulphuric acid, isopropanol, n-hexane, acetone, and methylene chloride are major extractants in astaxanthin extraction. It is essential to select the most appropriate polar reagent due to the varied polarity of the solvent molecules. Acetone was chosen as the best AST extractant because it has several carbonyl groups in its structure that are quite comparable to those found in AST. The recovery of AST extracted with acetone was the highest (44%) compared with other solvents such as methanol, ethanol, and acetonitrile [18].
Furthermore, the extraction rates of single organic solvents and combined solvents were evaluated by researchers who found that the extraction rate of mixed solvents (isopropanol: hexane = 1:1) was higher [9]. Acetone and other organic solvents, on the other hand, have low boiling temperatures, are volatile, and poisonous, and can pose a safety and health risk during food processing. The benefit of this approach is that it is easy to operate, but it also has the disadvantage of low extraction rate. Around 2020, Casella et al. attempted to apply commercial activated carbon into the purification of astaxanthin from microalgae. It was shown that the use of activated carbon as a downstream technique for astaxanthin purification can be considered an effective strategy [19]. Additionally, they suggested that for improving astaxanthin purification, one strategy is to selectively desorb astaxanthin by adjusting the operating parameters and using a solvent with greater polar affinity.
3.2. Biological enzyme
Recently, researchers have tried a new method of purification. Treated by biological enzyme at first, astaxanthin is extracted from shrimp shells. Then, operate macroporous adsorption resin chromatography to continue the purification of astaxanthin [20]. After protease efficient and stable hydrolysis of proteins can destroy the esters formed by astaxanthin and fatty acids with proteins and bio-calcium to form a reticular cross-linked structure, thus releasing astaxanthin and astaxanthin esters, further improving the extraction rate. Macroporous resin is appropriate to produce astaxanthin in industry because it has the advantages of large adsorption capacity, strong adsorption capacity and high mechanical strength. XDA-8 resin was considered as the ideal material for the separation and purification of astaxanthin due to its excellent ability to improve purity of astaxanthin which could reach 87.34% after the secondary purification process [20]. The purification method has the benefits of high separation capacity and reusability.
The effective separation of astaxanthin from other impurities such as proteins is a potential breaking point for the development of astaxanthin purification methods to start with. In the future, the development of purification methods with greater separation capacity and more economical and environmentally friendly will make an essential contribution to the production of astaxanthin.
3.3. Chromatography
Chromatography is also an important approach for the isolation and purification of astaxanthin. In general, thin layer chromatography (TLC) is often used in research laboratories, which can isolate astaxanthin from Haematococcus pluvialis but has the shortage of being incapable of expanding the production volume [21-22]. Bauer & Minceva had successfully separated astaxanthin from the fermentation liquid using a liquid-liquid chromatography column [23]. In recent study, five astaxanthin monoesters were separated from microalgae Haematococcus pluvialis by using high performance counter-current chromatography (HPCCC), in which the lower phase of a two-phase solvent system was used as the mobile phase [22].
HPCCC can offer excellent loading capacity and recovery for it uses two immiscible liquid phases without any solid support [24]. One of the two liquid phases (stationary phase) is retained in the column by centrifugal force, while the other one (mobile phase) is pumped through the column. Separation is based on the difference in partitioning of each target compound between these two immiscible phases [25]. In their study, astaxanthin monoesters were isolated from H. pluvialis biomass by HPCCC using a multiple injection system, followed by a final purification process using high performance liquid chromatography (HPLC) [22]. They developed a multi-input HPCCC method by combining two elution modes (reversed phase and downstream) to further improve process productivity.
4. Encapsulation of astaxanthin and applications
Despite possessing variety of biological activities, AST has hydrophobicity and instability that form barriers to AST’s application. It is accepted that an effective way to increase bioavailability of AST is the delivery system, shown by previous studies [26]. Various delivery systems have been developed to enhance the stability and bioavailability of AST in recent years, such as liposome, nanoparticles, emulsion system and microcapsule (Figure 3).
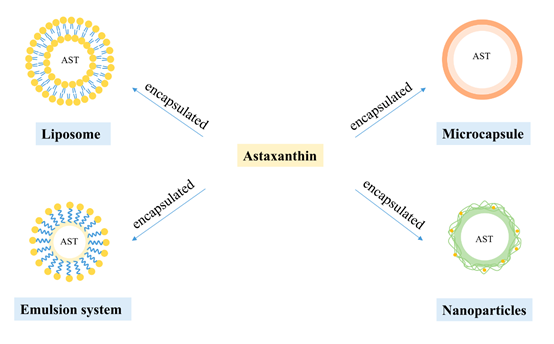
Figure 3. Encapsulation of astaxanthin by four different carriers.
4.1. Liposome
First described in the 1960s, lipid vesicles were later named liposomes [27]. Consisting of a hydrophilic head and a hydrophobic tail, liposome, microscopic phospholipid vesicle with a bilayer membrane, owns both hydrophilic and lipophilic properties. Encapsulating a wide range of bioactive compounds becomes possible because of this. As a lipid-soluble carotenoid, AST possesses multiple bioactivities and can be encapsulated by liposomes to improve its bioactivity [2,28]. Additionally, lipid-based delivery systems are regarded as promising carriers for natural products because liposomal molecules are considered orally safe and biodegradable [2].
Biocompatibility, sustained-release characteristic, target capacity and the potential to encapsulate hydrophilic and lipophilic components are specific advantages of liposomes [29]. However, liposomes also have certain disadvantages that they are extremely sensitive to the external environment, and unstable which are subjected to degradation, aggregation, fusion, and leakage of core materials. The physical stability of liposomes is related to the balance between attractive van der Waals forces and electrostatic repulsive forces [30]. To improve its stability, studies have been initiated in the field of electrostatic layer technology. Whey protein isolate (WPI) coatings for negatively charged ASX liposomes have been designed [31-32]. It has been shown that WPI coatings can adsorb on the membrane surface by electrostatic forces and effectively improve the physical stability of liposomes, preventing liposome aggregation, discoloration, and core material leakage [29]. Moreover, it was found that chitosan hydrochloride (CH) and lactoferrin (LF) are also available materials same as WPI which can be use as membrane surface modifiers to protect astaxanthin-loaded liposomes [33]. In addition, the development of nanoliposomes has also promoted this delivery system. Differing from the larger particle size of regular liposomes (around 1-100 μm), the small particle size of nanoliposomes allows them to have better penetration [28]. Pan et al. used soybean phosphatidyl choline as the material to encapsulate astaxanthin, which proved that nanoliposomes can enhance the thermal stability of astaxanthin obviously [34].
4.2. Nanoparticles
Nanoparticles, consisting of wall and core materials, are spherical in shape. Common nanoparticles can be divided into single-core nanoparticles, double-walled nanoparticles, multinuclear nanoparticles, and composite nanoparticles. In general, DNA, polysaccharides and proteins make up wall materials, which are different from liposomes but also have bio-friendly properties of non-toxicity and good affinity [2].
Nanoparticles as another method of encapsulation can improve properties of astaxanthin. Nanoparticles can enhance the stability and solubility of astaxanthin. Wang et al. built AST nanoparticles in 2017 [35]. To load AST, the wall material they used was a DNA/chitosan co-assembly system. After they built up the AST-loaded DNA/chitosan nanoparticles successfully by stirring wall material with AST ethanol solution and rotary evaporation, they analyzed both physicochemical characterization of prepared AST nanoparticles and in vitro experiments which showed that the AST nanoparticles were in uniform size and were easy to be preserved and absorbed by cells. Besides, the antioxidant activity of AST nanoparticles is promoted too because of its stronger clearance than natural AST [35]. Researchers also found different polysaccharides will have different effects on the stability of AST nanoparticles, which indicated that the molecular structure and chemical properties of polysaccharides are the significant reasons affecting the physical stability of AST nanoparticles [36]. In the research conducted by Jiang et al., the AST composite nanoparticles they prepared by using maize alcoholic protein and oligomeric chitosan showed an encapsulation rate of 94.34% [37]. It is positive that the UV and storage stability and DPPH radical scavenging ability of AST were significantly improved after encapsulation, and the solubility of AST in food matrices such as wine, apple cider vinegar and rice vinegar was also significantly improved [37]. Owing to the protection of maize alcoholic proteins and oligomeric chitosan against environmental stresses and their good solubility, the application of AST in the food industry is offered more opportunities to expand [2].
4.3. Emulsion system
Apart from two system mentioned above, another essential delivery system is the emulsion system, which uses the dispersion effect dissolved in the organic phase and aqueous phase [2]. Better storage stability is the specific advantage of emulsion system. Ribeiro et al. developed AST O/W (oil-in-water) emulsions by repeated premixed membrane emulsification with materials including the carrier oil, whey isolates and emulsifiers [38]. The process of premixed membrane emulsification is quite complicated and high demanding. Because of the sensitivity to high temperatures of AST, it is necessary to control the exposure time in the heat exchanger. The AST emulsion in a homogeneous particle size distribution is formed after three times membrane emulsifications. The research showed a result that the storage stability of AST emulsion is better than natural AST because the degradation rate of it is around 30% during 3-week storage [2].
Around 2019, people’s interest aroused by novel solid self-emulsifying delivery systems (S-SEDS). Self-emulsifying drug delivery systems (SEDS), one of the most promising technologies in the field of drug delivery especially for poorly soluble and poorly bioavailable drugs [39], is consist of an oil, a surfactant, a co-surfactant and a drug [40]. This type of system is usually dispersive and thus form fine oil-in-water emulsions or microemulsions (nanoemulsions) spontaneously [41]. It is accepted that the transition from liquid SEDS to solid formulations offers better stability [42], lower production costs, precise dosing, ease of handling and storage [43]. Most powders are prepared by spray drying technology. In a stream of hot air, the technique effectively converts the liquid phase into a dry granular phase. Considering that the properties of solids (e.g., low costs of production, easy process control, well stability and reproducibility, and good patient compliance) and self-emulsifiers (e.g., increased solubility and bioavailability), solid self-emulsifying delivery systems can enhance the bioavailability of a drug through the formation of a large specific surface area [44]. Moreover, a solidification technique with adsorption onto a solid carrier was used to prepare new formulation. In vitro studies on the physicochemical properties of astaxanthin S-SEDS are important for the application of astaxanthin in functional foods [44].
4.4. Microcapsule
The last delivery system is microencapsulation, which is an effective method to protect active part by coating materials. The coating materials are divided into natural products such as chitosan [45] and hydroxypropyl [46], and synthetic polymers such as poly lactic acid (PLA) and poly(lactic-co-glycolic) acid (PLGA), which play a key role in improving stability, enabling controlled release, and enhancing biocompatibility of encapsulated substance [47]. According to the benefits of microencapsulation, astaxanthin was encapsulated with chitosan [48] and hydroxypropy-b-cyclodextrin [46] to enhance its stability against high temperature, light, and oxygen conditions. The microcapsules consist of a wall material and a core material. The wall material forms a film around the core material, which protects the drug from unnecessary exposure [49]. However, the disadvantages these coating materials have are that it cannot prevent astaxanthin from damaging by acidic circumstance because of the lack of pH- responsiveness in matrixes [50]. Not only can microencapsulation improve the stability of astaxanthin, but also it can be allowed to be dispersed in aqueous media. Microencapsulation of light, oxygen, temperature and moisture sensitive substances is known to be widely used in healthcare products. Spray drying applied to microencapsulation is an effective method for the maintenance of natural dyes as it is inexpensive, has a short drying time, is flexible and gentle on thermally unstable compounds [51-52]. The research by Zhao et al. was able to successfully prepare astaxanthin microcapsules using spray drying technique with maltodextrin (MD) and gelatin as coating materials. It was also confirmed that the astaxanthin microcapsules retained their antioxidant activity after spray drying. The research shown that MD/gelatin and glycerol monostearate/sucrose fatty acid esters could be regarded as good composite carrier agents and emulsifiers for astaxanthin, which would increase the efficiency of microencapsulation and resistance to oxidation [53]. Moreover, Takeungwongtrakul et al. studied stability of encapsulated astaxanthin oleoresin using different wall materials at different ratios, which can be derived that the combination of astaxanthin and appropriate wall materials can improve oxidative stability of astaxanthin. After comparing the stability of astaxanthin oleoresin encapsulated by gum arabic (GA) and whey protein (WP) alone or in combination with maltodextrin (MD) or inulin (IN) as wall materials in conditions of different temperature and pH, they concluded that using 100% WP as wall material was the most stable plan among other plans in their research because of its better color presenting and higher antioxidant stability [54]. In conclusion, astaxanthin microencapsulation has attractive properties and low cost which can be applied in food industry [53].
5. Applications
Benefiting from its antioxidant capacity and red colour, astaxanthin can be applied in the food industry as a natural colorant and antioxidant. ESFA in 2014 stated that encapsulated astaxanthin novel food ingredient available in fermented liquid dairy products, non-fermented liquid dairy products, fermented soy products and juice beverages for healthy adults at a maximum admixture level of 1.6 mg of astaxanthin in 100 g or 100 ml [55]. Several drinks containing astaxanthin are available in Japan at dose levels ranging from 0.5 to 15 mg per serving [55]. On the other hand, Silva et al. studied the use of a microencapsulated A. platensis powder derived by spray drying added to yoghurt [56]. The results showed that the yoghurt combined with encapsulated A. platensis had a more uniform look, paler color, reduced strong odor and maintained the nutrient content and antioxidant activity of yoghurt during storage [56]. In addition, Mezquita et al. added astaxanthin to milk with different fat contents to determine the stability of astaxanthin in food matrices. It was demonstrated that the pigment maintenance of astaxanthin was inversely proportional to the fat content of the milk and showed low dispersion during storage indicating a high stability of astaxanthin in the matrix [57].
Astaxanthin is an emerging food supplement with immense potential for development and application. Its capacity to provide antioxidant properties and colour is what makes it so functional for application in food. The production and delivery of astaxanthin need to be further developed in the future to find more efficient, economical, environmentally friendly and sustainable methods. As a conclusion, continued research on the properties of astaxanthin application in food including safety, stability, color and taste are needed to be taken. Moreover, further innovations and developments are needed in the production of astaxanthin and astaxanthin applications in food.
Author contributions
Dai wrote the original manuscript, Zang reviewed and edited the manuscript; Lv supervised and revised the manuscript.
Conflict of interest
All authors declared no conflict of interest.
Kuhn, R., & Sörensen, N. A. (1938). Über astaxanthin und ovoverdin. Berichte Der Deutschen Chemischen Gesellschaft, 71(9), 1879-1888.
View ArticleZhao, T., Yan, X., Sun, L., Yang, T., Hu, X., He, Z., Liu, F., & Liu, X. (2019). Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends in Food Science & Technology, 91, 354-361.
View ArticleKittikaiwan, P., Powthongsook, S., Pavasant, P., & Shotipruk, A. (2007). Encapsulation of haematococcus pluvialis using chitosan for astaxanthin stability enhancement. Carbohydrate Polymers, 70(4), 378-385.
View ArticleLim, K. C., Yusoff, F. M., Shariff, M., & Kamarudin, M. S. (2018). Astaxanthin as feed supplement in aquatic animals. Reviews in Aquaculture, 10(3), 738-773.
View ArticleMaoka, T. (2011). Carotenoids in marine animals. Marine Drugs, 9(2), 278-293. PMid:21566799
View Article PubMed/NCBIEFSA. (2005). Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the safety of use of colouring agents in animal nutrition - PART I. General Principles and Astaxanthin. EFSA Journal, 3(12), 1-40.
View ArticleShahidi, F., & Synowiecki, J. (1991). Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. Journal of Agricultural and Food Chemistry, 39(8), 1527-1532.
View ArticleMeyers, S. P., & Bligh, D. (1981). Characterization of astaxanthin pigments from heat- processed crawfish waste. Journal of Agricultural and Food Chemistry, 29(3), 505-508. PMid:7251979
View Article PubMed/NCBISachindra, N. M., Bhaskar, N., & Mahendrakar, N. S. (2005). Carotenoids in different body components of indian shrimps. Journal of the Science of Food & Agriculture, 85(1), 167-172.
View ArticleSu, F., Huang, B., & Liu, J. (2018). The carotenoids of shrimps (Decapoda: Caridea and Dendrobranchiata) cultured in China. Journal of Crustacean Biology, 38(5), 523-530.
View ArticleLiu, X. B., & Osawa, T. (2007). Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochemical and Biophysical Research Communications, 357(1), 187-193. PMid:17416351
View Article PubMed/NCBIYang, C., Zhang, L. F., Zhang, H., Sun, Q. R., Liu, R. H., Li, J., Wu, L. Y., & Tsao, R. (2017). Rapid and efficient conversion of all-E-astaxanthin to 9Z- and 13Z-isomers and assessment of their stability and antioxidant activities. Journal of Agricultural and Food Chemistry, 65(4), 818-826. PMid:28071055
View Article PubMed/NCBISchiedt, K., Leuenberger, F. J., & Vecchi, M. (1981). Natural occurrence of enantiomeric and meso-astaxanthin .5. ex wild salmon (salmo salar and oncorhynchus). Chemischer Informationsdienst, 12(25), 449-457.
View ArticleArmenta-López, R., Guerrero, I. L., & Huerta, S. (2002). Astaxanthin extraction from shrimp waste by lactic fermentation and enzymatic hydrolysis of the carotenoprotein complex. Journal of Food Science, 67(3), 1002-1006.
View ArticleBi, W., Tian, M., Zhou, J., & Row, K. H. (2010). Task-specific ionic liquid-assisted extraction and separation of astaxanthin from shrimp waste. Journal of Chromatography B, 878(24), 2243-2248. PMid:20638917
View Article PubMed/NCBIDe Holanda, H. D., & Netto, F. M. (2006). Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. Journal of Food Science, 71(5), C298-C303.
View ArticleCirino, P., Brunet, C., Ciaravolo, M., Galasso, C., Musco, L., Fernández, T. V., Sansone, C., Toscano, A. (2017). The sea urchin arbacia lixula: a novel natural source of astaxanthin. Marine Drugs, 15(6), 187. PMid:28635649
View Article PubMed/NCBIRuen-ngam, D., Shotipruk, A., & Pavasant, P. (2010). Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Separation Science and Technology, 46(1), 64-70.
View ArticleCasella, P., Musmarra, D., Matteo, S. D., Chianese, S., & Molino, A. (2020). Purification of astaxanthin from microalgae by using commercial activated carbon. Chemical Engineering Transactions, 79, 2020.
Wang, L., Hu, J., Lv, W., Lu, W., Pei, D., Lv, Y., Wang, W., Zhang, M., Ding, R., & Lv, M. (2021). Optimized extraction of astaxanthin from shrimp shells treated by biological enzyme and its separation and purification using macroporous resin. Food Chemistry, 363, 130369. PMid:34274882
View Article PubMed/NCBIKamath, B. S., Srikanta, B. M., Dharmesh, S. M., Sarada, R., & Ravishankar, G. A. (2008). Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. European Journal of Pharmacology, 590(1-3), 387-395. PMid:18602387
View Article PubMed/NCBIFábryová, T., Tůmová, L., Correia da Silva, D., Pereira, D. M., Andrade, P. B., Valentão, P., Hrouzek, P., Kopecký, J., & Cheel, J. (2020). Isolation of astaxanthin monoesters from the microalgae Haematococcus pluvialis by high performance countercurrent chromatography (HPCCC) combined with high performance liquid chromatography (HPLC), Algal Research, 49, 101947.
View ArticleBauer, A., & Minceva, M. (2019). Direct extraction of astaxanthin from the microalgae haematococcus pluvialis using liquid-liquid chromatography. RSC Advances, 9, 22779-22789.
View ArticleBerthod, A., Maryutina, T., Spivakov, B., Shpigun, O., & Sutherland, I. A. (2009). Countercurrent chromatography in analytical chemistry (IUPAC technical report). Pure and Applied Chemistry, 81(2), 355-387.
View ArticleIto, Y. (2005). Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. Journal of Chromatography A, 1065(2), 145-168. PMid:15782961
View Article PubMed/NCBIKotake-Nara, E., & Nagao, A. (2012). Intestinal absorption and metabolism of carotenoid. Journal of Oil Chemists Society Japan, 12(10), 495-501.
View ArticleFarokhzad, O. C., & Langer, R. (2009). Impact of nanotechnology on drug delivery. ACS Nano, 3(1), 16-20. PMid:19206243
View Article PubMed/NCBILima, S., Freire, M., Oliveira, V., Solisio, C., & Lima, D. (2021). Astaxanthin delivery systems for skin application: a review. Marine Drugs, 19(9), 511. PMid:34564173
View Article PubMed/NCBIPan, L., Zhang, X., Fan, X., Li, H., Xu, B., & Li, X. (2020). Whey protein isolate coated liposomes as novel carrier systems for astaxanthin. European Journal of Lipid Science and Technology, 122(4), 1900325.
View ArticleSemenova, M. G., Antipova, A. S., Anokhina, M. S., Belyakova, L. E., Polikarpov, Y. N., Grigorovich, N. V., & Tsapkina, E. N. (2012). Thermodynamic and structural insight into the underlying mechanisms of the phosphatidylcholine liposomes - casein associates co-assembly and functionality. Food & Function, 3(3), 271-28. PMid:22358145
View Article PubMed/NCBIManca, M. L., Valenti, D., Sales, O. D., Nacher, A., Fadda, A. M., & Manconi, M. (2014). Fabrication of polyelectrolyte multilayered vesicles as inhalable dry powder for lung administration of rifampicin. International Journal of Pharmaceutics, 472(1-2), 102-109. PMid:24928129
View Article PubMed/NCBIManconi, M., Manca, M. L., Valenti, D., Escribano, E., Hillaireau, H., Fadda A. M., Fattal, E. (2017). Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. International Journal of Pharmaceutics, 525(1), 203-210. PMid:28438698
View Article PubMed/NCBIQiang, M., Pang, X., Ma, D., Ma, C., & Liu, F. (2020). Effect of membrane surface modification using chitosan hydrochloride and lactoferrin on the properties of astaxanthin-loaded liposomes. Molecules, 25(3), 610. PMid:32019205
View Article PubMed/NCBIPan, L., Wang, H., & Gu, K. (2018). Nanoliposomes as vehicles for astaxanthin: characterization, in vitro release evaluation and structure. Molecules, 23(11), 2822. PMid:30380797
View Article PubMed/NCBIWang, Q., Zhao, Y., Guan, L., Zhang, Y., Dang, Q., Dong, P., Li, J., & Liang, X. (2017). Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chemistry, 227, 9-15. PMid:28274463
View Article PubMed/NCBIAnarjan, N., & Tan, C. P. (2013). Physico-chemical stability of astaxanthin nanodispersions prepared with polysaccharides as stabilizing agents. International Journal of Food Sciences and Nutrition, 64(6), 744-748. PMid:23590613
View Article PubMed/NCBIJiang, G., & Zhu, M. (2019). Preparation of astaxanthin-encapsulated complex with zein and oligochitosan and its application in food processing. LWT, 106, 179-185.
View ArticleRibeiro, H. S., Rico, L. G., Badolato, G. G., & Schubert, H. (2006). Production of O/W emulsions containing astaxanthin by repeated premix membrane emulsification. Journal of Food Science, 70(2), E117-E123.
View ArticleYadava, S. K., Naik, J. B., Patil J. S., Mokale, V. J., & Singh, R. (2015). Enhanced solubility and bioavailability of lovastatin using stabilized form of self-emulsifying drug delivery system. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 481, 63-71.
View ArticleAboulfotouh, K., Allam, A. A., El-Badry, M., & El-Sayed, A. M. (2018). Role of self-emulsifying drug delivery systems in optimizing the oral delivery of hydrophilic macromolecules and reducing interindividual variability. Colloids Surf B: Biointerfaces, 167, 82-92. PMid:29627681
View Article PubMed/NCBIVasconcelos, T., Marques, S., & Sarmento, B. (2018), Measuring the emulsification dynamics and stability of self-emulsifying drug delivery systems. European Journal of Pharmaceutics and Biopharmaceutics, 123, 1-8. PMid:29133172
View Article PubMed/NCBIBhattacharjee, A., Verma, S., Verma, P. R. P., Singh, S. K., & Chakraborty, A. (2017). Fabrication of liquid and solid self-double emulsifying drug delivery system of atenolol by response surface methodology. Journal of Drug Delivery Science and Technology, 41, 45-57.
View ArticleMandić, J., Pobirk, A. Z., Vrečer, F., & Gašperlin, M. (2017). Overview of solidification techniques for self-emulsifying drug delivery systems from industrial perspective. International Journal of Pharmaceutics, 533(2), 335-345. PMid:28528850
View Article PubMed/NCBIMao, X., Sun, R., Tian, Y., Wang, D., Ma, Y., Wang, Q., et al. (2019). Development of solid self‐emulsification delivery system for the oral delivery of astaxanthin. European Journal of Lipid Science & Technology, 121(5), 1800258.
View ArticleBulmer, C., Margaritis, A., & Xenocostas, A. (2012). Encapsulation and controlled release of recombinant human erythropoietin from chitosan-carrageenan nanoparticles. Current Drug Delivery, 9(5), 527-537. PMid:22812393
View Article PubMed/NCBIYuan, C., Jin, Z., Xu, X., Zhuang, H., & Shen, W. (2008). Preparation and stability of the inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Food Chemistry, 109(2), 264-268. PMid:26003346
View Article PubMed/NCBIMartins, I., Barreiro, M. F., Coelho, M., & Rodrigues, A. E. (2014). Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chemical Engineering Journal, 245, 191-200.
View ArticleAmornset, T., Thanchanok, U., Luadthong, C., & Wanichwecharungruang, S. (2009). Preventing the thermal degradation of astaxanthin through nanoencapsulation. International Journal of Pharmaceutics, 374(1-2), 119-124. PMid:19446768
View Article PubMed/NCBIRé, M. I. (2006). Formulating drug delivery systems by spray drying. Drying Technology, 24(4), 433-446.
View ArticleZhang, X., Yin, W., Qi, Y., Li, X., & He, G. (2017). Microencapsulation of astaxanthin in alginate using modified emulsion technology: Preparation, characterization, and cytostatic activity. Canadian Journal of Chemical Engineering, 95(3), 412-419.
View ArticleGharsallaoui, A., Roudaut, G., Chambin, O., Voilley, A., & Saurel, R. (2007). Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Research International, 40(9), 1107-1121.
View ArticleRocha, G. A., Fávaro-Trindade, C. S., & Grosso, C. R. F. (2012). Microencapsulation of lycopene by spray drying: Characterization, stability and application of microcapsules. Food and Bioproducts Processing, 90(1), 37-42.
View ArticleZhao, X., Liu, H., Zhang, X., Zhang, G., & Zhu, H. (2019). Astaxanthin from haematococcus pluvialis microencapsulated by spray drying: Characterization and antioxidant activity. Journal of the American Oil Chemists' Society, 96(1), 93-102.
View ArticleTakeungwongtrakul, S., Benjakul, S., & H-kittikun, A. (2014). Wall materials and the presence of antioxidants influence encapsulation efficiency and oxidative stability of micro-encapsulated shrimp oil. European Journal of Lipid Science and Technology, 117(4), 450-459.
View ArticleEFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). (2014). Scientific opinion on the safety of astaxanthin-rich ingredients (astareal a1010 and astareal l10) as novel food ingredients. EFSA Journal, 12(7), 3757.
View ArticleSilva, S. C., Fernandes, I. P., Barros, L., Fernandes, A., Alves, M. J., Calhelha, R. C., Pereira, C., Barreira, J., Manrique, Y. A., Colla, E., Ferreira, I. C., Barreiro, M. F. (2019). Spray-dried Spirulina platensis as an effective ingredient to improve yogurt formulations: Testing different encapsulating solutions. Journal of Functional Foods, 60, 103424.
View ArticleMezquita, P. C., Huerta, B. E. B., Ramírez, J. C. P., & Hinojosa, C. P. O. (2015). Milks pigmentation with astaxanthin and determination of colour stability during short period cold storage. Journal of Food Science & Technology, 52, 1634-1641. PMid:25745234
View Article PubMed/NCBIMerdzhanova, A., Dobreva, D. A., & Georgieva, S. (2016). Nutritional evaluation of aquaculture mussels (M. galloprovincialis) from the Black Sea, Bulgaria. Ovidius University Annals of Chemistry, 27(1), 1-7.
View ArticleSwastawati, F. (2018). The changes of astaxanthin content and chemical characteristics of tiger prawn (Penaeus monodon) due to processing: boiling, smoking and frying. IOP Conference Series Earth and Environmental Science, 139(1), 012050.
View Article