Costantine F. Daher
E-mail: cdaher@lau.edu.lb
Phone: 009613998985
Fax: 0096195466262
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 7 ISSUE: 2
Page No: 442-455
Costantine F. Daher
E-mail: cdaher@lau.edu.lb
Phone: 009613998985
Fax: 0096195466262
Maria George Eliasa, Yves Najm Mrada, Bilal Nehmeha, Carole Dagherc, Mohamad Mrouehb, Robin I. Taleba, Costantine F. Dahera,*
aDepartment of Natural Sciences, School of Arts and Sciences, Lebanese American University, P.O. Box 36, Byblos, Lebanon.
bSchool of Pharmacy, Department of Pharmaceutical Sciences, Lebanese American University, P.O. Box 36, Byblos, Lebanon.
cSchool of Medicine, Lebanese American University, Byblos, Lebanon
Asmat Batool(semee_uca@yahoo.com)
Shuai Ren(ren.313@osu.edu)
Maria George Elias, Yves Najm Mrad, Bilal Nehmeh, Carole Dagher, Mohamad Mroueh, Robin I. Taleb, Costantine F. Daher, Wild Daucus Carota Oil Extract: An Innocuous Preventative and Chemotherapeutic Agent Against Chemically Induced Breast Cancer (2022) Journal of Food Science & Technology 7(2) :442-455
Daucus carota L. ssp. carota oil extract (DCOE) was shown to possess potent antitumor activity. This study investigates the chemopreventive and chemotherapeutic effects of DCOE against breast cancer induced by 7,12-Dimethylbenz(a)anthracene (DMBA) in rats. In the chemopreventive study, animals were pre-treated with DCOE (25 mg/kg; 1 week) followed by 14 weeks of treatment post-cancer induction with DMBA. In the chemotherapeutic study, animals were allocated into control, DCOE (25 mg/kg) and Cisplatin (2.5mg/kg) groups. When tumor size reached a diameter of 14-15 mm, the animals were divided into three groups each injected by either a vehicle (thrice a week), DCOE (thrice a week) or Cisplatin (twice a week), for 8 weeks to compare and assess the therapeutic value of DCOE with respect to Cisplatin and the vehicle. At 24 weeks, the experiment was terminated. DCOE pre-treatment protected against DMBA-induced toxicity and reduced tumor incidence. While 18% of control died few days post DMBA treatment, 100% survival in DCOE pre-treated group was observed just before week 8. In the chemotherapeutic experiment, treatment with either DCOE or Cisplatin caused significant inhibition of tumor growth. Unlike DCOE, Cisplatin caused drastic decrease in body weight with 75% and 100% death rates at week 6 and 8 respectively. DCOE produced a significant increase in Bax/Bcl2 ratio, Cytochrome c and cleaved caspase 3 proteins. DCOE may be considered a safe chemopreventive and/or chemotherapeutic agent, an effect that is partly due to the induction of the intrinsic apoptotic pathway.
Keywords:Daucus Carota, Wild carrot, ?-2-Himachalen-6-ol,7,12Dimethylbenz(a)anthracene, Breast cancer, Apoptosis
Abbreviations: DCOE, Daucus Carota Oil Extract; DMBA, 7,12-Dimethylbenz(a)anthracene; H&E, Haematoxylin and Eosin
ABSTRACT IMAGE
Cancer, which accounted for almost 10 million deaths in 2020, is one of the most prevalent diseases [1]. It is a multifactorial disease that targets various tissues in the body leading to more than 200 different types of cancers [2]. Among women, breast cancer is the most common type of cancer and a major cause of death and in 2020, it was responsible for about 15.1% of all cancer deaths [3]. Around 83% of all breast cancer patients survive a minimum of 10 years after their diagnosis [4]. Development and progression of breast tumor include a multifaceted sequence of events, that encompasses, dysregulation of cellular differentiation, excessive proliferation, and resistance to apoptosis. These processes are regulated by different genes, and lately ion channels have emerged as novel control mechanisms of primary and secondary tumorigenesis [5]. Although the exact causes of breast cancers are unclear, there are several factors that increase the risk of breast cancer occurrence such as fat and alcohol intake, smoking, weight, radiation, family history, genetic risk factors, among others [6]. Many approaches are used to control breast cancer depending on the phase and type of tumor, such as lumpectomy or mastectomy, chemotherapy and/or radiation therapy and gene therapy a designated effective technique, either by inactivating oncogenes or augmenting tumor suppressor genes [7].
Daucus Carota ssp. Carota, commonly known as “Wild Carrot”, part of the Umbeliferae family is a tall robust biannual spiny-fruited herb, which is widely distributed throughout the world [8-9]. Found and consumed along the Lebanese coast, its essential oil is used in folk medicine to protect against diabetes mellitus, gastric ulcer, muscle pain, back pain, urinary tract infections, cystitis, and prostatitis, along with liver enhancing, antilithic, carminative, antibacterial, anti-inflammatory, antiseptic and diuretic properties [10-13]. Rich in monoterpenes, sesquiterpenes, and phenylpropanoids, Daucus carota L. ssp. carota oil extract (DCOE) was reported to be cytotoxic against a panel of cancer cells including colon, breast [14] and acute myeloid leukaemia cells [15-17]. The pentane diethyl ether fraction of DCOE was shown to have similar in vitro anticancer activity, [18-19] which led to the isolation of its major compound, unique to the Lebanese wild carrot, β-2-himachalen-6-ol (HC), which is the active compound [14]. The cytotoxic effect of HC was confirmed against the previously tested cancer cells [20-21]. DCOE, pentane diethyl ether fraction and HC showed a protective effect against DMBA/TPA skin induced carcinogenesis in murine model [22-24].
DCOE has been previously shown to induce apoptosis through both intrinsic and extrinsic pathways, as well as via inhibition of the MAPK and PI3K pathways. Accordingly, the present study aims to evaluate the chemopreventive and chemotherapeutic effect of an HC-rich DCOE against 7,12-Dimethylbenz(a)anthracene (DMBA) induced breast cancer and elucidate the possible mechanism of action involved.
2.1. Chemicals and reagents
The 7,12-Dimethylbenz(a)anthracene (DMBA), Dimethyl Sulfoxide (DMSO), protease inhibitors cocktail and MG132, Benzonase nuclease, Tris-buffered saline with Tween 20® (TBST), sodium dodecyl sulfate (SDS), Tris-Base, Triton X-100, Glycine, Methanol,
Bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, USA). The BioRad protein assay kit, Laemmli sample buffer and ?-mercaptoethanol, horseradish peroxidase (HRP)-coupled secondary antibodies, stacking gel, resolving gel, semi-dry trans-blot turbo transfer system and ECL chemiluminescence kit were purchased from Bio-Rad (Hercules, CA, USA). PVDF membranes, (Thermo Fisher Scientific, Rockford, IL, USA). Actin, Bax, Bcl-2, Cytochrome C, and Cleaved Caspase-3 antibodies were purchased from Abcam (Cambridge, MA, USA) unless otherwise stated.
2.2. Plant collection and oil extraction
D. carota L. ssp. carota matured umbels were collected at the post flowering season between July and August from various coastal regions across Lebanon. The plant was distinguished according to the description in the “Handbook of Medicinal Herbs” [9] and approved by Dr. A. Houri, an expert in Lebanese plants at the Lebanese American University. Umbels were air dried in the shade, chopped into small pieces and extracted with 1:1 acetone: methanol upon soaking for 72 h [22]. The extract was filtered and then evaporated to dryness under reduced pressure at 40°C. The residual oil (3.5%) was dried over anhydrous sodium sulfate and stored in a closed amber bottle at -20°C until use.
2.3 Gas Chromatography and Mass Spectrometry (GC-MS)
DCOE was analysed using GC-MS (QP2010 SE) fitted with a fused silica HP5-MS 5% phenyl methyl siloxane cap column (30m·0.25mm i.d., film thickness 0.25) and directly coupled to the MS. The carrier gas was helium with splitless injection and the flow rate of 1.2 mL/min was applied. The temperature program was as follows: 2.0 min at 70°C, from 70°C to 130°C at 8°C/min and hold for 5 min, from 130°C to 180°C at 2°C/min and hold for 10 min, from 180°C to 220°C at 15°C/min and hold for 2 min, and then from 220°C to 280°C at 15°C/min and hold for 22 min. The components were identified by correlating their mass spectra with the literature (NIST11 and Wiley9). GC peak areas were used to compute percentage composition [25].
2.4 Experimental animal
Adult female Sprague Dawley rats (150 - 200 g; 2 months old) were obtained from the animal facility of the Natural Sciences Department at the Lebanese American University. Animals were maintained under optimum conditions (temperature 22 ± 2 °C; humidity 50 ± 5%; 12 h alternating light/dark cycle) and supplied with standard laboratory chow diet and water. All experimental protocols complied with the Guide for the Care and Use of Laboratory Animals (National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011).
2.5 Breast Cancer induction
Breast cancer was chemically induced with an 80 mg/kgbody weightdose of DMBA instilled intragastrically [26].
2.6 Chemopreventive and chemotherapeutic studies
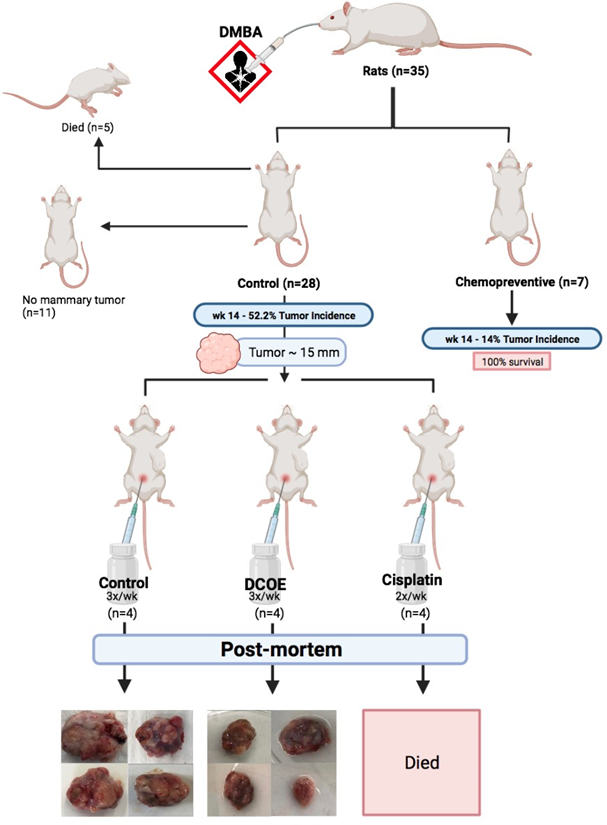
While all animals (n = 35) were subjected to breast cancer induction, seven animals were pre-treated with DCOE (25 mg/kg; dissolved in 50% DMSO and 50% saline) a week prior to cancer induction, and treatment continued twice weekly for a period of 14 weeks (chemoprevention). The remaining 28 animals were further allocated to various groups upon the development of a tumor with a diameter of approximately 14-15 mm: Control group (no treatment; vehicle; IP thrice a week), DCOE group (25 mg/kg body weight; IP; thrice a week for 8 weeks), and Cisplatin group (2.5 mg/kg body weight; IP; twice a week for 8 weeks). Tumor incidence was monitored by palpation over the study period, whilst body weight was measured monthly and mammary tumors were measured every 14 days. Animals were observed day-to-day, and all the necessitous data were recorded. All experimental groups were terminated by the 24th week post DMBA treatment and all animals were sacrificed by cervical dislocation after an overnight fast. At the time of necropsy, tumors were removed and measured in all dimensions with a Vernier calliper to confirm measures taken whilst in vivo. Isolated tumors were rinsed in physiological saline, dried and weighed. Experimental design is shown in figure 1.
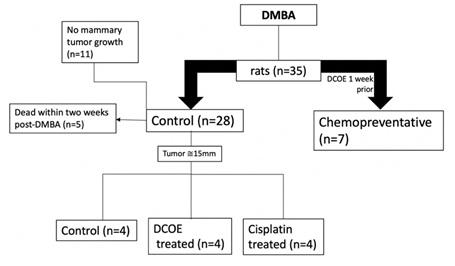
Figure 1. Experimental design showing animal allocation and treatment. Chemopreventive (n=7) animals were given DCOE a week prior to DMBA and continued for 14 weeks after DMBA. The remaining animals (n=28) given DMBA, were split according to tumor appearance of approximately 15 mm and moved to DCOE or cisplatin treated groups.
2.7. Histopathology
Breast tumors were fixed at the time of necropsy in 10% neutral formalin and then embedded in paraffin. Sections from paraffin-embedded blocks were taken and stained with Haematoxylin and Eosin (H&E). Slides were examined under light microscope (Zeiss, St. Louis, MO, USA) by a board-certified pathologist. Tumor samples were analysed for the presence of invasive carcinoma, inflammation or tumoral necrosis.
2.8. Protein extraction and western blot analysis
Breast tumor samples (200 mg) were cut into small pieces on ice and homogenized in 500 µL of radioimmunoprecipitation assay buffer (RIPA buffer; 50 mM of Tris-Cl, 1% Triton-X, 1% SDS, 1 mM disodium EDTA, 500 mM NaCl; pH = 7.4) containing protease inhibitors cocktail and MG132 (proteasome inhibitor), followed by Benzonase nuclease digestion for 15 min (Hoffman, 1990). The homogenate was then transferred into a new eppendorf tube and an equal volume of 2× Laemmli sample buffer and 3% (v/v) of ?-mercaptoethanol were added to the sample and heated at 100 °C for 10 min. The protein content of samples was determined using the Bio-Rad protein assay. Equal protein concentrations of each sample were then subjected to SDS-PAGE (5% stacking gel, 10% separating gel and running buffer: 0.3% Tris Base, 1.4% glycine, 20% SDS, pH = 8.3) at 70 V for 30 min and then at 90 V for 2 h. Post run, proteins were then transferred to a PVDF membrane with a semi-dry trans-blot turbo transfer system and using a transfer buffer (25 mM Tris base, 0.2M glycine, 20% methanol, pH 8.5) at 25V for 30 min. Blocking buffer (TBS containing, 0.1% Tween-20 and 5% BSA) was then used to block the membranes for 45 minutes. Primary rabbit monoclonal antibodies to Bax, Bcl-2, Cytochrome c and active Caspase-3 (cleaved caspase) proteins, and rabbit polyclonal antibodies to actin were used to probe the membranes for 2h. Afterwards, membranes were washed three times with TBS-Tween buffer (TBST) for 10 min each and then treated with horseradish peroxidase (HRP)-coupled secondary antibodies in blocking buffer for 1h. After washing with TBST for half an hour, proteins were detected using the ECL chemiluminescence kit and analysed with the image lab Software (Bio-Rad, Chemidoc Imaging Instrument, USA). [27-28]
2.9. Statistical analysis
Values of the different tested parameters within each group are presented as mean ± SEM. Data were analysed with GraphPad prism version 8. Statistical comparison between groups was assessed using one-way analysis of variance (ANOVA) with Bonferroni post hoc test and significant difference was considered when p-value < 0.05.
3.1. DCOE Chemical composition
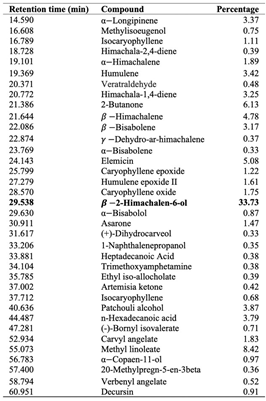
The composition of DCOE was assessed using GC/MS analysis. Data presented in Table 1 shows that the major compound identified in the oil is β-2-Himachalen-6-ol (33.73%). Other major identified compounds include methyl linoleate (8.26%), 2-butanone (5.95%), elemicin (4.93%), β-himachalene (4.63%), n-hexadecanoic acid (3.72%), humulene (3.27%), αlongipinene (3.22%), himachala-1,4-diene (3.09%) and β- bisabolene (3.01%).
Table 1. Main components of Daucus carota L. ssp. Carota. 0.2%
3.2. Induction of breast cancer
A gavage introduction of 80 mg DMBA/kg body weight resulted in tumor appearance within 10-14 weeks. Data have shown that 18% (5 out of 28) of the control group died within the first 2 weeks post DMBA treatment. Only 52.2% of remaining control animals (12 out of 23) developed breast tumor (figure 1).
3.3. Chemopreventive effect of DCOE
Pre-treatment of animals with DCOE extended animals’ survival till week 8 post-DMBA treatment (first 2 weeks in control) after which 1 animal (14% vs 18% in control) died (figure 2.A). Animal body mass were similar in both control and DCOE treated groups (figure 2.B). While the first tumor incidence started in the control group at week 10, the DCOE group had its first and only tumor at week 14 (figure 2.C). The 1/7 rats which showed tumor-like protrusion, revealed a different texture post-mortem. As it ruptured it showed to have a very thin membrane, releasing blackish-filled fluid, explained to be the result of necrotic tissue, as shown in supplementary results. Prior to burst, it was weighed to be 1.2 g vs. the average 13 g recorded from the control group, which showed a large polymorphic solid structure. The pre-treatment sample showed partial cystic degeneration with the formation of a cyst lined by tumor cells. The remaining areas in this specimen showed tubular and solid architecture with large areas of tumoral necrosis. As for the control, the sample presented tubules lined by malignant cells, featuring marked nuclear atypia, hyperchromatic nuclei, a high nuclearcytoplasmic ratio, and pleomorphism with large areas of tumoral necrosis (figure 3).
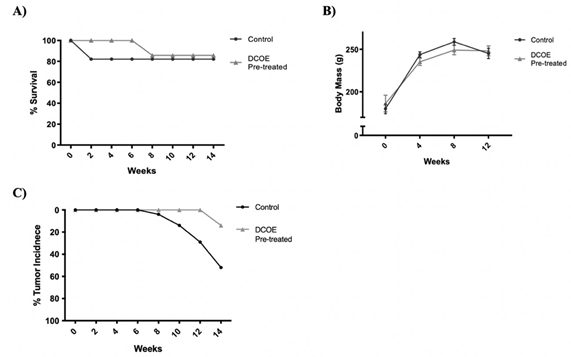
Figure 2. Chemopreventive effect of DCOE. Animals were pre-treated with DCOE one week before cancer induction with DMBA. Treatment with DCOE (25 mg/kg) was continued for 14 weeks. A) Percentage animal survival post DMBA administration. B) Mean body mass. C) Percentage tumor incidence. Data are mean ± SEM (n = 28 control; n = 7 DCOE).
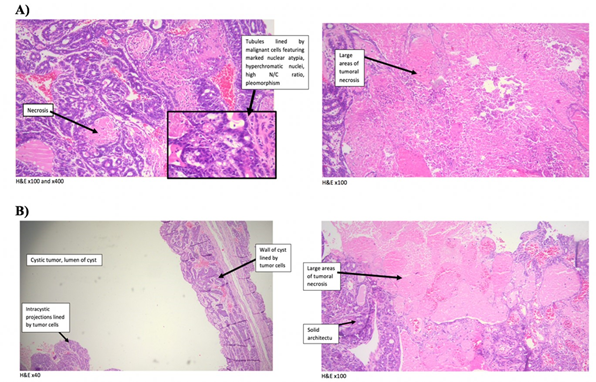
Figure 3. Histological analysis of mammary tumors induced by DMBA isolated at week 14. (A) Control (solid tumors) and (B) One-week pre-treated with DCOE (25 mg/kg) and then treatment continued for 14 weeks’ post DMBA (cystic tumor). Slides were stained with H&E and observed under the microscope.
3.4. Therapeutic effect of DCOE
Experimental animals were subjected to either DCOE or Cisplatin treatment (8 weeks) whenever the tumors reached a size of around 15 mm in diameter. Both control and DCOE treated animals showed 100% survival till the end of the treatment period; however, animals treated with Cisplatin started showing mortality at week 6 with 100% death recorded at week 8 (figure 4.A). DCOE treatment did not affect body weight, unlike cisplatin which showed a drastic decrease in body weight (22.9%) after four weeks of treatment (figure 4.B). The tumour volume of the control group, increased considerably at week 4 compared to that of the DCOE and Cisplatin treated groups (figure 4.C). At the end of the treatment period, the tumor mass of control was about 4 folds larger than that of DCOE group (figure 4.D and 5). Control and treated tumors were both classified as ductal carcinoma, with tubular and some solid architecture. The cells exhibited malignant features with marked nuclear atypia, hyperchromatic nuclei, high nuclear-cytoplasmic ratio, and pleomorphism. Tumoral necrosis was present in the three controls as a sign of the high turn-over of cells. Two of the DCOE treated samples showed severe chronic inflammation surrounding carcinomatous nests (figure 6).
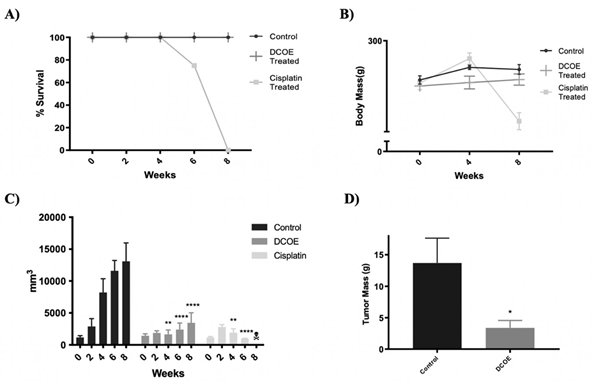
Figure 4. Chemotherapeutic effect of DCOE. Animals were treated with DCOE or Cisplatin for 8 weeks after tumor size reached roughly 15mm after cancer induction with DMBA. A) Percentage of animal survival during DCOE or Cisplatin Administration B) Mean body mass. C) Tumor volume. D) Tumor mass of control vs. DCOE group at the end of the experiment. Data are mean ± SEM (n=4 control; n=4 DCOE; n = 4 Cisplatin). * denotes p<0.05, *** denotes P <.001 and **** P <.0001 versus Control group, as measured by two-way ANOVA.
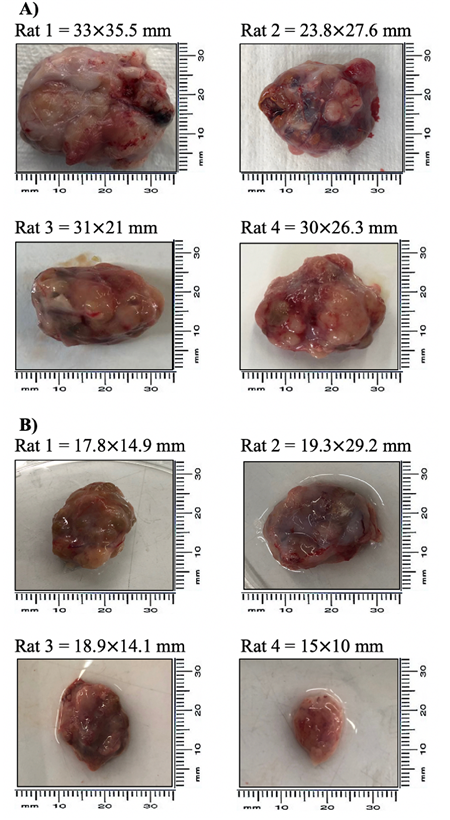
Figure 5. Rat mammary tumor images. Tumor size (mm) (A) DMBA group (n = 4) and (B) DCOE treated group (n = 4).
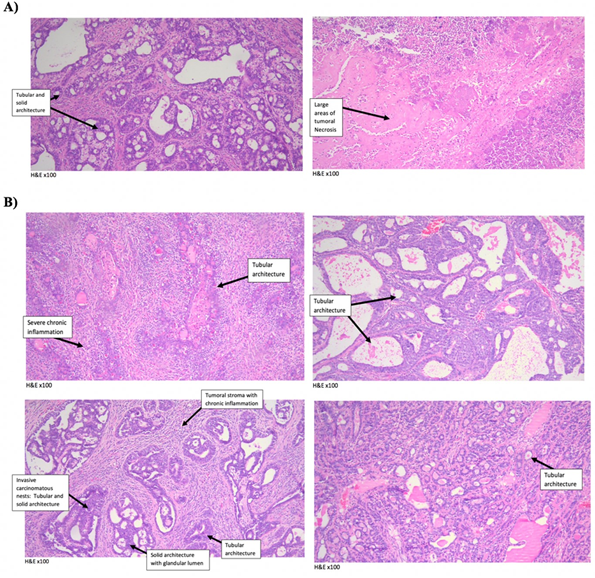
Figure 6. Histological analysis of mammary tumors induced by 7,12-dimethylbenz(a)anthracene (DMBA) treatment in rats removed after 8 weeks. (A) Control (solid tumors) and (B) DCOE (25 mg/kg) treated for 8 weeks starting at the size of approximately 15mm in diameter (solid tumors). Slides were stained with H&E and observed under the microscope.
3.5. Western Blot
The effect of DCOE on pro- and anti-apoptotic proteins was also investigated (figure 7). Data revealed a significant increase in Bax, Cytochrome C and cleaved caspase-3 protein expression with DCOE treatment. However, DCOE caused a significant decrease in Bcl2 protein expression. Calculation of the Bax/Bcl2 ratio showed a 14-fold increase upon DCOE treatment.
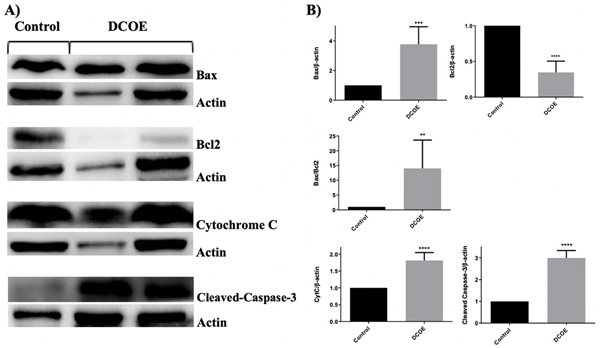
Figure 7. Western blot analysis of apoptosis related proteins in Tumors of SD rats treated with DCOE. (A) Effect of ?-2-himachalen-6-ol on the levels of Bax, Bcl-2, Cleaved Caspase-3 and Cytochrome C. ?-Actin levels were used as an internal protein loading control. Rats with tumors the size of 15 mm were treated with DCOE (25 mg/kg) crude oil three times a week and compared with control rats given one dose of 80mg/kg DMBA. (B) Densitometer intensity data of the proteins of each blot are presented as mean SEM from three independent experiments. ** P <.01, *** P <.001 and **** P <.0001 versus Control group, as measured by one-way ANOVA.
7,12-dimethylbenz(a)anthracene (DMBA), a polycyclic aromatic hydrocarbon (PAH) produces free radicals and oxygenated metabolites, subsequently forming oxidative stress, creating damaging effects by originating lipid peroxidation [29]. If PAH compounds are not bio-transformed into hydrophilic metabolites, which can be completely excreted, they can attack several tissues including breast [30]. Plentiful studies have proven that experimental mammary tumors in Sprague Dawley rats can be induced by DMBA oral administration [26, 31]. Previous studies have shown 100% tumor incidence via gavage introduction of 80 mg DMBA per kg body weight in Sprague Dawley rats [26, 32-33]. However, in the present study, only 52.2% of animals developed breast tumors 14 weeks post-treatment with a similar dose of DMBA. In another study, the use of a 400 mg/kg dose of DMBA caused a comparable percentage (40%) of breast tumors by week 14 [34]. Furthermore, another report showed that a 20 mg/kg dose of DMBA resulted in 85% tumor incidence by week 11 [35]. Unfortunately, no data is yet available to explain such large discrepancy in tumor incidence among various studies. Potential reasons could be attributed to difference in age of animals, body weight, environmental conditions, and strain of animal.
Earlier studies in our laboratory have shown that the Daucus carota pentane-based fraction arrests the cell cycle and increases apoptosis in MDA-MB231 breast cancer cells [36]. The pentane-based fraction also showed an in vitro anti-proliferative and apoptotic effects on Human colon adenocarcinoma (HT-29) cells by inhibiting the MAPK and PI3K pathways [25]. The DCOE was also tested in vitro on Acute Myeloid leukaemia (AML) cells and found to induce apoptosis in ML1, ML2 and U937 cells [17]. The preventative effects of DCOE have been studied in a DMBA-induced squamous cell carcinoma mice model, at which DCOE was introduced intraperitoneally, topically, or gavagly. Intraperitoneal and topical treatment exhibited significant reduction in tumor size and incidence [22]. β-2-Himachalen-6-ol, the major compound in DCOE, was also investigated in a similar animal model. Intraperitoneal and topical treatment caused a significant reduction in yield, incidence and volume of the papilloma. The β-2-himachalen-6-ol treated groups showed a threefold increase in survival in reference to cisplatin treated group. Results also showed pro and anti- apoptotic markers to reinforce apoptosis, as well as a significant surge in p53 protein [21]. Himachalen-6-ol treatment (topical; IP) of animals bearing tumors induced by a topical administration of DMBA/TPA caused a significant reduction in papilloma yield and volume. The significant tumor shrinkage was partially attributed to an apoptotic response to the inhibition of the MAPK/ERK and PI3K/AKT pathways [37].
In the present study, 25 mg/kg DCOE was selected for prevention and treatment studies, due to its significant anti-cancerous effect according to previous reports [23]. Using Dixon’s up and down model, the LD50 of β-2-himachalen-6-ol was previously found to be 6945 mg/kg, [20] which is in excess of 1000-folds higher than that of cisplatin reported to be 6-12 mg/kg bw in rodents [38]. Applying the same Dixon’s model to DCOE in the present study showed that the LD50 was not attained even after reaching a dose of 10,000 mg/kg.b.w. The latter dose did not show any signs of abnormal behaviour nor physical changes over a one-week course. DCOE was also found to protect against DMBA toxicity by delaying animal death (6-8 weeks) and significantly reducing tumor incidence compared with control group. While all control tumors showed solid architecture, the histopathological studies of the pre-treated animal tumor revealed cystic degeneration and necrosis, an effect that could be attributed to DCOE treatment. DCOE was also used to treat rats after growing tumors of roughly 15mm in diameter. DCOE treatment did not affect body weight, unlike those treated with cisplatin, which showed a drastic decrease in body weight after four weeks of treatment. While the control group exhibited a drastic significant increase in tumor volume at week 4, the DCOE treated group did not, indicating that DCOE was controlling tumor growth. A similar effect on tumor volume was also observed in the cisplatin treated group, however, cisplatin was highly toxic as all animals died by week 8 unlike DCOE where 100% animal survival was recorded. The antitumor activity of DCOE is most likely attributed to HC, which according to GCMS analysis constituted 33.73% of the oil extract. As former studies in our laboratory have demonstrated the major component of DCOE being HC, the compound was isolated and studied in a range of cancerous cell lines revealing its prospective to be a multi-mechanistic chemotherapeutic drug with high potency and safety [20]. HC has also been shown to be effective against a chemically induced skin carcinogenesis model, depicting safety, with no significant toxicity and efficacy with a significant decrease in tumor incidence and size. Tumor protein has also been studied post-mortem exhibiting apoptosis [21 & 37]. The antitumor activity of HC has also been observed in chemically induced colon cancer showing persistent chemotherapeutic potential and safety. [21]
In order to explore the potential mechanism behind the antitumor activity of DCOE treatment, apoptotic markers chiefly Bcl-2 and Bax, which are members of the Bcl-2 family of proteins, were assessed using western blot. While Bcl-2 is known to be an anti-apoptotic protein, Bax is recognized for promoting apoptosis [39-41]. Western blot analysis on tumor samples revealed that DCOE caused a significant increase in the Bax/Bcl-2 expression ratio favouring apoptotic cell death. This is in accordance with previous studies conducted on HC [23,37]. In addition, cytochrome c and cleaved caspase 3 protein expression showed a pronounced increase in DCOE treated animals, hence confirming the activation of the intrinsic apoptotic pathway. The caspase family, which includes caspase 3, has a key role in the regulation and execution of apoptosis, [25] which triggers the proteolytic cleavage and subsequent activation of several downstream protein targets leading to the activation of apoptosis [42].
Post-mortem, the tumors that were treated with DCOE after being around 15mm, were pathologically classified as ductal carcinoma, showing tubular and some solid architecture. The cells displayed abnormal cell nuclei, nuclear hyperchromasia; characteristic of malignancy [43]. Tumors also displayed a high nuclear-cytoplasmic (N/C) ratio, a great indicator of malignancy[44]. Tumors also show nuclear pleomorphism representing initial stages of breast carcinoma [45]. Two of the DCOE treated tumors represented severe chronic inflammation, which surrounded carcinomatous nests, which may be the result of DNA damage induction, [46] and a way to the control of tumor growth of the treated tumors, in comparison to the controls.
Tumors of the control group showed tumoral necrosis, an indicator of high turn-over of cells [46-47].
In conclusion, the present study supports that DCOE holds a potentially safer and more effective anti-tumor adjunct that could help in protecting from and treating breast cancer. The observed anticancer activity was shown to be partially attributed to a direct effect of DCOE on pro- and anti-apoptotic proteins that have key roles in inducing an intrinsic apoptotic pathway.
We would like to acknowledge financial support from the Department of Natural Sciences at the Lebanese American University and the Lebanese National Council for Scientific Research. Special thanks to Mr. Jean Karam for handling the animals.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethics approval
The Animal Care and Use Committee at the Lebanese American University have approved research ethics.
World Health Organization. Cancer in 2020. (2021, August 4)
View ArticleLodish H, Berk A, Zipursky SL. 2000. Tumor Cells and the Onset of Cancer. Molecular Cell Biology. 4th edition. New York: W. H. Freeman. 24(1).
Ginsburg O, Bray F, Coleman M P, Vanderpuye V, Dvaladze A, Gralow J, Yeates K, Taylor C, Oomman N, Krishnan S, Sullivan R, Kombe D, Blas MM, Parham G, Kassami N, Conteh L. 2017. The global burden of women's cancers: a grand challenge in global health. Lancet. 389(10071): 847-860. 31392-7
View ArticleSoerjomataram I, Louwman M W, Ribot J G, Roukema J, Coebergh JW. 2008. An overview of prognostic factors for long-term survivors of breast cancer. Breast cancer research and treatment. 107(3): 309-330. PMid:17377838
View Article PubMed/NCBITuszynski J, Tilli T M, and Levin M. 2017. Ion Channel and Neurotransmitter Modulators as Electroceutical Approaches to the Control of Cancer. Current pharmaceutical design. 23(32): 4827-4841. PMid:28554310
View Article PubMed/NCBIMcPherson K, Steel C M, and Dixon J M. 2000. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ (Clinical research ed.). 321(7261): 624-628. PMid:10977847
View Article PubMed/NCBIMorris L G, and Chan T A. 2015. Therapeutic targeting of tumor suppressor genes. Cancer. 121(9): 1357- 1368. PMid:25557041
View Article PubMed/NCBIMitich L. 1996. Intriguing world of weeds: wild carrot (Daucus carota L.). Weed Technol. 10: 455-457.
View ArticleVan Wyk B-E, Wink M. 2004. Medicinal Plants of the World. Briza Publications, Pretoria, South Africa, 124.
Tohme G, Tohme H, Illustrated Flora of Lebanon, National Council for Scientific Research, Beirut, Lebanon, 2014.
Barnes J. Herbal medicine. 1998. Pharm J. 260: 344-348.
Hoffman D, The New Holistic Herbal, 1990, Element Books Ltd., Rockport, Massachusetts.
Yahyaa M, Ibdah M, Marzouk S, Ibdah M. 2018. Profiling of the Terpene Metabolome in Carrot Fruits of Wild (Daucus carota L. ssp. carota) Accessions and Characterization of a Geraniol Synthase. J Agric Food Chem. 66(10):2378-2386. PMid:27673494
View Article PubMed/NCBIShebaby WN, El-Sibai M, Smith KB, Karam MC, Mroueh M, Daher CF. 2013. The antioxidant and anticancer effects of wild carrot oil extract, Phytotherapy. 27(5): 737-744. PMid:22815230
View Article PubMed/NCBIGonny M, Bradesi P, Casanova J. 2004. Identification of the components of the essential oil from wild Corsican Daucus carota L. using 13C- NMR spectroscopy. Flavour Frag J. 19(5): 424-433.
View ArticleStaniszewska M, Kula J, Wieczorkiewicz M, Kusewicz D. 2005. Essential oils of wild and cultivated carrots-the chemical composition and antimicrobial activity. J Essent Oil Res. 17(5): 579-583.
View ArticleTawil M, Bekdash A, Mroueh M, Daher C, Abi-Habib RJ. 2014. Wild carrot oil extract is selectively cytotoxic to human acute myeloid Leukemia cells, Asian Pac. J. Cancer Prev. 16(2): 761-767. PMid:25684522
View Article PubMed/NCBIShebaby WN, Bodman-Smith K, Mansour A, Mroueh M, Taleb RI, El-Sibai M, Daher CF. 2015. Daucus carota pentane-based fractions suppress proliferation and induce apoptosis in human colon adenocarcinoma HT-29 cells by inhibiting the MAPK and PI3K pathways, J. Med. Food. 18(7): 745-752. PMid:25599142
View Article PubMed/NCBIZgheib P, Daher CF, Mroueh M, Nasrallah M, Taleb RI, El-Sibai M. 2014. Daucus carota Pentane/Diethyl Ether fraction inhibits motility and reduces invasion of cancer cells. Chemotherapy. 60(5):302-309 PMid:26088465
View Article PubMed/NCBITaleb R I, Najm P, Shebaby W, Boulos JC, Demirdjian S, Hariri E, El-Sibai M, Daher C, Mroueh M. 2016. β-2-himachalen-6-ol: a novel anticancer sesquiterpene unique to the Lebanese wild carrot, J. Ethnopharmacol. (190): 59-67. PMid:27240746
View Article PubMed/NCBIDaaboul H E, Daher C F, Bodman-Smith K, Taleb R I, Daher CF. 2017. Antitumor activity of β-2himachalen-6-ol in colon cancer is mediated through its inhibition of the PI3K and MAPK pathways, Chem. Biol. (275): 162-170. PMid:28782499
View Article PubMed/NCBIZeinab R A, Mroueh M, Diab-Assaf M, Jurjus A, Wex B, Sakr A, Daher CF. 2011. Chemopreventive effects of wild carrot oil against 7, 12-dimethyl benz(a)anthracene-induced squamous cell carcinoma in mice, Pharm. Biol. 49(9):955-961. PMid:21777042
View Article PubMed/NCBIDaaboul H E, Daher CF, Taleb R I, Boulos J, Bodman-Smith K, Boukamp P, Shebaby WN, Dagher C, ElSibai M, Mroueh M. 2017. β-2-himachalen-6-ol protects against skin cancer development in vitro and in vivo. J. Pharm. Pharmacol. 69(11):1552-1564. PMid:28872682
View Article PubMed/NCBIShebaby W N, Mroueh M A, Boukamp P, Taleb R I, Bodman-Smith K, El-Sibai M, Daher CF. 2017. Wild carrot pentane-based fractions suppress proliferation of human HaCaT keratinocytes and protect against chemically-induced skin cancer, BMC Complement. Altern. Med. 17(1):36. PMid:28073348
View Article PubMed/NCBIShebaby W N, Daher C F, El-Sibai M, Bodman-Smith K, Mansour A, Karam MC, Mroueh M. 2015. Antioxidant and hepatoprotective activities of the oil fractions from wild carrot (Daucus carota ssp. carota), Pharm. Biol. 53(9):1285-94. PMid:25856705
View Article PubMed/NCBIGallo D, Giacomelli S, Cantelmo F, Zannoni GF, Ferrandina G, Fruscella E, Riva A, Morazzoni P, Bombardelli E, Mancuso S, Scambia G. 2001. Chemoprevention of DMBA-induced mammary cancer in rats by dietary soy. Breast Cancer Res Treat. 69: 153-164 PMid:11759821
View Article PubMed/NCBIElias M.G., Mehanna S, Elias E, Khnayzer R.S., Daher C.F. 2021. A photoactivatable chemotherapeutic Ru(II) complex bearing bathocuproine ligand efficiently induces cell death in human malignant melanoma cells through a multi-mechanistic pathway. Chemico-Biological Interactions. 348. PMid:34508709
View Article PubMed/NCBIMansour N, Bodman-Smith K, Khnayzer R, Daher C. 2020. A photoactivatable Ru (II) complex bearing 2,9diphenyl-1,10-phenanthroline: a potent chemotherapeutic drug inducing apoptosis in Triple Negative Human Breast Adenocarcinoma cells, Biol. Inorgan. Chem. PMid:33197429
View Article PubMed/NCBIRieder CR, Parsons RB, Fitch NJ, Williams AC. 2000. Human brain cytochrome P4501B1: immunohistochemical localization in human temporal lobe and induction by dimethylbenz(a)anthracene in astrocytoma cell line (MOG-G-CCM). Neurosci Lett. 278: 177-80. 00932-5
View ArticleKadir B, Uyumlu B, Satilmis B, Yildirim B, Yucel N, Demirtas H, Onkal R, Guzel RM, Djamgoz MB. 2012. Oxidative Stress in the in vivo DMBA Rat Model of Breast Cancer: Suppression by a Voltage- gated Sodium Channel Inhibitor (RS100642). Basic and Clinical Pharmacology and Toxicology Nordic Pharmacological Society. 111(2): 137-141.
Henry L, Narendra PS. 2006. Oral artemisinin prevents and delays the development of 7,12dimethylbenza[a]nthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 231: 43-4. PMid:16356830
View Article PubMed/NCBIAl-Dhaheri W S, Hassouna I, Al-Salam S, and Karam SM. 2008. Characterization of Breast Cancer Progression in the Rat. Annals of the New York Academy of Sciences. 1138(1): 121-131. PMid:18837892
View Article PubMed/NCBIKarnam K C, Ellutla M, Bodduluru L N, Kasala E R, Uppulapu SK, Kalyankumarraju M, Lahkar M. 2017. Preventive effect of berberine against DMBA-induced breast cancer in female Sprague Dawley rats. Biomedicine and pharmacotherapy = Biomedecine and pharmacotherapie. 92: 207-214. PMid:28544934
View Article PubMed/NCBIWalaszek Z, Hanausek-Walaszek M, and Webb T E. 1984. Inhibition of 7,12-dimethylbenzanthraceneinduced rat mammary tumorigenesis by 2,5-di-O-acetyl-D-glucaro-1,4:6,3-dilactone, an in vivo betaglucuronidase inhibitor. Carcinogenesis. 5(6):767-772. PMid:6202433
View Article PubMed/NCBIFunahashi H, Imai T, Tanaka Y, Tobinaga J, Kikumori T, Narita T, Takagi H. 1996. Suppressive effect of iodine on DMBA-induced breast tumor growth in the rat. Journal of Surgical Oncology. 61(3): 209-213. 1096-9098(199603)61:3<209::AID-JSO9>3.0.CO;2-F
View ArticleShebaby W N, Mroueh M, Bodman-Smith K, Mansour A, Taleb RI, Daher CF, El-Sibai M. 2014. Daucus carota pentane-based fractions arrest the cell cycle and increase apoptosis in MDA-MB-231 breast cancer cells. BMC complementary and alternative medicine. 14: 387. PMid:25300932
View Article PubMed/NCBIDaaboul H E, Dagher C, Taleb RI, Bodman-Smith K, Shebaby WN, El-Sibai M, Mroueh MA, Daher CF. 2018. The chemotherapeutic effect of β-2-himachalen-6-ol in chemically induced skin tumorigenesis. Biomedicine and Pharmacotherapy. 103: 443-452. PMid:29674280
View Article PubMed/NCBIMoayeri M, Wiggins J F, Lindeman R E, and Leppla, S. H. 2006. Cisplatin inhibition of anthrax lethal toxin. Antimicrobial agents and chemotherapy. 50(8): 2658-2665. PMid:16870755
View Article PubMed/NCBIKluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 275(5303): 1132-1136. PMid:9027315
View Article PubMed/NCBISedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ. 1995. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci. 92(17): 7834-7838. PMid:7644501
View Article PubMed/NCBIYoule R J, Strasser A. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9(1): 47. PMid:18097445
View Article PubMed/NCBILos M, Wesselborg S and Schulze-Osthoff K. 1999. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity,10(6):629-639 80062-X
View ArticleKim K Y, Kim J H, Hong A R, Seong M W, Lee KE, Kim SJ, Kim SW, Shin CS, Kim SY. 2016. Disentangling of Malignancy from Benign Pheochromocytomas/Paragangliomas. PloS one. 11(12) PMid:27992508
View Article PubMed/NCBIWhite F H, Jin Y, and Yang L. 1997. An evaluation of the role of nuclear cytoplasmic ratios and nuclear volume densities as diagnostic indicators in metaplastic, dysplastic, and neoplastic lesions of the human cheek. Histology and histopathology. 12(1): 69-77.
Dunne B, and Going J J. 2001. Scoring nuclear pleomorphism in breast cancer. Histopathology. 39(3): 259- 265. PMid:11532036
View Article PubMed/NCBILiu S, Edgerton S M, Moore D H, and Thor A D. 2001. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 7(6):1716-1723.
Lee S Y, Ju M K, Jeon H M, Jeong E K, Lee YJ, Kim CH, Park HG, Han SI, Kang HS. 2018. Regulation of Tumor Progression by Programmed Necrosis. Oxidative medicine and cellular longevity. 353. PMid:29636841
View Article PubMed/NCBI