Sastry S. Jayanty
Email: Sastry.jayanty@colostate.edu
Tel: 719-754-3594; Fax: 719-754-2619
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 6 ISSUE: 2
Page No: 345-355
Sastry S. Jayanty
Email: Sastry.jayanty@colostate.edu
Tel: 719-754-3594; Fax: 719-754-2619
Esam Emragi, David G. Holm and Sastry S. Jayanty*
San Luis Valley Research Center, Department of Horticulture and Landscape Architecture, Colorado State University,
0249 East County Road 9N, Center, Colorado 81125, USA
Les%c5%82aw Juszczak(rrjuszcz@cyf-kr.edu.pl)
Kumar N(nishantniftem@gmail.com)
Ghanem N(nassergo@agr.cu.edu.eg)
Sun H(sunhanjv@163.com)
Esam Emragi, David G. Holm, Sastry S Jayanty, The Effect of Field Heat Reduction Methods on Fresh and Processing Qualities of Red and Russet Potato Cultivars (2021) Journal of Food Science & Technology 6(2) :345-355
The effect of three field heat reduction methods, including temperature lowering stepwise (TLS), temperature lowering gradually (TLG), and temperature lowering immediately (TLI), after harvesting on the quality of Russet Norkotah 3 and red skin numbered line CO 07102-1R potatoes were investigated. The tubers were analyzed at harvest (0 time), when they reached 3 °C, and after 6 months of storage at 3 °C for physiological weight loss, firmness, wound healing, total phenolics content, reducing sugars, and color of french fries. The results indicated that weight and firmness losses were lower under the TLS reduction method after 6 months of storage than TLG and TLI methods. The weight loss in TLS is 12%, TLG 14% and TLI 17% in CO 07102-1R whereas Russet Norkotah TLS is 4%, TLG 6%, and TLI 8% respectively. Wound healing was more effective using the TLS reduction method, especially in Russet Norkotah 3. French fry color was lighter at harvest (USDA grade 0), while there were no differences in the color of French fries prepared from tubers stored under the TLS and TLG methods (both had USDA grade 2). French fries were darker for tubers from the TLI method (USDA grade 4). The total phenolic content and reducing sugars were significantly increased in the TLI field heat reduction method.
Keywords: Potato, Storage management, Wound healing, Weight loss, French fry
Potato (Solanum tuberosum L.) is the world’s fourth most important food crop and has considerable impacts on food security and economic growth (Zheng et al., 2020). Long-term storage is necessary to ensure year-round supply for the fresh market and processing industry (Wang et al., 2020a). The outermost layer of potato tubers is the periderm, which is susceptible to mechanical damage during harvest and handling, providing a pathway for pathogens, which infect tubers with several diseases, including pink rot, Pythium leak, late blight, soft rot, silver scurf, black dot, and early blight (Lulai, 2007; Barel & Ginzberg, 2008; Wang et al., 2020a). Potato tubers can heal their wounds by forming a wound periderm to prevent water loss and pathogen attack (Bernards, 2002). Wound healing is crucial in potato storage management, occurring in the first 2–3 weeks after harvest. Wound periderm formation is enhanced under a warm and humid environment and depends on various factors, such as cultivar, crop management, and tuber maturity(Wang et al., 2020b; Artschwager, 1927).
To reduce bruises during mechanical harvest, pulp temperatures of the tubers should be between 10-15 °C. Physiological tuber weight loss is maximum during initial storage time due to skinning and bruising. Potato piles in bins are ventilated to allow the wound healing process by reducing the field heat. Inside the potato storage bins, relative humidity should be maintained at above 95% for suberization. The temperature difference between the bottom and top of the piles was kept at a minimum (1-2 ºC) to avoid condensation on tubers with continuous ventilation inside the bin. Wound healing is necessary to reduce physiological weight loss in tubers. This is achieved by keeping tubers at optimal temperatures for an adequate duration to allow for rapid wound healing but minimize water loss and disease development (Wang et al., 2020b).
Phenylpropanoid metabolism plays a significant role in the healing process of tubers, providing suberin and lignin compounds involved in wound periderm formation (Dastmalchi et al., 2014). Formation of the wound periderm begins with cell division and suberin deposition in response to injuries (Dastmalchi et al., 2014). Primary suberization and wound periderm formation preferably occur at high relative humidity (RH) of 95–98%, in the presence of oxygen, and at temperatures of 10.0–15.6 °C. Warmer temperature enhances suberization, whereas cold temperatures slow it down (Lulai & Suttle, 2009; Morris et al., 1989). Consequently, the wound healing period at relatively high temperatures should be long enough to allow faster healing, but not too long to affect quality characteristics such as disease growth, weight loss, shelf-life, and frying quality (Daniels-Lake et al., 2014, Ellis et al., 2019).
The potato processing industry requires tubers with acceptable consumer preference qualities. In 2017, 50% of the potatoes produced in the United States were utilized as processed products, and approximately 45% were frozen French fry products (https://www.agmrc.org/commodities-products/vegetables/potatoesreference). Significant tuber quality considerations include sugar levels, dry matter content, cultivar, maturity stage, storage conditions, and exposure to the reconditioning process. The high content of reducing sugars in the tuber induces the Maillard reaction between reducing sugars and amino acids responsible for undesirable darkening in fried potato products.
Low reducing sugar levels are preferred as they result in the desired lighter color of fried potato products. Potato tuber reducing sugar levels are influenced by several factors, such as genotype, growing conditions, agronomic practices, and storage conditions (Agblor & Scanlon, 2002). The tubers’ physiological maturity is important, with immature tubers containing more sugar than mature tubers. During cold sweetening of potatoes stored at low temperatures, starch degradation occurs primarily through starch phosphorylase action and eventually through various enzymatic reactions, resulting in the accumulation of reducing sugars (Abong et al., 2009). Tubers are usually warmed for approximately two weeks, minimizing the reducing sugars before frying to reduce darkening (Li et al., 2007). Kim and Lee (1992) indicated that potato reconditioning improved chip color by reducing non-enzymatic browning during frying at high temperatures. Tubers are usually held at around 21 °C for three weeks before frying or blanching to leach soluble substances, reducing sugars before frying.
The present study aimed to investigate the effect of postharvest field heat reducing methods on the quality of Russet Norkotah 3 and CO 07102-1R potato tubers during storage and French fries produced after the reconditioning process.
2.1. Sampling
This study was conducted during the 2018–2019 growing season. The cultivars used were Russet Norkotah 3 and Red CO 07102-1R. All samples were obtained at harvest, mid-October, from the San Luis Valley Research Center in Colorado. Tubers were harvested mechanically, and disease-free tubers were selected for this study. The tubers were divided into several groups to maintain the number of tubers at 200 per treatment.
2.2. Chemicals
Folin Ciocalteu (FC) reagent, sodium carbonate, gallic acid, dinitro salicylic acid, crystalline phenol, sodium hydroxide, sodium sulfite, potassium sodium tartrate tetrahydrate, glucose, and metaphosphoric acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade.
2.3. Experimental design
The tubers were kept in mesh bags, divided into three sets, and placed in three separate storage units. Each storage unit’s temperature was managed differently, whereas relative humidity was maintained at 95% in all units. In the first and second storage units, the temperature was set to 2 °C below the pulp temperature at harvest (15 - 18°C) to simulate the commercial storage conditions. In the first storage unit, the temperature lowering gradually (TLG) method was employed, where the temperature was lowered by 1 °C every 2 days until 3 °C was reached and maintained for 1 month. In the second storage unit where the temperature lowering stepwise (TLS) method was adopted, the temperature was initially reduced by 1 °C every 2 days to 12 °C and maintained for 2 weeks. Afterward, the temperature was reduced by 1 °C every 2 days to 3 °C and held for 1 month. The third set of tubers in the third storage unit was subjected to the temperature lowering immediately (TLI) method, where after harvest, tubers were stored at 3 °C for 1 month. Before producing French fries, the tubers were maintained at 20 °C for 2 weeks in all treatments.
2.4. Determination of physiological weight loss
Tuber weight loss in each treatment during storage was measured by determining the change in tuber weight. Weight loss was expressed as the percentage of weight loss relative to the initial weight.
2.5. Firmness determination
The tuber firmness was measured using a Brookfield CT3 texture analyzer (Brookfield Engineering, Middleboro, MA, USA) according to the method developed by Crossen (2017), with some modifications. The texture analyzer was fitted with a spherical probe, and the force required (g) to pierce 3 mm on the surface of the tubers was measured. Approximately 30 measurements were taken from 15 tubers (two readings from each tuber stem and bud end) in each treatment to calculate the mean.
2.6. Wound healing
The wound healing process was evaluated visually. Tubers with mechanical damage on the skin owing to the harvest process were selected. When assessing wound healing, we rated tubers based on the color of the healed skin as follows: minimal healing (MH), average healing (AH), and perfect healing (PH).
2.7. French Fries
French fries were prepared according to the method described by Garmakhany et al., (2014). Fresh fries were made immediately after harvest (zero time) and when the tubers reached 3 °C without reconditioning. After storing the tubers at 3 °C for 1 month, the tubers were subjected to the reconditioning process, where potatoes were removed from cold storage and kept at 20 °C for 2 weeks to decrease the amount of reducing sugars that were increased during cold storage. Before frying, the tubers were peeled using an abrasive peeler and were cut into 6 × 1 × 1 cm3 pieces using a domestic stripper (Garmakhany et al., 2014). Blanching was then performed in hot water (93 °C) for 4 min, and the products were washed immediately with cold water. The samples were then fried at 350 °C for 2.5 min in a Tefal controlled temperature deep fryer (Tefal, Rumilly, France) filled with corn oil (2.5 L) (Mazola 100% Pure Corn Oil), purchased from a local market. The color standards for French fried potatoes (USDA, 1988) were used to determine the color grade of French fries.
2.8. The extraction procedure for estimating total phenolics and reducing sugars
Phenolic compounds and reducing sugars were extracted using the method described by (Perla et al., 2012) with some modifications. Potato tubers were collected from each treatment at zero time and when they reached 3 °C, and after 6 months of storage at 3 °C. All materials were then cut into small pieces, frozen, freeze-dried, and stored at -80 °C for further analysis. One gram of freeze-dried material was weighed in a 10-mL falcon tube, and 5 mL of 95% methanol was added. The mixture was vortexed for 1 min, and then the tubes were incubated overnight in an orbital shaker at 150 rpm at 25 °C. Afterward, the homogenates were centrifuged at 2800 RCF for 25 min, followed by filtration using Whatman filter paper (40 nm; Whatman plc, Maidstone, UK). The remaining tubes were re-extracted under the same conditions. The final volume was made up to 10 mL with methanol and kept at -80 °C for further analysis.
2.9. Determining total phenolics
The total phenolic content in potatoes was estimated based on the method developed by Perla et al. (2012). After placing 50 µL of distilled water in a 96-well flat-bottom assay plate (Costar 3370; Corning Inc., Corning, NY, USA), 20 µL of the extract was added. Next, 75 µL of commercial FCR solution (MP Biomedicals, Solon, OH, USA) was added and mixed for 1 min in a plate reader (Power Wave XS2; BioTek Instruments, Winooski, VT, USA). Eighty microliters of 75 g L-1 sodium carbonate solution were added and directly mixed using a pipette. The microplate was then shaken in a plate reader for 5 min. The absorbance of the samples was measured at 760 nm. For the standard, gallic acid in methanol was used, and the total phenolic content was quantified as µg of gallic acid equivalent per gram of dry weight of potato samples using a 7-point calibration curve with an R2 value 0.98.
2.10. Determination of reducing sugars
Reducing sugars were determined using a previously described 96-well microplate assay described by King et al. (2009) with some modifications. A dinitrosalicylic acid reagent was prepared first (10 g L-1 dinitrosalicylic acid, 2 g L-1 crystalline phenol, 10 g L-1 sodium hydroxide, and 0.5 g L-1 fresh sodium sulfite). Then, 20 µL of the dinitrosalicylic acid reagent was added to a PCR tube (BioExpress, Kaysville, UT, USA), where 120 µL of the extract was then added and mixed well. The mixture was heated in a water bath at 99 °C for 15 min, cooled at 4 °C for 1 min, and then held at 20 °C to prevent further reaction. After mixing the contents of each tube, 100 µL of the mixture was transferred to a 96-well flat-bottom microplate containing 40 µL of 400 g L-1 potassium sodium tartrate tetrahydrate solution. The plates were mixed well for 2 min in a plate reader, and the absorbance was measured at 570 nm. A glucose standard was prepared in 800 ml L-1 methanol, and reducing sugars were expressed as mg glucose per g of dry matter.
2.11. Statistical analysis
The total phenolics, total flavonoids (data not shown), and reducing sugars were measured in triplicate. The weight loss and firmness changes were measured in 7 and 30 replications, respectively. All results are expressed as mean ± standard deviation (SD). The data were subjected to analysis of variance (ANOVA), and Tukey’s test was performed to examine if differences between treatments were significant at P < 0.05. All statistical analyses were performed using R software version 3.4.3.
3.1. Physiological weight loss
The physiological weight loss of CO 07102-1R and Russet Norkotah 3 during the wound healing period and after 6 months of storage at 3 °C and 90 % RH are shown in Figures 1a and b. During wound healing, there was no significant difference in the rate of physiological weight loss in Russet Norkotah 3 under all methods of field heat reduction. The lowest weight loss was observed in the TLG reduction method, whereas the highest physiological weight loss was observed in the TLI reduction method. After 6 months of cold storage, the lowest physiological weight loss was observed in the TLS reduction method. There was no significant difference in Russet Norkotah 3 weight loss between the TLG and TLI reduction methods. However, in CO 07102-1R potatoes, there were significant differences between all methods, with the lowest weight loss in the TLS method and the highest loss observed in the TLI method.
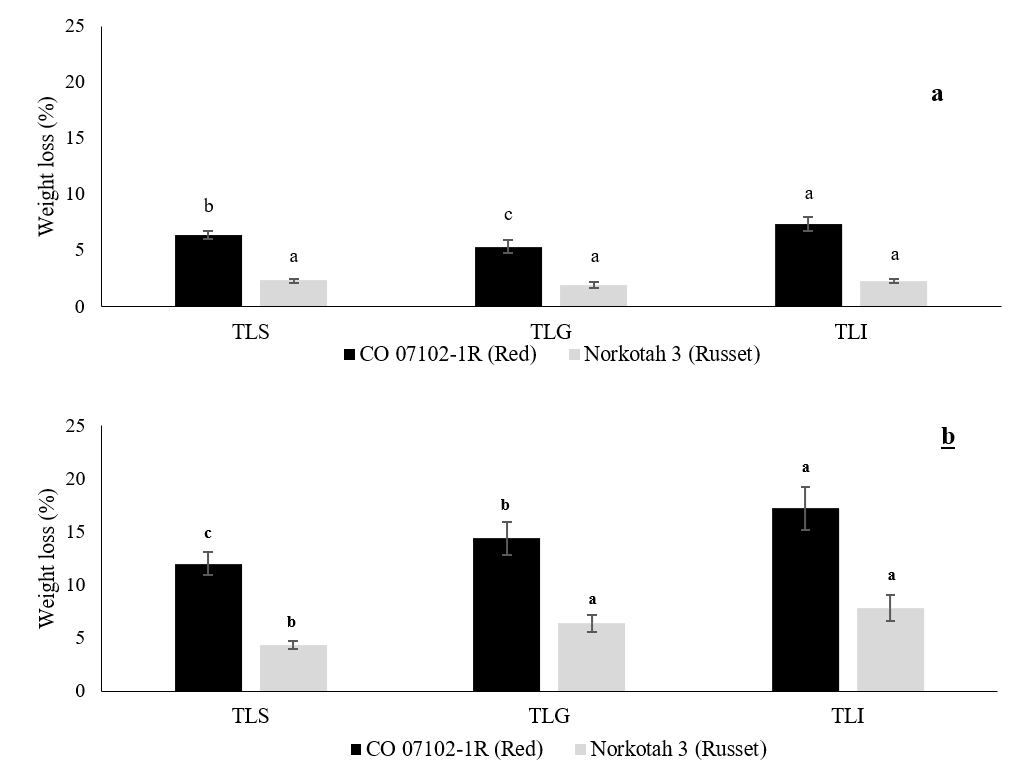
Figure 1. The effect of field heat reduction on the physiological weight loss (%) of potato tubers after reaching 3 °C (a) and after 6 months of storage at 3 °C (b). Data expressed as mean ± S.D., n = 7. The different letters are significantly different (P < 0.05).
3.2. Firmness change
The firmness changes of CO 07102-1R and Russet Norkotah 3 at zero time, during the wound healing period, and after 6 months of storage at 3 °C and 90% RH are shown in Figures 2 a and b. Firmness loss during the wound healing period was significant under the TLS method in both CO 07102-1R and Russet Norkotah 3, whereas it was lowest under the TLG and TLI reduction methods, with no significant difference between the methods. On the other hand, after 6 months of storage, the highest firmness loss was observed in the TLI method, followed by the TLG method. Firmness loss in Russet Norkotah 3 was significantly lower in the TLI method than the other two methods after six months. Firmness loss was low under the TLS method for both CO 07102-1R and Russet Norkotah 3.
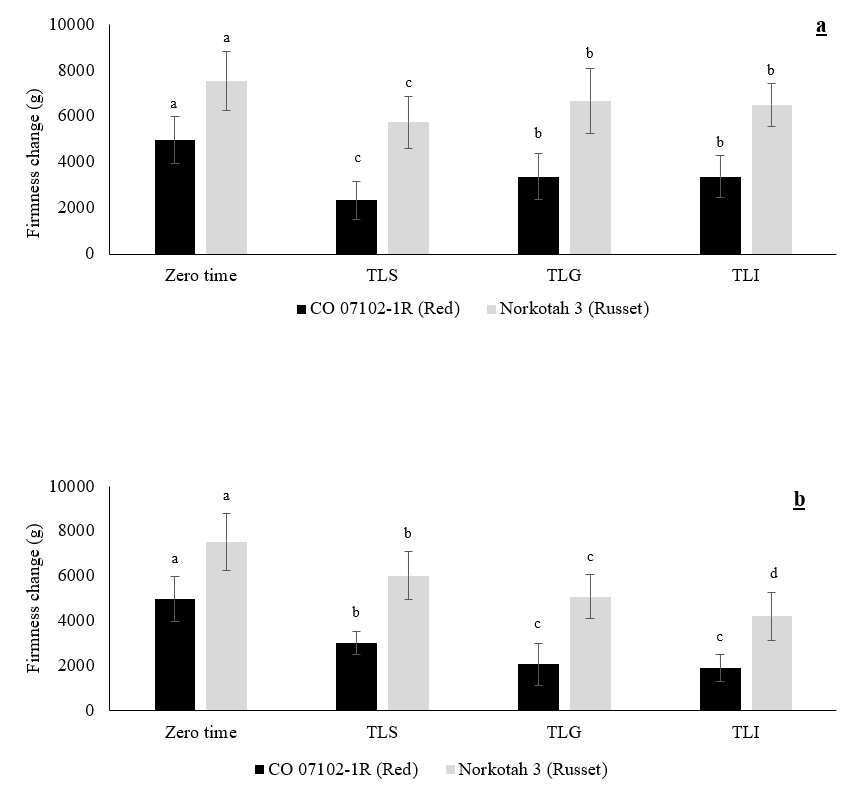
Figure 2. Effect of field heat reduction method on firmness change (g) of potato tubers after temperature reached 3 °C (a) and after six months of storage at 3 °C (b). Data expressed as mean ± S.D., n = 40. The different letters are significantly different (P < 0.05).
3.3. Wound healing
Table 1 shows the visual evaluation of the wound healing process in CO 07102-1R and Russet Norkotah 3. The TLS reduction method performed relatively better in wound skin healing than the TLG reduction method, whereas no healing occurred using the TLI reduction method. The best method for wound healing was TLS with Russet Norkotah 3, and the worst was TLI for both CO 07102-1R and Russet Norkotah 3.
Table 1. The effect of field heat reduction method on wound healing of potato tubers after reaching 3 °C. MH: minimal healing; AV: average healing; and PH: perfect healing.
|
|
CO 07102-1R |
Russet Norkotah 3 |
|
TLS when 3 °C was reached |
AH |
PH |
|
TLG when 3 °C was reached |
AH |
AH |
|
TLI when 3 °C was reached |
MH |
MH |
3.4. French fries
Table 2 and Figures 3a, b, c, and d show the visual evaluation of the color of French fries produced from CO 07102-1R and Russet Norkotah 3 at zero time, (harvest time) after wound healing, and after 2 weeks of reconditioning at 20 °C. The color of French fries at zero time was at USDA grade 1 in both cultivars. The lowest grade (USDA grade 4) was observed in the TLI method. The TLS and TLG methods resulted in French fries of USDA color grade 2 in both cultivars. All the samples were subjected to the reconditioning process for 2 weeks at 20 °C before the French fries were made.
Table 2. The effect of the field heat reduction method on the color of French fries produced from potato tubers after 1 month of storage at 3 °C and 2 weeks of reconditioning at 20 °C.
|
Time |
CO 07102-1R |
Russet Norkotah 3 |
|
Zero Time (at harvest) |
Grade 0 |
Grade 0 |
|
TLS at 3 °C and after 2 weeks of reconditioning at 20 °C |
Grade 2 |
Grade 2 |
|
TLG at 3 °C and after 2 weeks of reconditioning at 20 °C |
Grade 2 |
Grade 2 |
|
TLI at 3 °C and after 2 weeks reconditioning at 20 °C |
Grade 4 |
Grade 4 |
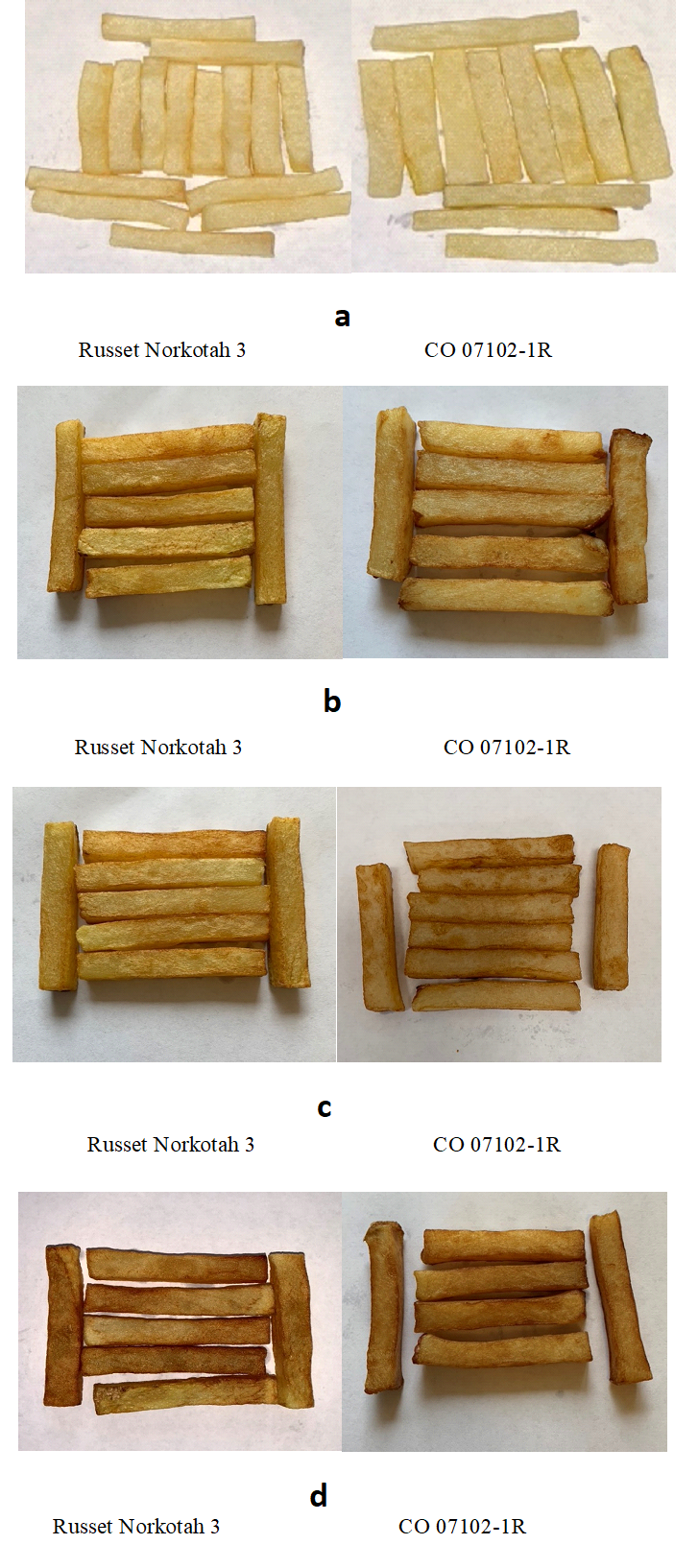
Figure 3. The color of French Fries made from Russet Norkotah 3 and CO 07102-1R potato tubers at zero time (a), the effect of field heat reduction TLS (b), TLG (c), and TLI (d) on the color of French fries made from potato tubers after 1 month of storage at 3 °C and 2 weeks of reconditioning at 20 °C.
3.5. Total phenolics and reducing sugars
The total phenolic content of CO 07102-1R and Russet Norkotah 3 at zero time, after wound healing, and after 6 months of storage at 3 °C and 90 % RH are shown in Figures 4 a and b. There was a significant difference in the effect of the field heat reduction methods on the total phenolic content during the wound healing period. The total phenolic compound concentration increased towards the end of the wound healing process. Total phenolic compounds were significantly higher in the TLG and TLI methods than in the TLS method. There was no significant difference between the TLG and TLI field heat reduction methods.
The reducing sugar content of CO 07102-1R and Russet Norkotah 3 at zero time, after wound healing, and after 6 months of storage at 3 °C and 90 % RH are shown in figure 5 a and b. The reducing sugar content was significantly higher in all field heat reduction methods at the end of the wound healing process than at zero time. However, there was no significant difference between the TLS and TLG field heat reduction methods in the CO 07102-1R cultivar, whereas there was a significant difference with Russet Norkotah 3. The highest reducing sugar content was observed in Russet Norkotah 3 under the TLI method.
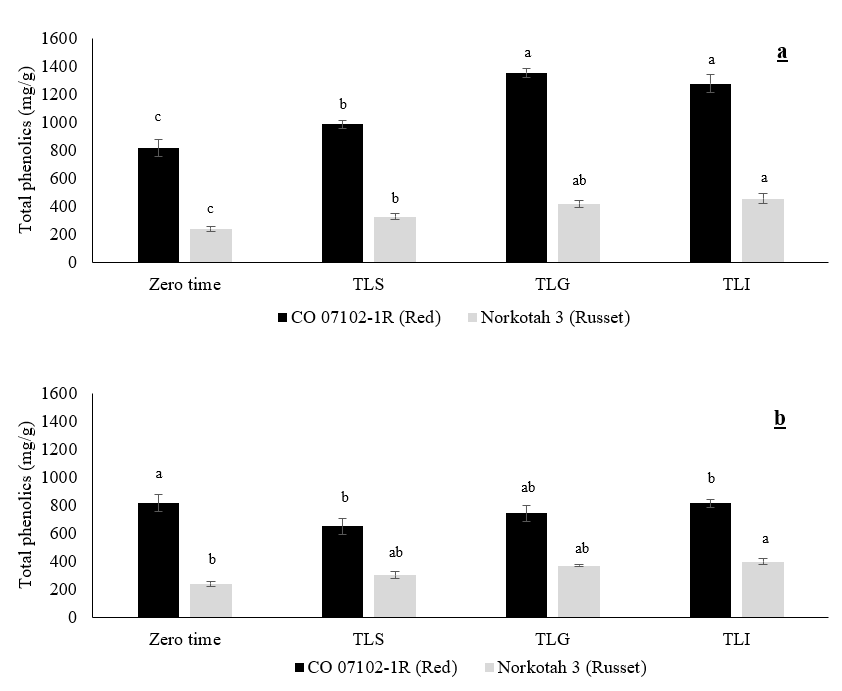
Figure 4. The effect of the field heat reduction method on total phenolics (mg/g) of potato tubers after reaching 3 °C (a) and after 6 months of storage at 3 °C (b). Data expressed as mean ± S.D., n = 3. The different letters are significantly different (P < 0.05).
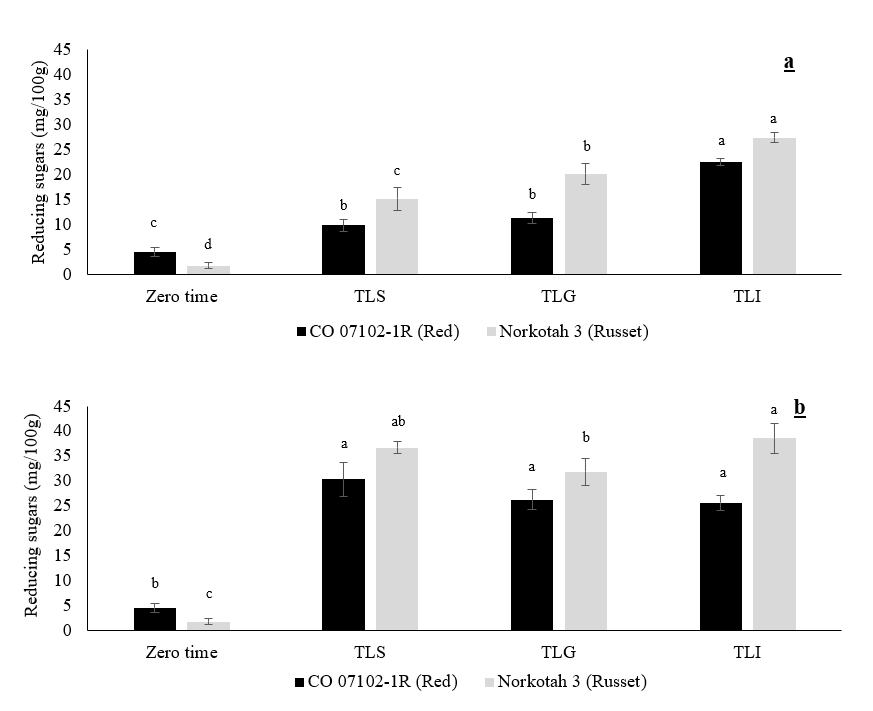
Figure 5. The effect of the field heat reduction method on reducing sugars (mg/100 g) of potato tubers after reaching 3 °C (a) after 6 months of storage at 3 °C (b). Data expressed as mean ± S.D., n = 3. The different letters are significantly different (P < 0.05).
Tuber harvest damage and storage issues can cause significant losses to potato growers (Dastmalchi et al., 2014). Moreover, incomplete wound healing can affect the long-term storability of potato tubers, and it is essential to maintain end product quality (Knowles et al., 1982). Weight loss is mainly due to the difference in vapor pressure between the tubers and the surrounding atmosphere. However, the most critical factors determining potato quality in long-term storage are wound healing, respiration rate, and storage management (Voss et al., 2001).
In response to injuries, potato tubers produce periderm layers impregnated with suberin and lignin to seal wounded areas so that they resist dehydration and prevent microbial infection. The differences in weight loss between the TLS and TLG methods could be due to holding tubers at high temperatures in the TLS method for a longer time than in the TLG method during the wound healing period. Cultivar differences might be due to genetic and agronomic practices. The weight loss at the end of the storage time was lower in the TLS method. The ability of tubers to heal wounds significantly declines with tuber age (Kumar & Knowles, 2003; Kumar et al., 2010). The tuber’s periderm is relatively immature at harvest, and as a result, water loss is more rapid than after periderm maturation when soluble waxes are deposited in the periderm (suberization), considerably reducing the rate of water loss (Schreiber et al., 2005). Wang et al. (2020a) indicated that higher temperatures are effective during the healing process, as the tuber’s metabolism is high. Following harvest, some red cultivars have been kept for healing at 29–32 °C and 90–95 % RH for a longer time; such warm temperatures are conducive to pathogen spread, increase water loss, and promote other physiological disorders such as pressure and blackspot bruises.
In the present study, we observed that there were differences in wound healing ability between cultivars. The Russet Norkotah 3 healed faster than CO 07102-1R tubers. The effect was evident at the end of the storage period when the weight loss of Russet Norkotah 3 was significantly lower than that of CO 07102-1R. Furthermore, the TLS method was more effective than the TLG method in terms of wound healing, where weight loss was significantly lower in both cultivars under the TLS method after 6 months of storage, consistent with Lulai (2007) and Herman et al. (2017), who reported that the ability of various potato cultivars to heal wounds depends on the levels of tuber metabolism. Carbohydrate metabolism is necessary to provide carbon skeletons for lignin biosynthesis in wounded tubers (Zheng et al., 2020). With the degradation of the intracellular structure and cell wall composition, horticultural products lose firmness. This softening phenomenon is a biochemical process involving enzyme hydrolysis of starch and other cell wall polysaccharides (Ali et al., 2010). The loss of firmness is often associated with water loss related to the substantial movement of water molecules from the tuber cell structure. The free and linked water molecules work together to hold the tuber firm (Taşdelen & Bayindirli, 1998). The textural changes noted in the current study are consistent with the hypothesis that textural loss is correlated with weight loss in potato tubers (Castleberry and Jayanty 2017).
In a study conducted by Wang et al. (2020a) on Russet Burbank potatoes, wound healing at a higher temperature led to lower stem-end glucose and, therefore, to a lighter fry color than wound-healing treatment at cooler temperatures. This is consistent with the current study, where the color of French fries was at USDA grade 1 at harvest when tubers had matured and reducing sugars were at the lowest level. In addition, the color of fries produced from tubers subjected to the TLS method was lighter than that of the fries made from tubers of the TLG method. Processors score fry color according to USDA grade standards. Groups range from USDA 0 (lightest fries) to USDA 4 (darker fries) (USDA,1988).
We demonstrated that reducing sugars were significantly lower at harvest than at the end of storage, where the level was considerably high due to the effect of the TLS (Edwards et al. 2002; Driskill et al. 2007; Pinhero et al., 2011). Furthermore, phenolic content was significantly higher at the end of storage than at zero time, which might be due to the tuber’s response to wound healing, as Wang et al. (2020b) observed in sweet potato, where they noticed that the concentration of total phenolics and total flavonoids significantly increased in the wound sites of sweet potato. Total phenolics and total flavonoids are considered anti-pathogen and oxidation-resistant substances, which the plant uses as a defense mechanism to maintain the postharvest quality and shelf life of potatoes. The total phenolics and reducing sugar content in the TLG and TLI treatments were higher than those in the TLS treatment at the end of the wound healing period. This may be attributed to faster field heat reduction; consequently, the response of potato tubers to low-temperature stress was faster than in tubers under the TLS treatment. The effect of TLS and reducing sugar accumulation occurred over a short time, and as a result, the total phenolic content increased since sugars are phenolic precursors.
This study demonstrates that the TLS temperature reduction method was more effective in reducing weight loss, firmness maintenance, wound healing, and fry color than the TLG and TLI methods. Weight loss was less when tubers reached 3 °C under the TLG method; however, weight loss increased significantly after 6 months of cold storage under the TLG method. In terms of fry color after the tubers were reconditioned, there was no difference between the TLG and TLS methods; unlike the TLI method, the fry color was darker ). Total phenolics and reducing sugar content significantly increased at 3 °C and after 6 months of storage, respectively, especially in TLI.
The TLS method gives the tubers more time to heal and synthesize the components responsible for wound healing. Our studies indicated that the TLG method is a better treatment in short-term storage when the tuber has more skin damage, while the TLS method is better for russet cultivars in long-term storage in terms of weight loss as the tuber has more time to heal.
We acknowledge Mr. Michael Gray for helping with the preparation of the samples used in this study. We recognize the San Luis Valley Research Center for providing the tubers used in this study. The Colorado Potato Administrative Committee (CPAC) Areas II and III partially provided funding for this project.
Abong', G. O., Okoth, M. W., Karuri, E. G., Kabira, J. N., & Mathooko, F. M. (2009). Levels of reducing sugars in eight Kenyan potato cultivars as influenced by stage of maturity and storage conditions. Journal of Animal & Plant Sciences, 2(2), 9.
View ArticleAgblor, A., & Scanlon, M. G. (2002). Effect of storage period, cultivar and two growing locations on the processing quality of french fried potatoes. American Journal of Potato Research, 79(3), 167-172.
View ArticleAli, A., Maqbool, M., Ramachandran, S., & Alderson, P. G. (2010). Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology, 58(1), 42-47.
View ArticleArtschwager, E. (1927). Wound periderm formation in the potato as affected by temperature and humidity. Journal of Agricultural Research, 35(11), 995-1000.
Barel, G., & Ginzberg, I. (2008). Potato skin proteome is enriched with plant defence components. Journal of Experimental Botany, 59(12), 3347-3357. PMid:18653692
View Article PubMed/NCBIBernards, M. A. (2002). Demystifying suberin. Canadian Journal of Botany, 80(3), 227-240.
View ArticleCastleberry, H. C., & Jayanty, S. S. (2017). Susceptibility to pressure flattening correlates with texture analysis of potato tubers. American Journal of Potato Research, 94(5), 556-566.
View ArticleCrossen, E. (2017). Textural, Color and Sensory Attributes of Fruits and Vegetables Dried Using Electric Forced-Air and Solar Dehydrators. Theses and Dissertations.
Daniels-Lake, B., Prange, R., Walsh, J., Hiltz, K., Bishop, S., & Munro-Pennell, K. (2014). Effects of simulated harvest injury and relative humidity during the first week postharvest on potato ( Solanum tuberosum L.) tuber weight loss during subsequent storage. The Journal of Horticultural Science and Biotechnology, 89(2), 167-172.
View ArticleDastmalchi, K., Cai, Q., Zhou, K., Huang, W., Serra, O., & Stark, R. E. (2014). Solving the Jigsaw Puzzle of Wound-Healing Potato Cultivars: Metabolite Profiling and Antioxidant Activity of Polar Extracts. Journal of Agricultural and Food Chemistry, 62(31), 7963-7975. PMid:24998264
View Article PubMed/NCBIDriskill, E. P., Knowles, L. O., & Knowles, N. R. (2007). Temperature-induced changes in potato processing quality during storage are modulated by tuber maturity. American Journal of Potato Research, 84(5), 367-383.
View ArticleEdwards, C. G., Englar, J. W., Brown, C. R., Peterson, J. C., & Sorensen, E. J. (2002). Changes in color and sugar content of yellow-fleshed potatoes stored at three different temperatures. American Journal of Potato Research, 79(1), 49-53.
View ArticleEllis, G. D., Knowles, L. O., & Knowles, N. R. (2019). Respiratory and low-temperature sweetening responses of fresh-cut potato (Solanum tuberosum L.) tubers to low oxygen. Postharvest Biology and Technology, 156, 110937.
View ArticleGarmakhany, A. D., Mirzaei, H. O., Maghsudlo, Y., Kashaninejad, M., & Jafari, S. M. (2014). Production of low fat french-fries with single and multi-layer hydrocolloid coatings. Journal of Food Science and Technology, 51(7), 1334-1341. PMid:24966427
View Article PubMed/NCBIHerman, D. J., Knowles, L. O., & Knowles, N. R. (2017). Heat stress affects carbohydrate metabolism during cold-induced sweetening of potato (Solanum tuberosum L.). Planta, 245(3), 563-582. PMid:27904974
View Article PubMed/NCBIKim, H. O., & Lee, S. K. (1992). Effects of curing and storage conditions on processing quality in potatoes. Physiological Basis of Postharvest Technologies 343, 73-76.
View ArticleKing, B. C., Donnelly, M. K., Bergstrom, G. C., Walker, L. P., & Gibson, D. M. (2009). An optimized microplate assay system for quantitative evaluation of plant cell wall-degrading enzyme activity of fungal culture extracts. Biotechnology and Bioengineering, 102(4), 1033-1044. PMid:18973283
View Article PubMed/NCBIKnowles, N. R., Iritani, W. M., Weiler, L. D., & Gross, D. C. (1982). Susceptibility of potatoes to bacterial rot and weight loss as a function of wound-healing interval and temperature. American Potato Journal, 59(11), 515-522.
View ArticleKumar, G. M., & Knowles, N. R. (2003). Wound-induced superoxide production and PAL activity decline with potato tuber age and wound healing ability. Physiologia Plantarum, 117(1), 108-117.
View ArticleKumar, G. N. M., Lulai, E. C., Suttle, J. C., & Knowles, N. R. (2010). Age-induced loss of wound-healing ability in potato tubers is partly regulated by ABA. Planta, 232(6), 1433-1445. PMid:20839005
View Article PubMed/NCBILi, X.-Q., Griffiths, R., Lagüe, M., DeKoeyer, D., Rothwell, C., Haroon, M., Stevens, B., Xu, C., Gustafson, V., Bonierbale, M., Regan, S., & Flinn, B. (2007). EST sequencing and analysis from cold-stored and reconditioned potato tubers. Acta Horticulturae, 745, 491-495.
View ArticleLulai, E. C. (2007). Chapter 22-Skin-Set, Wound Healing, and Related Defects. In D. Vreugdenhil, J. Bradshaw, C. Gebhardt, F. Govers, D. K. L. Mackerron, M. A. Taylor, & H. A. Ross (Eds.), Potato Biology and Biotechnology (pp. 471-500). Elsevier Science B.V.
View ArticleLulai, E. C., & Suttle, J. C. (2009). Signals involved in tuber wound-healing. Plant signaling & behavior, 4(7), 620-622. PMCid:PMC2710555
View ArticleMorris, S. C., Forbes-Smith, M. R., & Scriven, F. M. (1989). Determination of optimum conditions for suberization, wound periderm formation, cellular desiccation and pathogen resistance in wounded Solanum tuberosum tubers. Physiological and Molecular Plant Pathology, 35(2), 177-190. 90087-8
View ArticlePerla, V., Holm, D. G., & Jayanty, S. S. (2012). Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT-Food Science and Technology, 45(2), 161-171.
View ArticlePinhero, R., Pazhekattu, R., Marangoni, A. G., Liu, Q., & Yada, R. Y. (2011). Alleviation of low temperature sweetening in potato by expressing Arabidopsis pyruvate decarboxylase gene and stress-inducible rd29A: A preliminary study. Physiology and Molecular Biology of Plants : An International Journal of Functional Plant Biology, 17(2), 105-114. PMid:23573000
View Article PubMed/NCBISchreiber, L., Franke, R., & Hartmann, K. (2005). Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta, 220(4), 520-530. PMid:15378368
View Article PubMed/NCBITaşdelen, Ö., & Bayindirli, L. (1998). Controlled Atmosphere Storage and Edible Coating Effects on Storage Life and Quality of Tomatoes. Journal of Food Processing and Preservation, 22(4), 303-320.
View ArticleUSDA. (1988). USDA Color standards for frozen French-fried potatoes. 4th Ed. Munsell Color. Baltimore, MD.
Voss, R. E., Baghott, K. G., & Timm, H. (2001). Proper environment for potato storage. Vegetable Research and Information Center. The University of California.
Wang, C., Chen, L., Peng, C., Shang, X., Lv, X., Sun, J., Li, C., Wei, L., & Liu, X. (2020a). Postharvest benzothiazole treatment enhances healing in mechanically damaged sweet potato by activating the phenylpropanoid metabolism. Journal of the Science of Food and Agriculture, 100(8), 3394-3400. PMid:32147823
View Article PubMed/NCBIWang, Y., Naber, M. R., & Crosby, T. W. (2020b). Effects of Wound-Healing Management on Potato Post-Harvest Storability. Agronomy, 10(4), 512.
View ArticleZheng, X., Jiang, H., Bi, Y., Wang, B., Wang, T., Li, Y., Gong, D., Wei, Y., Li, Z., & Prusky, D. (2020). Comparison of wound healing abilities of four major cultivars of potato tubers in China. Postharvest Biology and Technology, 164, 111167.
View Article