Saji Gomez
Email: saji.gomez@kau.in
© 2019 Sift Desk Journals. All Rights Reserved
Saji Gomez
Email: saji.gomez@kau.in
Saji Gomez*, Bintu Kuruvila, Maneesha P.K and Meagle Joseph
Department of Post Harvest Technology, College of Agriculture, Kerala Agricultural University, Thrissur, Kerala, India-680656
Saji Gomez, Bintu Kuruvila, Maneesha P.K, Meagle Joseph, Indian blackberry (Syzigium cumini L.) nectar processed without chemical preservatives-changes in physico-chemical properties and bioactive constituents during storage(2022) Journal of Food Science & Technology 7(2) : 468-478
Indian blackberry fruit is widely acclaimed for the antidiabetic property. The fruits are highly perishable, having a shelf life of about two days. Therefore, an attempt was made to convert the fruits into nectar by pasteurization, without adding any chemical preservatives. Indian blackberry nectar having a total soluble solids content of 17 0 Brix and acidity of 0.253% without any chemical preservatives, was pasteurized at 100 0 C, for 10 minutes. Viscosity of the nectar recorded an upward trend throughout storage, irrespective of storage temperature. The L* and b* values rose with a corresponding decline of a* values during storage of the product. Pasteurized Indian blackberry nectar had an initial vitamin C content of 18.6 mg 100g- 1. After three months of storage, total phenolics in Indian blackberry nectar were 59.50 and 57.50 mg 100g-1 under ambient and refrigerated storage, respectively. The product held under refrigerated conditions had significantly higher anthocyanins (85.67 mg) than the nectar held at ambient temperature (78.72 mg 100g-1). Scavenging of DPPH radical of the nectar declined slightly (2.94 µg ml-1), compared to that in fresh fruits (2.75 µg ml-1).
Keywords: Indian blackberry, ascorbic acid, total phenolics, total anthocyanins, DPPH scavenging
Increase in disposable incomes and subsequent improvements in living standards have resulted in highly health conscious populations who are aware of their nutritional requirements which lead to choices in the types of food for consumption. Absence of synthetic food additives, particularly class II preservatives make the product more demanding and will therefore, creates an impression of being ‘chemical-free’. The demand for fruits and vegetables are on the rise as they are considered rich sources of health beneficial vitamins and bioactive substances. Fruits are undisputable commodities with respect to their antioxidant potential. The antioxidant ability of plant based foods could be due to their higher levels of phenolic compounds like flavonoids and anthocyanins along with carotenoids, vitamins C, E and due to the presence of minerals like Zn and Se (Wu et.al 2004). Several studies have revealed that the inclusion of fruits and vegetables rich in polyphenols in our diet could reduce incidence of cardiovascular problems (Pilijac-Zegarac et.al, 2009; Nowak et.al, 2011). Determination of antioxidant potential using the DPPH (2, 2-diphenyl-1-picrylhydrazyl) (method has become very popular owing to the stability of the radical in solutions containing alcohol, ease of determination and lower cost. Besides, no sophisticated equipment is needed for its determination. Novak et. al (2015) reported that DPPH method is currently the most commonly used one to determine the free radical quenching capacity of fruit and vegetables (Novak et.al, 2015).
Among fruits, berries are considered as richer sources of polyphenols. Indian gooseberry, popularly known as aonla is a potent source of phenolic compounds. Fruits like, chokeberry (aronia) is estimated to have about 40–100 g polyphenols per kg of fruit sample. Other fruits which are useful sources of polyphenols include blueberries, raspberries, cranberries, currants and strawberries (Kahkonen et.al, 2001) and Jakobek et. al (2012). Indian blackberry (Syzigium cumini L.), more popular by the name jamun, belongs to the Myrtaceae family. Other edible species in the genus Syzigium are S. aqueum, S. jambos, S. malacensis, S. densiflorum and S. samarangense. The fruit is indigenous to India and is known for its health protective properties, owing to the rich contents of bioactive compounds, especially phenolic compounds. Indian blackberry fruit is widely acclaimed for the antidiabetic property. Besides, it is also known to have anti-inflammatory properties along with immunity boosting benefits. Regular consumption of the fruit is advised to increase the haemoglobin content of blood. The fruit is also rich in important minerals such as calcium, potassium, iron etc. Jamun has substantially high vitamin C content, which helps in maintaining higher levels of immunity. Besides, it is also an important source of tocopherols, tocotrienols, phenols like catechins, isoflavones, flavones, flavons and anthocyanins (Gowri et.al, 2010). Due to lower levels of sugars and absence of sucrose, consumption of Indian blackberry fruits does not result in an immediate spike in sugar levels in blood and urine. The antidiabetic property of the fruit is well known because of the beneficial impact on the function of pancreas and regular intake of the fruit is found to boost insulin production and the seeds possess several bioactive glucosides like ellagic acid and jamboline that could slow down the conversion of starch into sugar and thereby alleviating the difficulties caused by high levels of glucose in blood (Giri et.al, 1985; Benherlal and Arumughan, 2007). Despite the excellent nutritional profile, the fruit has an inherent astringent taste that dominates sweetness which prevents it from being relished by consumers in the fresh form. Besides, the major bottleneck in the post harvest handling of this fruit is the huge size of the trees which poses problems in harvesting of the fruits and the very short shelf life, wherein the ripe fruits cannot be stored under ambient conditions for more than two to three days. Therefore, conversion of this highly perishable produce into a durable processed product with the retention of its nutritional quality and health protective properties is the need of the hour. As per the specifications of FSSAI (2011), the minimum total soluble solids and juice content in fruit nectars except that of orange and pineapple should be 15 0 Brix and 20 per cent, respectively while the maximum acidity expressed as citric acid should be 1.5 per cent. Therefore, an attempt was made to convert jamun fruits into nectar by pasteurization, without adding any chemical preservatives and to evaluate the qualitative changes in the product during storage.
2.1. Nectar preparation
Indian blackberry fruits collected from the huge trees grown in the Central Nursery of Kerala Agricultural University campus, after tying nets around the trunk of trees. Ripe fruits falling down on the nets were collected, sorted and graded to select sound fruits free of damages, diseases and pest infestations. Excellent quality fruits thus selected were washed in chlorinated water (50 ppm chlorine) to remove soil and other inert particles adhering to fruits. The cleaned fruits were transferred to a stainless steel utensil, followed by adding water (1: 0.25 proportions of fruit and water) and subsequently heated to 90 0 C for 1 minute to extract the firmly adhering pulp from the seed by simultaneously crushing the fruit during heating and also, to destroy the enzyme pectin methyl esterase, responsible for coagulation of colloidal particles. . This also ensured maximum extraction of pigment from the peel which has higher anthocyanins compared to those in the pulp. The smooth pulp thus obtained was strained through a stainless steel sieve of 0.2 mm mesh size, followed by removal of the hard seeds. Sugar syrup was added to the prepared pulp to adjust the TSS content of the nectar to 17 0 Brix, having an acidity of 0.253 per cent. The prepared hot nectar ( 70 0 C) was filled into 200 ml glass bottles, followed by sealing with metallic cap, leaving a headspace of 0.5 cm and were subsequently pasteurized at 100 0 C, for 10 minutes. Pasteurized bottles were immediately inverted and left as such to cool down. The nectar thus prepared from Indian blackberry fruits were held under ambient (32 ± 2 0C) as well as low temperature (5± 2 0C) to study the changes in the quality of the product over a storage period of six months. Observations were recorded at monthly intervals.
2.2. Determination of quality characteristics and bioactive compounds
Titratable acidity was determined by diluting the sample to 100 ml with distilled water, followed by titration against 0.1 N NaOH using phenolphthalein indicator as described by AOAC (1998). Determination of pH was done with a pH meter (Scientific Tech, S72025). Viscosity of nectar was determined at 25 0 C, using a Brookfield viscometer ( Brookfield DVE, Digital Viscometer, USA) and viscosity of the sample was recorded in centipoise (cp) as suggested by Karangwa et.al (2010). Determination of colour values was done by the reflectance measurement using a Minolta CM-3600D spectrophotometer (Konica Minolta Sensing, Inc., Osaka, Japan). Reference light source used was a D 65 lamp (Hajare et. al, 2006). The L* (lightness), a* (redness) and b* (yellowness) of samples were determined using JAYPAK 4808 software. Total soluble solids (TSS) content was measured using a digital refractometer (ATAGO, PAL 1 & 2) manufactured in Japan. Ascorbic acid (vitamin C) content was measured by dissolving the sample in 3 per cent metaphosphoric acid, followed by titration against 2, 6-dicholorophenol indophenol dye (AOAC, 1998). Total phenolics contents were estimated using Folin-Ciocalteau reagent as described by Asami et al. (2003). 5 mL of the extracted sample solution containing 80 per cent ethanol (1mL) to which 0.3 mL of Folin-Ciocalteau reagent was added, followed by addition of 10 mL of 7 per cent sodium carbonate solution after six minutes. The entire solution mixture was left for 2 hours. The reaction between phenols and phosphomolybdic acid resulted in a blue coloured complex. The absorbance was read at 740 nm on a UV-Visible 1800 spectrophotometer manufactured by Shimadzu, Kyoto, Japan. Gallic acid formed the standard for the calibration curve to estimate total phenolics. Phenolics content was expressed as mg gallic acid equivalent (GAE) in 100 g of sample. Total anthocyanins were estimated by the pH differential method as suggested by Lee et. al (2016). Two different dilutions of the sample, one with KCl buffer of pH 1.0 and another with CH3COONa of pH 4.5. The absorbance was measured at 510 and 700 nm. Total anthocyanins in the sample were calculated using the formula
TAC = (Ax MW x DF x Ve x1000) / e x 1 x M
Where A denotes the absorbance difference between pH buffers 1.0 and 4.5. MW indicates molecular weight of cyanidin-3-glucoside, which is 449 g/ mol and DF stands for dilution factor. Ve denotes the extract volume and, e stands for molar extinct coefficient (cyaniding-3-glucoside), whereas M indicates the Indian blackberry fruit mass extracted. DPPH method of radical scavenging activity of samples was found out based on the method suggested by Braca et al. (2001). 1.3 mL DPPH was reacted with the sample extracted using methanol solution. The absorbance of the reaction mixture was read at 517 nm. Percentage of radical scavenging activity was determined from the formula
% Inhibition of radical = (Control - Sample x 100) / Control
Gallic acid formed the standard and concentration of sample providing 50 % inhibition of radical i.e., IC50 values in µg ml-1 were calculated from the formula given above.
2.3. Statistical analysis
Analysis of the data obtained from the experimental results was done using the CRD (completely randomized design) having five replications. The mean values are presented in two- way ANOVA to examine the significant difference between treatments namely, storage period and temperature, using Web Based Agricultural Statistics Software Package (WASP). Comparison of mean values at 95 % confidence level i.e., p =0.05, was done by adopting the Duncan’s multiple range test.
3.1 Changes in physico-chemical properties
3.1.1. Titratable acidity
Acid content in fruit beverages plays a crucial role in contributing to the flavour of fresh as well as processed fruit products, which ultimately indicates preference of consumers. Higher the acidity, lower will be the preference, but higher acidity is beneficial in prolonging the keeping quality of food samples. The initial acidity in Indian blackberry nectar was 0.253 % (Table 1). Titratable acidity of Indian blackberry nectar increased throughout the storage period, irrespective of temperature at which product was stored (Table 2). However, the nectar held under refrigerated conditions showed lower values in comparison with the sample held at ambient temperature. After three months of storage, acidity of jamun nectar held under ambient and low temperature was 0.670 and 0.402 %, respectively. Temperature and duration of storage significantly affected the titratable acidity of Indian blackberry nectar. Degradation of pectin to pectinic acid or fermentation of sugars might have increased the acidity in nectar during storage. Lower levels of acidity in refrigerated nectar compared to the one held at ambient temperature could happen because of lower rates of chemical reactions at low temperature. This finding is in conformity with the one reported by Hosseini et. al (2015) in orange nectar added with stevioside to make it low in calorific value. Hussain et. al (2011) also reported a rise in acidity of blended juice of apple and apricot, during storage of 90 days.
Table 1. Proximate composition of Indian blackberry fruits
|
Sl. No |
Parameters |
Results |
|
1 |
Titratable acidity (%) |
0.804 |
|
2 |
pH |
3.0 |
|
3 |
Ascorbic acid (mg 100g-1) |
27.90 |
|
4 |
Total phenolics (mg 100g-1) |
280.0 |
|
5 |
Total anthocyanins (mg 100g-1) |
147.88 |
|
6 |
Total sugars (%) |
9.85 |
|
7 |
Reducing sugars (%) |
7.29 |
|
8 |
Non-reducing sugars (%) |
2.56 |
|
9 |
TSS (o Brix) |
8.0 |
|
10 |
DPPH radical scavenging activity (IC50 µl ml-1) |
2.75 |
|
11 |
Moisture content (%) |
78.85 |
Table 2. Quality characteristics of Indian blackberry nectar during storage
|
Storage |
Titratable acidity (%) |
pH |
TSS (o Brix) |
|||
|
AMBIENT
|
LOW
|
AMBIENT
|
LOW
|
AMBIENT
|
LOW
|
|
|
INITIAL |
0.253 |
0.253 |
3.16 |
3.16 |
17.0 |
17.0 |
|
1MAS |
0.504 |
0.402 |
3.02 |
3.12 |
16.0 |
16.0 |
|
2MAS |
0.580 |
0.402 |
2.98 |
3.10 |
15.5 |
16.0 |
|
3MAS |
0.670* |
0.402 |
2.93* |
3.06 |
15.0* |
15.0 |
|
4MAS |
- |
0.670 |
- |
3.02 |
- |
15.0 |
|
5MAS |
- |
0.670 |
- |
3.00 |
- |
15.2 |
|
6MAS |
- |
0.670 |
- |
2.93 |
- |
16.0 |
|
CD FACTOR A |
0.001 |
0.010 |
0.102 |
|||
|
CD FACTOR B |
0.002 |
0.019 |
0.190 |
|||
|
CD FACTOR A*B |
0.003 |
0.027 |
0.269 |
|||
|
SE(m) |
0.001 |
0.009 |
0.089 |
|||
*- Unmarketable
3.1.2. pH
Quality of foods, particularly the shelf life is directly related to pH. The type of microorganism associated with spoilage of food items is determined to a great extent by pH. Foods having high pH will be of poorer shelf life and will also have higher microbial proliferation. The initial pH of Indian blackberry nectar was 3.16 (Table 1). pH of the product fell throughout the storage period under ambient as well as in refrigerated storage (Table 2) and Indian blackberry nectar held under refrigerated condition had higher pH throughout storage. Storage conditions and duration had a significant impact on pH of Indian blackberry nectar. After three months of storage, the pH of nectar under ambient and refrigerated conditions was 2.93 and 3.06, respectively. Fall in pH of the product was due to the rise in acidity of the nectar imparted by formation of pectinic acid and fermentation of sugars during storage. These chemical reactions might have been slower in the refrigerated samples, which recorded lower pH than the samples at ambient temperature. Decline in pH and retention of higher pH values in refrigerated fruit juices over a storage period of twelve days were recorded by Kaddumukasa et. al (2017) who noticed that the pH values of samples of different fruit juices under ambient (24 0 C) storage ranged between 3.87 and 3.80 in passion fruit, while in pineapple it was between 3.05 to 3.03 and from 3.25 to 3.13 in mango juice whereas in refrigerated samples (4 0 C), it ranged from 3.39 to 3.20 in passion fruit, 3.96 to 3.86 in pineapple and from 4.19 to 3.90 in mango juice, respectively. A pH value of 3.5 was reported in pineapple variety Smooth Cayenne by Tortoe et. al (2013) whereas a value of 3.86 was recorded in pineapple pulp stored in dark bottles under refrigerated conditions ( 4 0 C) by Silva et. al (2015).
3.1.3. TSS (Total soluble solids)
TSS of fruit juice beverages is an indication of the sweetness of the product which ultimately determines the consumer acceptability as well. The initial TSS of Indian blackberry nectar was adjusted to 17 0 Brix. Decline in TSS content of Indian blackberry nectar was noticed under both ambient and refrigerated storage (Table 2). After three months, TSS in the nectar fell to 15 0 Brix under ambient as well as in refrigerated storage. However, there was a slight rise in TSS of nectar stored at low temperature, towards the final stage of storage. Storage period and temperature significantly influenced TSS content of the nectar. Fall in TSS content of nectar may be due to conversion (fermentation) of sugars in the nectar into ethanol, carbon dioxide, water etc. A general decline in 0 Brix values was reported by Kaddumukasa et. al (2017) in fresh and unpasteurized fruit juices of mango, passion fruit and pineapple, when stored for 12 days under ambient as well as in refrigerated conditions. Upward trend in TSS of nectar towards the final stage of storage may be due to the formation of simple sugars such as glucose and fructose caused by inversion of sucrose. Sandi et. al (2004) noticed that the sucrose concentration declined and that of reducing sugars (glucose and fructose) increased in juice of yellow passion fruit when held for 120 days under ambient storage ( 25 ± 50 C) as well as in refrigerated samples (5 ± 10 C).
3.1.4. Viscosity
The body or consistency of fruit juice beverages, which is informally known as “thickness” is referred to as viscosity. It also plays a key role in determining consumer preference. Nectar prepared from Indian blackberry had an initial viscosity of 3.0 cP (centipoise). Viscosity of the nectar recorded an upward trend throughout storage, irrespective of storage temperature. (Table 3). However, the rise in viscosity in the nectar stored at ambient temperature was higher than the one under refrigerated storage. Storage time and temperature significantly influenced the viscosity of jamun nectar. After three months of storage, viscosity of the product was 4.80 and 4.10 centipoises under ambient and refrigerated conditions, respectively. Retention of higher viscosity of the product may be due to the heat denaturation of enzymes like pectin methylesterase and polygalacturonase during thermal treatments which might have retarded degradation of pectic substances. The results are in accordance with those reported by Nindo et. al (2005) wherein as solids content of red raspberry juice rose from 10 to 65 per cent at 20 0 C , the viscosity also increased from 1.8 to 224 mPas (megaPascals). They also reported that in case of blueberry juice, the viscosity increased from 2.9 to 184 mPas. However, the results for viscosity in this study on Indian blackberry nectar contradict the findings of Hosseini et. al (2015) who observed a 10 per cent reduction in viscosity during storage of stevioside containing orange nectar of low calorific value.
Table 3. Viscosity(Cp) and colour values (L*, a*, b*) of Indian blackberry nectar during storage
|
Storage |
Viscosity (Cp) |
L* |
a* |
b* |
||||
|
AMBIENT
|
LOW
|
AMBIENT
|
LOW
|
AMBIENT
|
LOW
|
AMBIENT
|
LOW
|
|
|
INITIAL |
3.00 |
3.00 |
44.39 |
44.39 |
44.35 |
44.35 |
7.92 |
7.92 |
|
1MAS |
4.50 |
3.50 |
55.79 |
50.08 |
36.27 |
38.62 |
15.63 |
14.01 |
|
2MAS |
4.62 |
3.86 |
58.80 |
52.16 |
33.61 |
38.62 |
15.92 |
14.01 |
|
3MAS |
4.80* |
4.10 |
61.59* |
58.55 |
29.39* |
36.57 |
16.31* |
15.30 |
|
4MAS |
- |
4.90 |
- |
60.94 |
- |
35.51 |
- |
17.66 |
|
5MAS |
- |
5.30 |
- |
61.20 |
- |
31.20 |
- |
18.08 |
|
6MAS |
- |
5.60 |
- |
17.66 |
- |
18.08 |
- |
19.23 |
|
CD FACTOR A |
0.010 |
0.010 |
0.010 |
0.010 |
||||
|
CD FACTOR B |
0.019 |
0.019 |
0.019 |
0.019 |
||||
|
CD FACTOR A*B |
0.027 |
0.027 |
0.027 |
0.027 |
||||
|
SE(m) |
0.009 |
0.009 |
0.009 |
0.009 |
||||
*- Unmarketable
3.2. Changes in bioactive compounds
3.2.1. Ascorbic acid
vitamin C, which is chemically known as ascorbic acid, contributes immensely to antioxidant properties of foods and its immunity boosting power is also a thoroughly researched aspect. Pasteurized Indian blackberry nectar had an initial vitamin C content of 18.6 mg 100g-1, compared to 27.9 mg 100g-1 in fresh fruits, indicating that the thermal treatment involved in developing the product could retain about 67 per cent vitamin C (Figure 1a). This reduction in the content of ascorbic acid in the nectar can occur due to thermal treatments during processing of the nectar as well as due to photo-oxidation, as vitamin C is thermo-labile as well as photo-sensitive. Ascorbic acid content of jamun nectar showed a declining trend throughout storage, under ambient as well as in refrigerated conditions, although the retention was higher at low temperature. Vitamin C content of the product was significantly influenced by storage time and temperature. After three months of storage, the vitamin C retention in the nectar was 10.24 and 15.01 mg 100g-1 under ambient and refrigerated storage, respectively. The results are in conformity with the ones observed by Hosseini et. al (2015) in orange nectar of low calorific value prepared with stevioside, during storage. Chia et. al (2012) reported that degradation of ascorbic acid during long storage durations was due to the presence of dissolved oxygen. They also reported that ascorbic acid loss could occur because of exposure to light, peroxides formed during heating and due to the presence of enzymes like ascorbate oxidase, peroxidase etc. Further, temperature during storage, processing method and type of packaging material used can also determine the extent of degradation/ retention of ascorbic acid. According to Chia et. al (2012), fruit juices retaining 50 % of their initial vitamin C content could be considered as termination of their shelf life. In the present study, Indian blackberry nectar could retain 50 % of its vitamin C content up to three and five months under ambient and refrigerated storage, respectively when stored for six months.
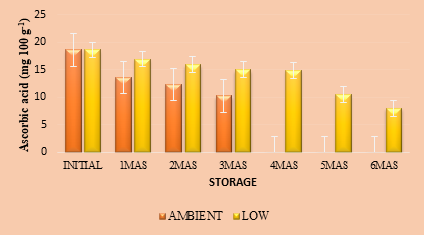
Fig.1. a. Ascorbic acid (mg 100 g-1) in Indian blackberry nectar
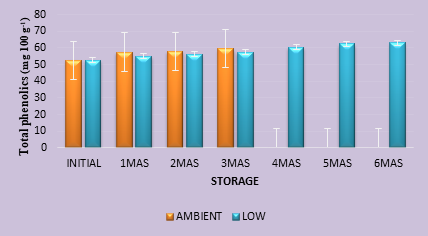
Fig.1. b. Phenolic compounds (mg 100 g-1) in Indian blackberry nectar
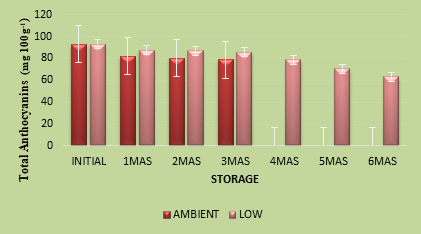
Fig.1. c. Anthocyanins (mg 100 g-1) in Indian blackberry nectar
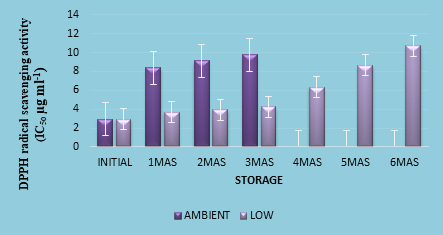
Fig.1.d. Radical (DPPH) scavenging ability of Indian blackberry nectar
3.2.2. Total phenolic content
Many fruits are rich sources of phenolic compounds which have beneficial health effects owing to their potent antioxidant capacities. The type and concentration of phenolic compounds differ with plants and fresh Indian blackberry fruits had a total phenolic content of 280.0 mg 100g-1. Thermal treatments adopted during development of the nectar brought down the phenolic content to 52.50 mg 100g-1 (Figure 1b). Reduction in the level of phenolics content might have occurred due to the thermal and oxidative deterioration leading to polymerization of polyphenols during processing of the product. Aguilar-Rosas et. al (2007) observed a reduction of about 32% in the total phenolic compounds in apple juice preserved through pasteurization by adopting high temperature and short time method at a temperature of 90 0 C and 30 seconds, as compared to the untreated juice. Similar reduction in total phenolics was also reported by Akhila and Hiremath (2018) in jam, squash and nectar prepared from jamun fruits. The effect of thermal pasteurization (90 0 C, for 30 seconds) on phenolic compounds, particularly flavonoids in orange juice was negligible (Sentandreu et. al, 2007). However, the total phenolic content rose during storage, with higher amounts in orange juice stored under ambient conditions than those stored at low temperature. Higher phenolic compounds in the juice kept under ambient storage were because of faster chemical reaction rates at higher temperature. Total phenolics content in Indian blackberry nectar was significantly affected by storage time and temperature. After three months of storage, total phenolics in Indian blackberry nectar were 59.50 and 57.50 mg 100g-1 under ambient and refrigerated storage, respectively. Findings of this study corroborates with those reported by Piljac-Zegarac et. al (2009) wherein the total phenolics in six dark coloured fruit juices were seen rising when kept for 29 days under refrigerated storage.
3.2.3. Total anthocyanin content
Anthocyanins are one of the largest groups of water soluble pigments concentrated in cell vacuoles and these pigments impart diverse colours to plant organs. Besides their antioxidant capacity, anthocyanins have been found to possess health protective properties, particularly in combating cardiovascular disease and, against several types of cancer. The purple colour of Indian blackberry is deeper in the peel than in the pulp of the fruit. Total anthocyanin content of the nectar was 93.15 mg 100g-1, which was lower than that in fresh fruits (147.88 mg 100g-1) (Figure 1c). Reduction in anthocyanin content in the nectar may be due to thermal and oxidative degradation during processing of the product. Reduction in anthocyanin content of products from jamun (jam, squash and nectar) was also reported by Akhila and Hiremath ( 2018) which contained 132, 128 and 97 mg 100g-1, respectively as compared to the content in fresh fruits (157 mg 100g-1). Total anthocyanin content of Indian blackberry nectar declined during storage. Three months after storage, the nectar kept at low temperature had significantly higher anthocyanins (85.67 mg) in comparison with the one held at ambient temperature (78.72 mg 100g-1). Anthocyanin pigments are less stable and therefore, can easily be degraded. Fall in level of anthocyanins in the nectar could be due to the combined action of temperature, presence of light, pH level, and presence of enzymes and availability of oxygen during storage. The inactivation of the enzyme polyphenol oxidase during thermal processing might have had an impact on total anthocyanin content during subsequent storage. Lingli Zhang et. al (2019) also reported on the impact of thermal pretreatments processing on total anthocyanin retention in blueberry juice during storage. They reported that the rate of retention of total anthocyanins was 23.77 % in the juice subjected to hot water bath treatment, while it was 30.71 % in steam treated juice whereas, the retention was only 19.91 % in the control sample.
3.2.4. DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical-scavenging activity
Ability to scavenge radicals indicates the antioxidant capacity of food items. Plant metabolites like phenolic substances, carotenoids, ascorbic acid, vitamin E etc. are some of the potent bioactive compounds imparting antioxidant activity. Higher levels of these compounds in fruits and vegetables and in their processed products are indicators of better health protective attributes in food samples. A lower IC50 value denotes greater potential to scavenge free radical compounds. The IC50 value of fresh Indian blackberry fruits was 2.75 µg ml-1. Akhila and Hiremath (2018) reported an antioxidant activity of 92 mg/ 100 g vitamin C equivalent in fresh jamun fruits. A slight decline in DPPH radical scavenging activity was noticed in the nectar (2.94 µg ml-1) during the initial analysis of the product. This decline in IC50 value in the product can be attributed to the partial destruction of phenolic substances and vitamin C due to thermal and oxidative degradation during processing of the product. Similar fall in radical scavenging activity was also reported by Akhila and Hiremath (2018) in processed products of jamun (jam, squash and nectar). DPPH radical scavenging activity of the nectar declined during storage. After three months of storage, the nectar kept at refrigeration temperature had significantly superior DPPH scavenging ability (4.23 µg ml-1) when compared to the one kept at ambient temperature (9.75 µg ml-1) (Figure 1d). Fall in radical scavenging activity of the nectar was noticed throughout the storage period which might have been caused by decline in the contents of compounds contributing to antioxidant capacity such as total phenolics, total anthocyanins and ascorbic acid. Lingli Zhang et. al (2019) reported similar trend in the antioxidant capacity of blueberry juice, wherein the juice subjected to hot water bath treatment had an antioxidant capacity of 37.16 %, while it was 51.81 % for steam-treated juice and the control sample retained only 23.38 % antioxidant activity, when stored for a period of 10 days. Ferric reducing antioxidant power (FRAP) of nectar prepared from orange, apricot, peach and sour cherry were evaluated by Ilkay Tosun and Sule Ustun (2003). They reported that sour cherry nectar had the highest antioxidant power and they attributed this property of sour cherry nectar to the higher levels of total phenolics and anthocyanins than those in nectar prepared from orange, apricot and peach.

The study revealed that pasteurization of Indian blackberry nectar at 100 0 C for 10 minutes and its subsequent storage, without adding any chemical preservatives, resulted in a shelf life of three months at ambient temperature and the nectar could be stored beyond six months at low temperature. This finding is relevant in the context of health conscious consumers preferring to have ‘chemical-free’, safe to eat food items. The method of processing adopted in the preparation of Indian blackberry nectar, though brought down the levels of bioactive compounds in the product as compared to those in the fresh fruits and prevented the loss of these compounds in a substantial level during storage. Low temperature storage of Indian blackberry nectar was beneficial in retaining the physico-chemical attributes and enabled maintenance of higher levels of bioactive compounds in the product. The technique could be adopted as a viable method of processing to minimize the huge post harvest losses occurring in this health protective, nutritious fruit.
Aguilar-Rosas, S. F., Ballinas-Casarrubias, M. L., Nevarez-Moorillon, G. V., Martin-Belloso, O., Ortega-Rivas, E., 2007. Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. J. Food Eng. 83(1), 41-46.
View ArticleAkhila, H., Hiremath, U.S., 2018. Physico-chemical properties of jamun (Syzigium cumini L.) fruits and its processed products. Int. j. pure appl. Sci. 6 (6): 1317-1325.
View ArticleAOAC. 1998. Official method of analysis of AOAC International (16 Ed.). Association of Official Agricultural Chemists, Washington DC, p899.
Asami, D.K., Hong, Y.J., Barret, D.M., Mitchel, I., 2003. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air dried marionberry, strawberry and corn grown using conventional, organic and sustainable agricultural practices. J. Agric. Food Chem (51):1237-1241. PMid:12590461
View Article PubMed/NCBIBenherlal, P.S., Arumughan, C., 2007. Chemical composition and in vitro antioxidant studies on Syzgium cumini fruit. J. Sci. Food Agric. 87:2560-2569. PMid:20836162
View Article PubMed/NCBIBraca, A., De Tommasi., Di Bar, L., Pizza, C., Politi, M., Morelli, I.,2001. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 64: 892-95. PMid:11473417
View Article PubMed/NCBIChia, S.L., Rosnah, S., Noranizan, M.A., Wan Ramli, W.D., 2012. The effect of storage on the quality attributes of ultraviolet-irradiated and thermally pasteurised pineapple juices. Int. Food Res J. 19:1001-1010.
Fang, Z.; Zhang, M.; Sun, Y.; Sun, J., 2006. How to Improve Bayberry (Myrica Rubra Sieb. Et Zucc.) Juice Color Quality: Effect of Juice Processing on Bayberry Anthocyanins and Polyphenolics. J. Agr. Food Chem. 54, 99-106. PMid:16390184
View Article PubMed/NCBIFSSAI. 2011. Food Safety and Standards Authority of India, Ministry of Health and Family Welfare, Government of India.
Giri, J., Sathidevi, T., Dushyanth, N., 1985. Effect of jamun seed extract on alloxan induced diabetes in rats. Journal of the Diabetic Association of India. 25:115-119.
Gowri, S.S., Vasantha, K., 2010. Phytochemical screening and antibacterial activity of Syzygium cumini (L.) (Myrtaceae) leaves extracts. Int. J Pharmtech Res. 2:1569-1573.
Hajare, S., Dhokane, V., Shashidhar, R., Saroj, S.D., Sharma, A., & Bandekar, J.R.(2006).Radiation processing of minimally processed pineapple (AnanascomosusMerr.): Effect on nutritional and sensory quality. J. Food Sci. 71(6): 501-505.
View ArticleHosseini, S., Hossein Goli, S.A., Keramat, J., 2015. Production and characterization of low-calorie orange nectar containing stevioside. J. Food Sci. Technol. 52 (10) 63-65. PMid:26396381
View Article PubMed/NCBIHussain, I., Zeb, A., Ayub, M., 2011. Evaluation of apple and apricot blend juice preserved with sodium benzoate at refrigeration temperature. World J Dairy Food Sci.; 6:79-85.
Ilkay Tosun., Sule Ustun, N., 2003. An investigation about antioxidant capacity of fruit nectars. Pak J Nutr. 2 (3): 167-169.doi: 10.3923/pin.2003.167.169.
View ArticleJakobek, L.; Seruga, M., 2012. Influence of Anthocyanins, Flavonols, and Phenolic Acids on the Antiradical Activity of Berries and Small Fruits. Int. J. Food Prop.15, 122-133.
View ArticleKaddumukasa, P.P., Imathiu, S.M., Mathara, J.M., Nakavuma, J.L., 2017. Influence of physicochemical parameters on storage stability: Microbiological quality of fresh unpasteurized fruit juices. Food Sci Nutr. 5:1098- 1105. PMid:29188037
View Article PubMed/NCBIKähkönen, M.P.; Hopia, A.I.; Heinonen, M., 2001. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 49, 4076-4082. PMid:11513713
View Article PubMed/NCBIKarangwa, E., Khizar, H., Rao, L., Nshimiyimana, D.S., Foh, M.B.K., Li, L., Xia, S.Q., Zhang, X.M., 2010. Optimization of processing parameters for clarification of blended carrot-orange juice and improvement of its carotene content. Adv J Food Sci Technol. 2:268-278.
Lee, S. G.; Vance, T. M.; Nam, T. G.; Kim, D. O.; Koo, S. I.; Chun, O. K. 2016. Evaluation of Ph Differential and HPLC Methods Expressed as Cyanidin-3-Glucoside Equivalent for Measuring the Total Anthocyanin Contents of Berries. J. Food Meas. Charact. 10, 562-568.
View ArticleLingli Zhang., Guangsheng, Wu., Wenbo Wang., Junyang Yue., Pengxiang Yue., Xueling Gao., 2019. Anthocyanin profile, color and antioxidant activity of blueberry (Vaccinium ashei) juice as affected by thermal pretreatment. Int. J. Food Prop. 22:1, 1035-1046.
View ArticleNindo, C.I., Tang, J., Powers, J.R., Singh, P., 2005. Viscosity of blueberry and raspberry juices for processing applications. J. Food Eng. 69: 343-350.
View ArticleNowak Dariusz. 2011. The Role of Natural Antioxidants Present in Foods in the Prevention and Treatment of Cardiovascular Disease and Cancer. Czasopismo Aptekarskie 2011, 8-9, 65-71.
Nowak Dariusz., Michał Gośliński., Elżbieta Wojtowicz., 2016. Comparative Analysis of the Antioxidant Capacity of Selected Fruit Juices and Nectars: Chokeberry Juice as a Rich Source of Polyphenols. Int. J. Food Prop.19:6, 1317-1324,
View ArticlePiljac-Zegarac, J.; Valek, L.; Martinez, S.; Belscak, A., 2009. Fluctuations in the Phenolic Content and Antioxidant Capacity of Dark Fruit Juices in Refrigerated Storage. Food Chem. 209, 113, 394-400. PMid:19627210
View Article PubMed/NCBIReque, P. M.; Steffens, R. S.; Jablonski, A.; Flores, S. H.; Rios, A. D. O.; De Jong, E. V., 2014. Cold Storage of Blueberry (Vaccinium Spp.) Fruits and Juice: Anthocyanin Stability and Antioxidant Activity. J. Food Compos. Anal. 2014, 33, 111-116.
View ArticleSandi, D., Chaves, J.B.P., de Sousa, A.C.G., Ferreira, J., Parreiras, M., da Silva, M.T.C., Constant, P.B.L., 2004. Hunter color dimensions, sugar content and volatile compounds in pasteurized yellow passion fruit juice (Passiflora edulis var. flavicarpa) during storage. Braz Arch Biol Technol. 47:233-245.
View ArticleSentandreu, E., Navarro, J. L., Sendra, J. M., 2007. Effect of technological processes and storage on flavonoids content and total, cumulative fast-kinetics and cumulative slow-kinetics antiradical activities of citrus juices. Eur. Food Res. Technol. 225(5-6): 905-912.
View ArticleSilva, C. E. F., da Silva, I. C. C., Abud, A. K. D. S., 2015. Acidulants in tropical fruit pulp: Physicochemical and sensory changes. Chem. Eng. Trans. 44: 1-6.
Tortoe, C., Johnson, P. N. T., Slaghek, T., Miedema, M., Timmermans, T., 2013. Physicochemical, proximate and sensory properties of pineapple (Ananas sp.) syrup developed from its organic side-stream. Food Nutr Sci. 4: 163-168.
View ArticleWu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L.,2004. Lipophilic and Hydrophilic Antioxidant Capacities of Common Foods in the United States. J. Agric. Food Chem. 52: 4026-4037. PMid:15186133
View Article PubMed/NCBI