Sastry S. Jayanty
E-mail: Sastry.jayanty@colostate.edu
Contact Number:719-754-3594 x 11
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 6 ISSUE: 2
Page No: 356-370
Sastry S. Jayanty
E-mail: Sastry.jayanty@colostate.edu
Contact Number:719-754-3594 x 11
Mansor Hameda, Michael Bartolob, and Sastry S. Jayantya *
aSan Luis Valley Research Center, Department of Horticulture and Landscape Architecture, Colorado State University, 0249 East County Road 9N Center, CO 81125, USA.
bArkansas Valley Research Center, Department of Horticulture and Landscape Architecture Colorado State University, 27901 Co Rd 21, Rocky Ford, CO 81067, USA.
Jianquan Kan(kanjianquan@163.com)
Anilkumar C(anilcgpb@gmail.com)
Zamljen T(tilen.zamljen@bf.uni-lj.si)
Apiradee Uthairatanakij (apiradee.uth@kmutt.ac.th)
Mansor Hamed, Michael Bartolo, Sastry S Jayanty, Studying the Influence of Storage Conditions, 1-MCP, and Packaging Films on Quality of Sweet Dalilah Green and Red Stage Peppers (Capsicum annuum L.) (2021) Journal of Food Science & Technology 6(2): 356-370
Peppers are a popular fresh market commodity but have a limited shelf life. The present study evaluated the effects of storage time, packaging films, and 1-methylcyclopropene (1-MCP) on weight loss, firmness, respiration rate, ethylene production, ascorbic acid, antioxidant activity, and bioactive compounds of Sweet Delilah (Capsicum annuum). Four packaging films were tested in this study: polypropylene (P12F), laminated polynylon (30 NV), coextruded vacuum pouch (30 NVC), and polyethylene (P15G). Collectively, packaged peppers showed less weight loss than the control. When stored at the red stage, the firmness loss was 13 % in peppers that were treated with 1-MCP compared to 25% loss in the control samples. The most significant reduction in respiration rate in the red stage peppers was 0.88 ml kg-1 h-1 when packaged with 30NVC and 0.91 ml kg-1 h-1 when packaged with P15G, compared to 1.22 ml kg-1 h-1 for the control. The ranges of total phenolic and total flavonoid compounds were 3782 and 5090, respectively, in the green stage and 519 and 647 µg/g, respectively, in the green and red stages. When Sweet Delilah peppers that were treated with 1-MCP maintained higher levels of phenolic and flavonoid compounds than the control samples. Overall, the largest phenolic and flavonoid losses occurred from the control samples, while the smallest phenolic and flavonoid losses occurred from the packaged peppers. The highest ABTS activity was 150 µmol TE/g when packaged with P12G film, whereas the lowest ABTS activity was 143 µmol TE/g in the control samples in the red stage. Peppers packaged with 30NVC films retained higher ascorbic acid levels than peppers that were packaged with other films and the control samples.
Keywords: Peppers, Phenolics, Flavonoids, Ascorbic acid, Antioxidant activity, Packaging.
Peppers are grown worldwide and have been incorporated into the cuisines of many cultures. Peppers are valued for their diverse flavors and nutritional content. In addition to their use in foods, peppers are used for coloring and valued for their medicinal properties (Ramchiary et al., 2013; Sora et al., 2015). Peppers are excellent sources of phytochemicals such as anthocyanins, vitamins, phenolic acids, flavonoids, carotenoids, and capsaicinoids (Kumar et al., 2009). These compounds act as primary antioxidants or free-radical terminators and are considered to be among the main phytochemicals that are related health benefits that include antioxidant, anti-inflammatory, and antimicrobial activities, reduced prevalence of type 2 diabetes and obesity, protection against hypercholesterolemia, and reduced prevalence of atherosclerotic cardiovascular diseases (Alvarez-Parrilla et al., 2011; Hervert-Hernández et al., 2010; Sora et al., 2015).
Peppers are often harvested at both the immature green and mature red stages. Peppers are highly perishable and require suitable postharvest handling practices to maintain food quality and decrease storage losses (Mahajan et al., 2010; Erin et al., 2018). Several factors cause pepper fruit losses after harvest, including increased respiration rate, hormone production such as ethylene, physiological disorders, and senescence (Chitravathi et al., 2016). Pepper nutritional quality is affected by the stage of fruit development, the type of processing, and the postharvest conditions during storage, transport, and handling (Chung et al., 2012). In addition, pepper nutritional quality can be affected by water loss, shriveling, tissue softening, physiological disorders, and fungal infection (Ilic et al., 2017).
There are several ways to improve pepper shelf life, such as lower storage temperature, increased humidity, and packaging with films to preserve product consistency during storage time (Sharma et al., 2013). During storage time, different treatments such as physical, chemical, and gaseous can be applied to delayed fruit ripening and preserve the high nutritional quality of peppers (Ilić et al., 2012; Mahajan et al., 2010). Studies have suggested that optimum storage temperature and high relative humidity may slow down the water loss and increase the shelf life of peppers fruits (Sharma et al., 2013). A lower storage temperature and increased humidity can extend pepper shelf life (Ilić et al., 2012). However, low temperatures may cause chilling injuries (Lim et al., 2007).
Recently, packaging films have been used to extend the shelf life and storability of perishable commodities (Manolopoulou et al., 2012). There are different types of packaging films such as Polypropylene, Laminated Poly-Nylon, Coextruded Vacuum Pouch, and polyethylene that have been used to extend the shelf life of many commodities such as vegetables and fruits. These films differ in barrier properties and selective permeability based on thickness and material. Packaging films, one of the most important techniques that have been successfully used to delay physiological processes such as water loss, respiration rate, transpiration, ethylene production, and softening and prevent decay in various vegetables and fruits (Sahoo et al., 2014; Barbosa et al., 2020). In peppers, the packaging films extend the shelf life by reducing respiration rates, chilling injury, physiological disorders (Gonzalez et al., 1999).
In addition to packaging, the 1-methylcyclopropene (1-MCP) application can have beneficial effects on fruit quality. 1-MCP is an ethylene perception inhibitor that can bind ethylene receptor molecules and delay the ripening process (Mahajan et al., 2010; Oz et al., 2011). Moreover, 1-MCP can delay ripening and senescence processes, such as pigment color changes, cell wall softening, and processes that affect nutritional properties (Huang et al., 2003; Oz et al., 2011).
Many pepper cultivars have been released in Colorado by the peppers breeding program in Arkansas Valley Research Center Colorado (AVRC), such as Joe Parker, Flavorburst, Canrio, Aristotle, and Sweet Delilah. The specialty variety Sweet Delilah is known to have a high level of bioactive compounds such as phenolics, flavonoids, and ascorbic acid but no capsaicinoid compounds (Hamed et al., 2019). It is a flavorful pepper with a short shelf life. This project's primary objective is to evaluate the effects of several packaging films and 1-MCP applications on Sweet Delilah pepper quality and to extend the shelf life to capture its true marketing potential. We measured weight loss, firmness, respiration rate, bioactive compounds, antioxidant activities, and ascorbic acid content in Sweet Delilah peppers with different packaging and storage conditions.
2.1. Chemicals
Ascorbic acid, Folin Ciocalteu Reagent (FC Reagent), sodium carbonate, gallic acid, potassium chloride, sodium acetate, DPPH (2,2-diphenyl-1-picrylhydrazyl) reagent, ABTS [2,2’-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid)], 1-MCP, potassium persulfate, Trolox, Quercetin, and all other reagents were purchased from Sigma Aldrich.
2.2 Pepper cultivars
The peppers varieties that were used in this study were collected from the AVRC during the 2019 season at the mature green and red stages. Pepper fruits were harvested and placed in polyethylene bags in a cold container and transferred to the San Luis Valley Research Center. For evaluation of various quality parameters, three peppers were placed in four packaging films with different thicknesses. To estimate the content of bioactive compounds, antioxidant activity, and ascorbic acid content, three to five peppers were cut into small pieces and placed in a freeze drier until completely dry (LABCONCO New York, USA). The freeze-dried pepper samples were ground to a fine powder using a mortar and pestle and stored at −20 °C until further analysis.
2.3 Packaging films and storage conditions
Four different packaging films with different thicknesses were tested in this study polypropylene flat bag (P12F), laminated poly-nylon (30NV), coextruded vacuum pouch (30NVC), and polyethylene (P15G) with 0.038, 0.0762, 0.038, 0.036 mm thicknesses, respectively. Three peppers were placed into their respective packaging films; then, peppers were sealed by a sealer and stored at 7.2 °C and 90 % relative humidity (RH) for 21 days to estimate the quality. Unpackaged peppers (control) were stored in trays without packaging films. To simulate retail marketing conditions, peppers were kept for four days at 15.5 °C and 75 % RH without packaging films. Three peppers were placed in each bag and we did three bag replications in each treatment. Three replicates of each packaging film were used in this study. A total of nine technical replicates were analyzed.
Table. 1 Relative permeability values for four types of commercial packaging
|
Type of package |
Thickness (mm) |
Permeabilities |
Density, g/cm 3 |
|
|
O2 |
CO2 |
|||
|
Polypropylene (P12F) |
0.0380 |
4.3 |
13.6 |
0.87 |
|
Laminated Poly-Nylon (30NV) |
0.0762 |
3.4 |
18.4 |
0.90 |
|
Coextruded Pouch (30NVC) |
0.0381 |
3.1 |
10.7 |
0.89 |
|
Polyethylene (P15G) |
0.0364 |
3.2 |
11.9 |
0.88 |
2.4 Application of 1-MCP
Pepper fruits in the red stage had been treated with 60 nl L-1 1-MCP by placing the fruits in a closed container in warm water for 24 hours at 20 °C. A small fan was mounted inside the container with a battery for the gas's circulation around the fruits. Control and green stage peppers weren't treated with 1-MCP. We did not observe ethylene production in green peppers.
2.5 Weight loss
The weight loss (%) of peppers was calculated as the percentage of each sample's initial mass using an electronic scale (Giantex, San Diego, CA, USA). The weight loss of each packaging film P15G, P12F 30NV, 30NVC, and control samples were recorded at the beginning of the experiment, 7th, 14th, 21st, and four days simulated marketing conditions.
2.6 Firmness
The firmness of peppers in green and red stages was measured by using texture analyzer equipment (Brookfield CT3, Middleboro, MA, USA). The firmness measurement was carried out using a cylindrical stainless-steel probe of 2 mm in diameter, and the speed of the probe was set to 1 mm/s. The measurement of firmness was carried out on 20 discs of peppers, and the firmness was measured at the beginning of the experiment, 7th, 14th, 21st, and four days simulated marketing conditions.
2.7 Respiration rate and Ethylene levels
The Respiration rate and ethylene production of peppers were measured by using a Gas Analyzer (F-900 Felix Instruments Place, Camas, USA. The measurements of the gases were carried out at the beginning of the experiment, 7th, 14th, 21st, and four days simulated marketing conditions.
2.8. Color measurement
The color was measured with MiniScan Chromameter (Reston Virginia, U.S.A.) on CIE L*a*b* chromatic space. The instrument was initially calibrated using a white standard and black standard. Color measurements were taken on opposite sides of each pepper sample. The chroma values of the green and red stage were calculated by using the chroma values equation. C* = (a*2+b*2)0.5. C* chroma value described the intensity of color in a sample (+a*) describe the degree of red, (-a*) the degree of green while (-b*) describe blue and (+b*) describe the degree of yellow color (Manolopoulou et al., 2010)
2.9. Extraction and analysis of the phenolic and flavonoid compounds
Pepper samples weighing 0.500 g were mixed with 15 ml (80 % methanol) and homogenized for 5 min. The supernatants of pepper extract were filtered to evaluate total phenolics and total flavonoids. Total phenolics and flavonoids were estimated by spectrophotometric methods, using a Costar 3370 spectrophotometer (Corning, NY, USA). The total phenolic compounds were calculated according to the method declared by Kalita and Jayanty. (2014) with modifications. FC reagent solution was added to pepper extract, and sodium carbonate was added to the 96 microplates. The total phenolic compounds of pepper samples were calculated as gallic acid (μg/g). The colorimetric method was used for total flavonoid compounds to evaluate the total flavonoid compounds in pepper samples. Aluminum chloride was added to the pepper extract in 96 microplates. The total flavonoid compounds of pepper samples were expressed as quercetin (μg/g).
2.10 Extraction and analysis of ascorbic acid
Ascorbic acid was extracted using meta-phosphoric acid from ground samples and estimated using a method defined by Watada et al. (1982). The ascorbic acid quantification was performed using a Waters 2695 HPLC system fitted with a Photodiode Array Detector and column C18. In unknown samples, the concentration of ascorbic acid was determined from the standard curve.
2.11. Antioxidant activity
2.11. 1 DPPH assa
DPPH activity of peppers extract was estimated according to the method described by Kalita and Jayanty. (2014). Pepper's extract (25 μl) was added to 15 μl distilled water in a 96-well microplate. Then, the DPPH solution was added to pepper extract, and the absorbance of the reaction was set at 515 nm. The DPPH activity has been calculated using the following formula: DPPH activity (%) = [(A control – A sample/ A control)] ×100.
2.11.2 ABTS assa
The ABTS radical cation-scavenging activity of the pepper sample was estimated using the method described by Kalita and Jayanty. (2014) with modifications. ABTS solution (280 µl) was added to 10 µl sample extract in 96 microplates. Then, the absorbance of the reaction was set at 734 nm. To evaluate pepper samples' antioxidant activity, Trolox was used as a standard, and the antioxidant capacity was expressed as μmol TE/g.
2.12 Statistical Analysis
The effects of packaging films, and storage time on weight loss, firmness, respiration rate, ethylene levels, antioxidant activity, vitamin C, and bioactive compounds were determined by analysis of variance (ANOVA) using the R software. The results were reported as mean ± standard deviation (SD.) values. Tukey's test was performed to determine whether differences between means were significant at P <0.05. All statistical analyses were performed with R software version 3.4.3 for Windows. The correlation analysis among the means of respiration rate and weight loss by using IBM SPSS Statistics version 28.0.0.0 (190) at α = 0.05. Pearson’s correlation test was used to assess correlations between the means.
3.1 Weight loss
Weight loss is one of the most important quality parameters that determine the shelf life of fruits and vegetables (Castro et al., 2002). Weight loss in fruits and vegetables during storage is primarily due to water loss, respiration, and evaporation, which depends on the temperature, relative humidity, and storage conditions (Awole et al., 2011). Our studies observed that packaging films and storage conditions affected the quality of pepper fruits during storage time (Fig. 1A). In the green and red stages, the weight loss in packaged peppers is less when compared to control at 7.2 °C and 90% RH for 21 days. Green peppers packaged with films lost 0.70 % with (P15G), 0.72 % with (P30NVC), 0.89 with % (P30NC), and 1.06 % with % (P12F) whereas control samples lost 3.37 % at 7.2 °C and 90 % RH for 21 days. Moreover, there was a significant difference between green pepper packaged with P15G films and P12 film compared to other packaging films. Green peppers packaged with P15G showed the lowest loss of weight compared to other packaging. Manolopoulou et al., (2010) reported that green peppers packaged with MDPE-30 lost 0.32 % of their weight, 0.65 % with LDPE-60, and 1.17 % with PVC unpackaged samples showed the largest weight loss (3.91 %). In the case of bell peppers that were packaged with low-density polyethylene, the weight loss was less than 2 % after 21 days of storage compared to that of the control samples.
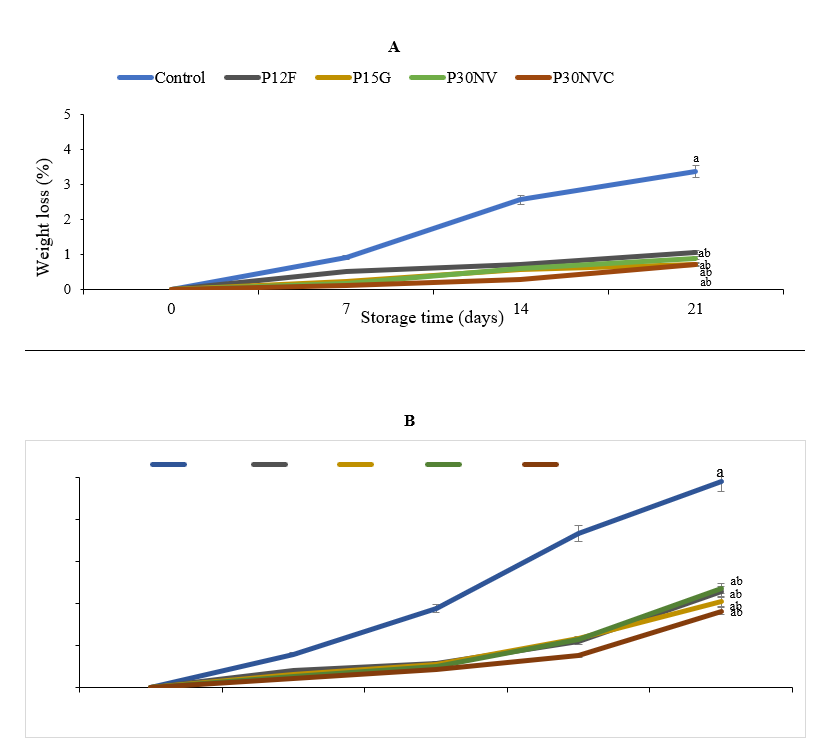
Fig. 1. Percent weight loss in Sweet Delilah at green stage (A) red stage (B) at 7.2 °C and 90 % humidity. Data are the mean of six replicates with standard deviation. Significant differences are denoted by different letters, while the same or shared letters indicate that they are not significant to each other. MC: marketing conditions (after 4 days).
Our findings exhibited that red peppers had a higher weight loss than green peppers (Fig.1B). Red peppers packaged with films lost 1.82 % (P30NC), 2.03 % (P15G), 2.35 % (P30NVC), and 2.28 % (P12F) whereas, control samples lost 4.91 %. The rate of weight loss depends on the type of crop andthe stage of maturity of a commodity. The difference in weight loss between green and red peppers, when stored under the same temperature and humidity conditions, may result from differences in physiological condition and respiration.
These results are similar to the findings of Mahajan et al., (2016), who reported that pepper fruits were stored at 18-20 °C with 90-95 % relative humidity packaged with various films such as heat shrinkable film (15μ), cling film (15μ) and low-density polyethylene film (LDPE25μ) showed the lowest weight loss compared to control samples.
There was a significant difference between red pepper treated 1-MCP stored in P30NC and peppers that were packaged with P15G films and P12 film. Furthermore, the lowest weight loss was 1.82 % in packaged peppers when treated with 1-MCP, whereas the highest weight loss was 4.91 % in control samples. These results agree with data described by Ilić et al., (2012), who studied the influence of 1-MCP on postharvest storage quality of bell pepper at 7 °C and 90 % RH for 18 days storage in dark conditions. They found that pepper fruits treated with 1-MCP exhibited less weight loss (3.2 %) compared to control samples (3.6 %). Similar results were obtained by Thakur et al., (2017), when peppers treated with 1000 ppb of 1-MCP. Our study showed that the quality attributes of peppers packaged with different films were observed to be higher than control fruits. These results suggested that pepper at red stage treated with 1-MCP showed to be the most effective treatment in maintaining fruit quality and minimizing deterioration during storage time of peppers.
3.2 Firmness
Firmness is a critical quality attribute for consumers. The use of packaging films in the food industry is essential because they provide excellent protection against changes in texture during storage time (Diane et al., 2010). Several studies have indicated that the change in firmness in fruits and vegetables is related to moisture loss through transpiration and enzymatic changes in the cell wall (Požrl et al., 2010).
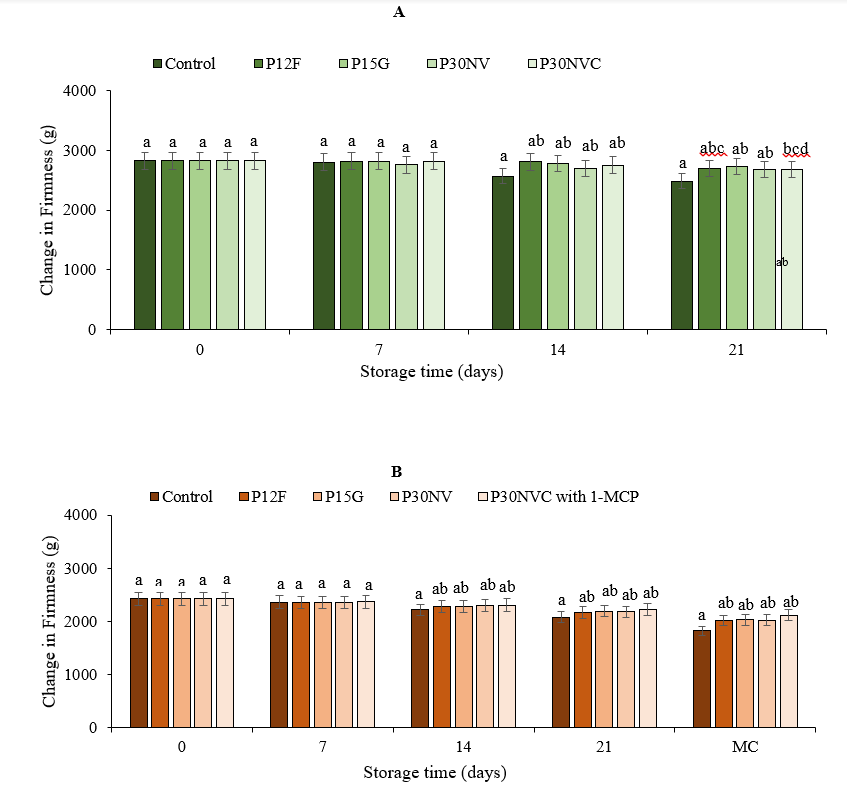
Fig. 2. Firmness levels in green stage (A) and red stage (B) of Sweet Delilah at 7.2 °C and 90 % humidity. Data are the mean of six replicates with standard deviation. Significant differences are denoted by different letters, while the same letters indicate that they are not significant to each other. MC: marketing conditions (after 4 days).
During storage, green pepper firmness was reduced by 3.11 % with P15G, 4.35 % with P12F, 4.84 % in P30NVC, 4.87 % with P30NV, and 12.10 % in the control peppers (Fig. 2A) whereas the firmness of red peppers was reduced to 13.04 % with P30NVC, 16.6 % with P15G, 16.8 % with P30NV, 16.9 % with P12F, and 25.07 % in control peppers (Fig. 2B). Tsegay et al. (2013), reported that the firmness of sweet bell pepper decreased with an increase in storage time. The results demonstrated that red peppers that were treated with 1-MCP and stored with P30NVC films maintained a higher level of firmness than peppers that were packaged with other packaging films and the control samples Thakur et al. (2017) found that pepper fruits that were treated with 1-MCP (1000 ppb) exhibited the highest mean texture compared to control fruits. Grzegorzewska et al., (2020) reported the treatment of pepper fruits with various concentrations of 1-MCP (1.0 μl·dm-3, 3.0 μl·dm-3, and 5.0 μl·dm-3) at 0 °C and 5 °C for up to 8 days slowed softening compared to untreated fruits. In our study, peppers treated with 1-MCP maintained a high level of firmness throughout 21 days at 7.2 °C and four days in retail marketing conditions.
3.3 Respiration rate and ethylene levels
Respiration is one of the most important physiological processes that occur in all commodities. Decreasing the respiration rate is one of the primary objectives after harvest to delay the ripening process and preserve the quality of vegetables and fruits. We estimated the levels of respiration rate in green peppers and red peppers during storage time and compared them with control samples (Fig 3).
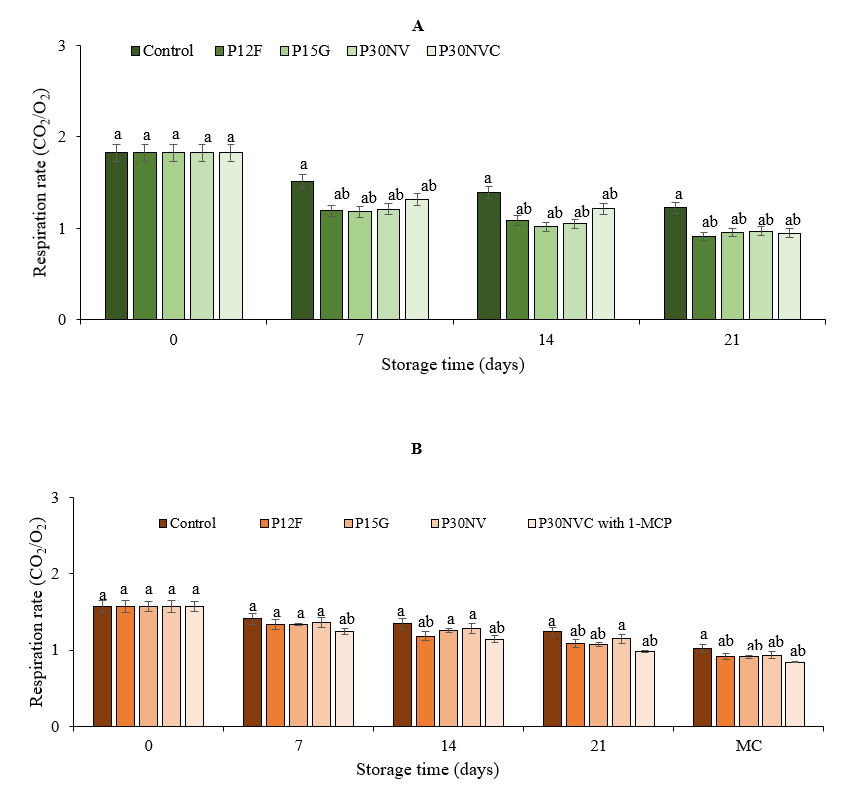
Fig. 3. Respiration rate in Sweet Delilah green stage (A) and red stage (B) at 7.2 °C and 90 % humidity. Data are the mean of six replicates with standard deviation. Significant differences are denoted by different letters, while the same letters indicate that they are not significant to each other. MC: marketing conditions (After 4 days).
In green peppers, the highest respiration rate was in control samples, whereas the lowest rate of respiration rate was in packaged peppers with (P12F) films. It was observed that the respiration rates in packaged peppers with films decreased with increasing storage time in both the green and red stages compared to those of the control samples. These results are in agreement with those of Hameed et al. (2013), who found that pepper fruits that were stored at 10 °C and 90-95 ±RH exhibited the lowest respiration rate compared to control samples. Similar observations were made by Manolopoulou et al. (2012), who observed that the levels of O2 were decreased in packaged peppers whereas the levels of CO2 were increased during storage. Singh et al., (2014), observed that storage temperatures influenced respiration rates, and the smallest decrease in respiration rate was measured at 10 °C during the storage period. Pearson correlation analysis of respiration rate and weight loss and was carried out (Table 3). The respiration rate and weight loss had a negative correlation in all packaging films in both green and red stages respectively. A significant difference was observed between red peppers treated with 1-MCP and packaged with P30NVC films and untreated red peppers that were packaged with P12 and 15G films after three weeks of storage. The largest decrease in respiration rate was 0.88 ml kg-1 h-1 in peppers that were treated with 1-MCP, whereas the lowest respiration rate was 1.02 ml kg-1 h-1 in the control samples. Thakur et al. (2017) reported that the lowest mean respiration rate was observed in pepper fruits that were treated with 1000 ppb 1-MCP at 10 °C and 90-95 % RH for 28 days of storage compared to control samples.
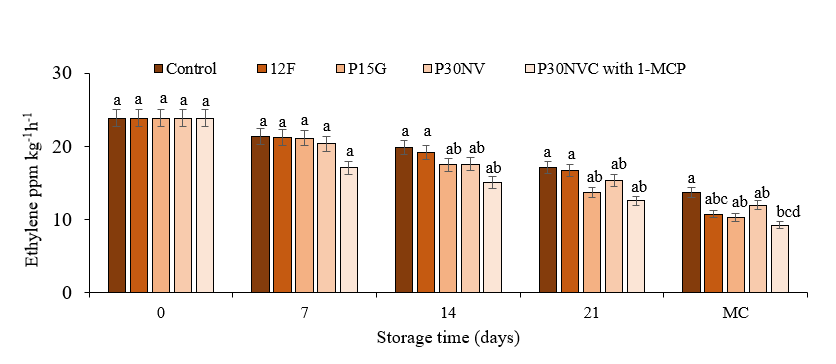
Fig. 4. Ethylene levels in Sweet Delilah at red stage at 7.2 °C and 90 % humidity. Data are the mean of six replicates with standard deviation. Significant differences are denoted by different letters, while the same or shared letters indicate that they are not significant to each other. MC: marketing conditions (After 4 days).
Studies have demonstrated that ethylene exhibits both beneficial and deleterious effects on produce. Shorter storage life, promotion of senescence, fruit softening, and discoloration are examples of deleterious effects of ethylene on peppers and other vegetables and fruits (Mahajan et al., 2016). The application of 1-MCP has been shown to delay the ripening process by slowing the respiration rate in several vegetables and fruits. Significant variations in ethylene production level were observed in peppers that were packaged and peppers that were treated with 1-MCP compared to the level of the control (Fig 4). We observed that ethylene production was decreased in all peppers that were packaged and peppers that were treated with 1-MCP during storage compared to that of the control. Our data show that the lowest ethylene production level was 9.25 ppm kg-1 h-1, measured in peppers treated with 1-MCP, whereas the highest ethylene production was 13.6 ppm kg-1 h-1, which was measured in the control samples. Fernández-Trujillo et al. (2009) reported that red pepper treated with 900 ppb of 1-MCP and storage at 8 °C in polypropylene package inhibited ethylene production. Huang et al. (2003) found that pepper fruits that were treated with 250 nmol/liter of 1-MCP and stored at 20 °C in polyethylene bags (0.02-mm thick) for 18 days showed less ethylene production than untreated fruits.
3.4 Color change
The change of color is an important indicator of the maturity and quality of fresh pepper. The color intensity in pepper fruits is one of the essential quality parameters that determine acceptance (Alcock and Bertling., 2013). Due to differences in carotenoid composition, peppers exhibit a range of colors, including green, yellow, orange, and red. Several studies have suggested that variations in color change can be attributed to physicochemical reactions, such as biochemical synthesis and metabolic interconversion of xanthophylls and carotenoids associated with the ripening process (Marcus et al., 1999). Therefore, we estimated the changes in color in packaged peppers with various films and peppers at the red stage that were treated with 1-MCP during the storage time.
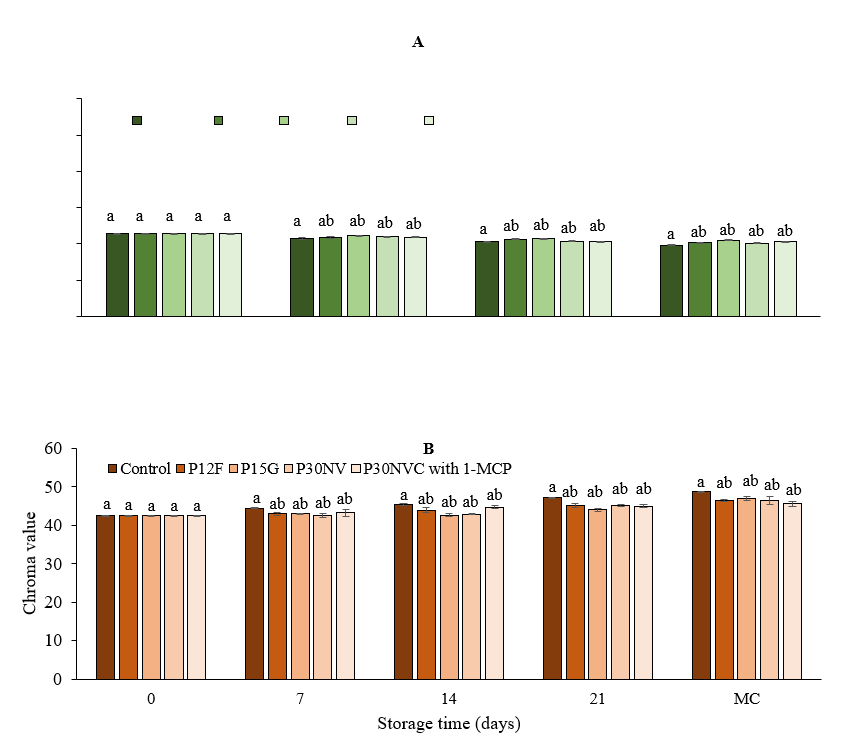
Fig. 5. Chroma values in Sweet Delilah at green stage (A) and red stage (B) at 7.2 °C and 90 % humidity. Data are the mean of six replicates with standard deviation. Significant differences are denoted by different letters, while the same or shared letters indicate that they are not significant to each other. MC: marketing conditions (After 4 days).
During storage time, chroma values of fruits and vegetables can change rapidly with storage conditions. At the end of storage time, the chroma values of green peppers were 20.19 with P30NVC 20.31 with P12F, 20.52 with P30NV, 20.92 withP15G, and 19.62 in control peppers (Fig. 5A). The chroma values at red stage peppers showed a significant difference during the storage time with control peppers (Fig. 5B). After two weeks of storage, there was a significant difference between green pepper packaged with P30NVC and P15G films. Overall, we observed that the chroma values of the green peppers decreased, whereas the chroma values of the red peppers increased. We found that the peppers that were packaged with films maintained their green color better than the controls. Significant differences were observed between red peppers treated with 1-MCP and stored in P30NVC and untreated peppers packaged with P12F. Lim et al., (2007) and Gonzalez-Aguilar et al. (1999) reported that the chroma values of green peppers that were stored in low-density polyethylene maintained green color even after four weeks.
The red peppers samples that were treated with 1-MCP showed a significant delay in the ripening processes and color change during storage. According to a study that was conducted by Fox et al. (2005), the chroma values increased from 24.2 to 29.9 in bell peppers that were stored at 20 °C with 90 % RH for ten days. The chroma values increased with increasing RH during storage at 15 °C according to the study conducted by (Nunes et al., 2012). Fernández-Trujillo et al. (2009) found that when peppers were treated with 900 ppb1-MCP and stored at 8 °C in polypropylene bags showed an increase in chroma values during storage. Various studies have suggested that 1-MCP treatment delays the color change of pepper fruits by inhibiting ethylene production during storage.
3.5 Phenolic, flavonoids and ascorbic acid
Peppers are an excellent source of bioactive compounds, including carotenoids, phenolics, and flavonoids. The total phenolic (TF) and total flavonoid (TF) contents of the green and red stages are presented in Table 1. At the beginning of the experiment, there were no significant differences in TP level among peppers that were packaged with various films and control samples in either stage. Substantial variations (p ≤ 0.05) in TP content were observed in green peppers that were packaged with various films. In the green stage, the smallest TP loss was 4.93 %, which was observed in peppers that were packaged with P30NV, whereas the largest TP loss was 11.98%, which was observed in the control samples. The results demonstrated that the TP levels decreased with increasing storage time, and the largest TP loss was established in the control samples. Red pepper fruits that were treated with 1-MCP in P30NVC films showed the smallest loss of phenolic compounds, whereas the control showed the largest loss of phenolic compounds.
The TF levels in green peppers that were packaged with the considered films followed the order P15G > P12F > P30NV > P30NVC > control samples. There was a significant loss in TF during storage; the largest loss of TF was 7.77 % in the control samples, whereas the smallest loss of TF was 3.79 % in P30NV.
The decrease in phenolic compounds is attributed to oxidation by polyphenol oxidase (Yamaguchi et al., 2003). Szwejda-Grzybowska et al. (2016) reported that pepper fruits that were stored at 5 °C for four days exhibited decreases in polyphenol content by 2–7 % in the Blondy variety and 11–20% in the Yecla variety. Many researchers reported similar results that TP in peppers decreases with increasing storage time (Barbagallo et al., 2012; Haishan et al., 2019; Iqbal et al., 2015). Chung et al. (2012) reported that peppers treated with 90 ppb1-MCP and stored at 10°C in polyethylene bags (50 μm) maintained high levels of phenolic content. The results demonstrated that peppers that were packaged with various films and treated with 1-MCP showed increased stability of bioactive compounds during storage. Additionally, the packaging of peppers with various films effectively slowed the decreases in the total phenolic and total flavonoid contents in both the green and red stages.
Vitamin C is one of the most important bioactive compounds in peppers which plays an essential role as an antioxidant compound (Zhang et al., 2003). A wide range of ascorbic acid concentrations have been reported in pepper cultivars; hence, the differences are related to the type of variety, genetic variation, ripening stage, and climatic conditions (Kumar et al., 2009). Several studies suggest that the level of ascorbic acid in fruits is associated with carbohydrate metabolism. The level of ascorbic acid is high in ripening fruits due to the accumulation of sugars during the advanced ripping process (Campos et al., 2013).
Table 2. Change in content of phenolic, flavonoid, and ascorbic acid content in green and red stages of Sweet Delilah at 7.2 °C and 90 % humidity. Values of total phenolic and total flavonoids are expressed as (µg\g). Data are mean of six replicates with standard deviation. Significant differences are denoted by different letters, while the same or shared letters indicate that they are not significant to each other. NM: not measurement. MC: marketing conditions (after 4 days).
|
Bioactive compounds |
||||||||||||||||
|
Total phenolic (µg/g) |
Total flavonoids (µg/g) |
Ascorbic acid (mg/100g) |
||||||||||||||
|
Stages |
Packaging films |
Zero Time |
7th |
14th |
21st |
MC |
Zero Time |
7th |
14th |
21st |
MC |
Zero Time |
7th |
14th |
21st |
MC |
|
Green |
P15G |
3785a±116 |
3715a±138 |
3693a±20 |
3576b±18 |
NM |
520a±31 |
517a±35 |
515a±16 |
505a±42 |
NM |
420a±25 |
418a±8 |
415a±21 |
415a±13 |
NM |
|
P12F |
3785a±116 |
3706a±120 |
3697a±36 |
3581b±28 |
NM |
520a±31 |
514a±39 |
509a±11 |
504a±31 |
NM |
420a±25 |
415a±8 |
413a±9 |
411a±6 |
NM |
|
|
P30NVC |
3785a±116 |
3717a±29 |
3684a±61 |
3568b±16 |
NM |
520a±31 |
518a±16 |
512a±4 |
503a±8 |
NM |
420a±25 |
417a±3 |
415a±25 |
405a±13 |
NM |
|
|
P30NV |
3785a±116 |
3726a±155 |
3655a±64 |
3598b±67 |
NM |
520a±31 |
515a±13 |
511a±49 |
502a±20 |
NM |
420a±25 |
417a±6 |
416a±4 |
421a±3 |
NM |
|
|
Control |
3785a±116 |
3694a±35 |
3548a±26 |
3331a±127 |
NM |
520a±31 |
510a±41 |
501a±4 |
490ab±19 |
NM |
420a±25 |
412a±9 |
389a±45 |
371ab±3 |
NM |
|
|
Red |
P15G |
5090a±242 |
5082a±279 |
4994a±29 |
4882a±34 |
4778a±47 |
647a±37 |
639a±31 |
633a±42 |
628a±52 |
622a±48 |
497a±9 |
491a±2 |
488a±3 |
482a±3 |
480a±3 |
|
P12F |
5090a±242 |
5067a±203 |
4915a±82 |
4850a±80 |
4807a±30 |
647a±37 |
642a±29 |
634a±36 |
625a±37 |
614a±41 |
497a±9 |
493a±4 |
485a±1 |
483a±6 |
480.4a±1 |
|
|
P30NVC + MCP |
5090a±242 |
5035a±267 |
5026a±85 |
4912a±61 |
4882a±52 |
647a±37 |
642a±45 |
634a±38 |
622a±33 |
615a±6 |
497a±9 |
489a±2 |
485.3a±1 |
482a±7 |
478a±4 |
|
|
P30NV |
5090a±242 |
5047a±257 |
5007a±290 |
4791a±133 |
4773a±75 |
647a±37 |
639a±33 |
636a±35 |
624a±37 |
610a±19 |
497a±9 |
491.2a±2 |
486a±1 |
484a±2 |
479a±1 |
|
|
Control |
5090a±242 |
4900a±230 |
4773a±190 |
4406ab±107 |
4304ab±24 |
647a±37 |
633a±43 |
627±a51 |
620ab±46 |
597ab±9 |
497a±9 |
487a±2 |
479a±1 |
475ab±1 |
472ab±0.3 |
|
A significant variation (p ≤ 0.05) was observed in ascorbic acid content between the packaged peppers and controls (Table 1). The results demonstrated that the red peppers had the highest level of ascorbic acid content at the beginning of storage time, whereas the green peppers had the lowest level. During storage, the largest loss of ascorbic acid was observed in the control samples, whereas the smallest loss of ascorbic acid was in peppers that were packaged with P30NVC film. Our data showed that the ascorbic acid level decreased as the storage time was increased in the packaged peppers and control samples. During the red stage storage period, the largest loss of ascorbic acid was 4.9 % in the control samples, whereas the smallest loss of ascorbic acid was 3.2 % in peppers that were packaged with P12G films. Various studies reported similar results on the loss of ascorbic acid in peppers during storage (Sahoo et al., 2014; Haishan et al., 2019; Chávez-Mendoza et al., 2015)
3.6 Antioxidant activity
Antioxidant compounds of fruits and vegetables play an essential role in reducing the risk of several chronic diseases (Singh et al., 2015). Antioxidant activity is associated with bioactive compounds such as vitamin C, phenolic, and flavonoid compounds. Antioxidant compounds can inhibit free radical compounds due to the redox properties of their hydroxyl groups (Palma et al., 2015). The difference in antioxidant activity between green and red peppers could be explained by their differences in carotenoid, phenolic, and flavonoid contents (Sun et al., 2007).
Table 3. Change of antioxidant activity in green and red stages of Sweet Delilah at 7.2 °C and 90 % humidity. Values of DPPH are expressed as percentage, and ABTS are expresses as (µmol TE/g). Data are mean of six replicates with standard deviation. Significant differences are denoted by different letters, while the same or shared letters indicate that they are not significant to each other. NM: not measurement. MC: marketing conditions (after 4 days).
|
Antioxidant Activity |
|||||||||||
|
|
|
DPPH (%) |
ABTS (µmol TE/g) |
||||||||
|
Stages |
Packaging films |
Zero time |
7th |
14th |
21st |
MC |
Zero time |
7th |
14th |
21st |
MC |
|
Green |
P15G |
68a±11 |
68a±11 |
67a±10 |
65a±11 |
NM |
122a±5 |
119a±8 |
115a±8 |
114a±1 |
NM |
|
P12F |
68a±11 |
67a±11 |
67a±12 |
65a±12 |
NM |
122a±5 |
118a±1 |
115a±4 |
114a±1 |
NM |
|
|
P30NVC |
68a±11 |
67a±11 |
67a±11 |
65a±12 |
NM |
122a±5 |
118a±2 |
116a±1 |
113a±2 |
NM |
|
|
P30NV |
68a±11 |
68a±11 |
66a±11 |
66a±11 |
NM |
122a±5 |
118a±1 |
115a±1 |
112a±3 |
NM |
|
|
Control |
68a±11 |
65a±11 |
64a±12 |
61a±13 |
NM |
122a±5 |
114a±1 |
113a±5 |
108a±7 |
NM |
|
|
Red |
P15G |
72a±11 |
71a±3 |
70a±4 |
66a±8 |
65a±6 |
156a±2 |
154a±1 |
153a±1 |
153a±1 |
148a±4 |
|
P12F |
72a±3 |
70a±4 |
69a±3 |
67a±2 |
66a±6 |
156a±2 |
154a±1 |
152a±2 |
151a±1 |
150a±1 |
|
|
P30NVC + MCP |
72a±3 |
71a±4 |
71a±4 |
67a±4 |
66a±1 |
156a±2 |
154a±1 |
151a±1 |
150a±2 |
149a±2 |
|
|
P30NV |
72a±3 |
70a±2 |
69a±3 |
68a±1 |
65a±1 |
156a±2 |
153a±2 |
152a±2 |
149a±2 |
148a±3 |
|
|
Control |
72a±3 |
70a±7 |
67a±5 |
64a±1 |
54ab±1 |
156a±2 |
153a±1 |
148a±3 |
146ab±2 |
143ab±3 |
|
The present study evaluated the antioxidant activities of green and red peppers that were packaged with films and those of control samples. The results of this study demonstrated that packaged peppers and control samples in the green stage did not show significant differences in DPPH or ABTS activity. Kevers et al. (2007) reported that green pepper storage does not negatively affect antioxidant capacity. Our results demonstrated that red peppers had higher DPPH activity than green peppers (p ≤ 0.05). This is due to the presence of more total phenolics and ascorbic acid in the red stage than in the green stage (Table 2). During storage, the largest loss of ABTS activity was 5.86 % in the control samples, whereas the smallest loss of ABTS activity was 1.9 % in the red peppers that were packaged with P15 films. The reduction in antioxidant activity could be related to the loss of antioxidant compounds, such as total phenol and L-ascorbic acid, during storage (Haishan et al., 2019). We found that the antioxidant activity decreased with advanced storage time in both the green and red stages, which agrees with the findings of (Chitravathi et al., 2015; Devgan et al., 2019). According to this study, pepper fruits packaged with various films and pepper fruits treated with 1-MCP showed higher antioxidant activity during storage.
Table 4. Pearson’s correlation coefficient analysis between respiration rate and weight loss in at green stage and red stages of Sweet Delilah packaged with different films.
|
Variables |
Green stage |
|||
|
Respiration Rate |
||||
|
Weight loss |
P12F |
P15G |
30NV |
30NVC |
|
P12F |
1 |
|
|
|
|
|
0.016 |
|
|
|
|
|
-0.99 |
|
|
|
|
P15G |
|
1 |
|
|
|
|
|
0.061 |
|
|
|
|
|
-0.95 |
|
|
|
30NV |
|
|
1 |
|
|
|
|
|
0.023 |
|
|
|
|
|
-0.792 |
|
|
30NVC |
|
|
|
1 |
|
|
|
|
|
0.025 |
|
|
|
|
|
-0.99 |
|
|
Red stage |
|||
|
|
P12F |
P15G |
30NV |
30NVC+MCP |
|
P12F |
1 |
|
|
|
|
|
0.065 |
|
|
|
|
|
-0.95 |
|
|
|
|
P15G |
|
1 |
|
|
|
|
|
0.004 |
|
|
|
|
|
-.091 |
|
|
|
30NV |
|
|
1 |
|
|
|
|
|
0.094 |
|
|
|
|
|
-0.989 |
|
|
30NVC+MCP |
|
|
|
1 |
|
|
|
|
|
0.150 |
|
|
|
|
|
-0.972 |
Sweet Delilah pepper in both green and red stages packaged with different films were tested during storage time and marketing conditions. Packaged peppers with P15G and P12F films presented significantly less weight loss, lower ethylene and respiration rates, and less texture change compared to the control peppers. Also, the results indicate that using 1-MCP can effectively delay weight loss, color change, maintain firmness, extend the shelf life and retain the nutritional value of peppers. Finally, packaged Sweet Delilah peppers treated with 1-MCP effectively slowed down the loss of total phenolic, total flavonoids, and ascorbic acid in the red stage. The data suggest that packaged peppers preserved more phenolics and retained antioxidant activity compared to control samples.
Alcock, C.M., & Bertling, I. (2013). Effect of light and fruit Mmaturity on postharvest colour change in green 'Sondela' peppers (Capsicum annuum L.). In: Hannweg, K., Penter, M. (Eds.), Ii All Africa Horticulture Congress. Leuven 1, International Society for Horticultural Science, 107, 171-177.
View ArticleAlvarez-Parrilla, E., De La Rosa, L.A., Amarowicz, R., & Shahidi, F. (2011) . Antioxidant activity of fresh and processed Jalapeno and Serrano peppers. J. Agric. Food Chem, 59, pp. 163-173.
View ArticleAwole, S., Woldetsadik, K., & Workneh, T.S. (2011). Yield and storability of greenfruits from hotpepper cultivars (Capsicumspp.). African Journal of Biotechnology, 10(56), 12662-12670.
View ArticleBarbosa, C., Machado, T., & Alves M.R. (2020). Fresh-Cut Bell Peppers in Modified Atmosphere Packaging: Improving Shelf Life to Answer Food Security Concerns. Molecules, 25(10),2323.
View ArticleBarbagallo, R.N., Chisari, M., & Patané, C. (2012). Polyphenol oxidase, total phenolics and ascorbic acid changes during storage of minimally processed 'California Wonder'and 'Quadrato d'Asti'sweet peppers. LWT-Food Science and Technology, 49(2), 192-196.
View ArticleCampos, M., Gómez, K., Moguel-Ordonez, Y., & Betancur, D. (2013). Polyphenols, Ascorbic Acid and Carotenoids Contents and Antioxidant Properties of Habanero Pepper (Capsicum chinense) Fruit. J. Food. Nutr Sci, 3 (4), 47-54.
View ArticleCastro, J. M., Avila, V.C.M., Rocha, F.M., Ochoa, M.A., & Gallegos, A.I. (2002). Effect of controlled atmospheres on quality of green pepper poblano (ancho). Proceedings of the 16th International Pepper Conference. November 10-12, Tampico, Tamaulipas, Mexico.
Chávez-Mendoza, C., Sanchez, E., Muñoz-Marquez, E., Sida-Arreola, J.P., & Flores-Cordova, M.A. (2015). Bioactive compounds and antioxidant activity in different grafted varieties of bell pepper. Antioxidants, 4(2), 427-446.
View ArticleChitravathi, K., Chauhan, O.P., & Raju, P.S. (2015). Influence of modified atmosphere packaging on shelf-life of green chillies (Capsicum annuum L.). J. Food Pack. Shelf Life, (4),1-9.
View ArticleChung, K.T., Zainon, M.A., Ismanizan, I., & Zamri, Z. (2012). "Effects of 1-Methylcyclopropene and Modified Atmosphere Packaging on the Antioxidant Capacity in Pepper "Kulai" during Low-Temperature Storage". The Scientific World Journal, (3-4), 1-10.
View ArticleDevgan, K., Kaura, P., Kumarm, N., & Kaur, A. (2019). Active modified atmosphere packaging of yellow bell pepper for retention of physico-chemical quality attributes. J. food. Sci. Techno, 56 (2), 878-888.
View ArticleDiane, M.B., John., C.B., & Rob, S. (2010). Color, Flavor, Texture, and Nutritional Quality of Fresh-Cut Fruits and Vegetables: Desirable Levels, Instrumental and Sensory Measurement, and the Effects of Processing. J. Food Sci. Nutrition, 50, 369-389.
View ArticleErin, M., O'D., David, A.B., Marian., J.M., Donald, A.H., & Ross, E.L. (2018). Sweet capsicum: postharvest physiology and technologies, New Zealand. J. Crop. Horti. Sci 46(4):269-297.
View ArticleFernández-Trujillo, J. P., J. M. Serrano., & Martinez, J.A. (2009). Quality of red sweet pepper fruit treated with 1-MCP during a simulated postharvest handling chain. J. Food Science. Technolo. International (FSTI), 15 (1), 23-30.
View ArticleFox, A. J., Del Pozo-Insfran, D., Lee, J.H., Sargent, S. A., & Talcott, S.T. (2005). Ripening-induced chemical and antioxidant changes in bell peppers as affected by harvest maturity and postharvest ethylene exposure. HortScience, 40(3), 732-736.
View ArticleGonzalez-Aguilar, G.A., Cruz, R., & Baez, R. (1999). Storage quality of bell peppers pretreated with hot water and polyethylene packaging. J. Food. Qual, 22, 287-299.
View ArticleGrzegorzewska, M., Badełek, E., Wrzodak, A., Fabiszewski, K., & Ciecierska, A. (2020). Quality and storage ability of fresh-cut pepper treated by 1-methylcyclopropene. Journal of Horticultural Research, 28(1), 101-110.
View ArticleHaishan, X., Shenghua, D., Hui, Z., Youjin, Y., Fangming, D., & Rongrong, W. (2019). Quality attributes and related enzyme activities in peppers during storage: effect of hydrothermal and calcium chloride treatment. International Journal of Food Properties, 22(1), pp.1475-1491.
View ArticleHamed, M., Kalita, D., Bartolo, M.E., & Jayanty, S.S. (2019).Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods. Antioxidants, 2019, 8(9), 364.
View ArticleHervert-Hernández, D., Sáyago-Ayerdi, S.G., & Goñi, I. (2010). Bioactive compounds of four hot pepper varieties (Capsicum annuum L.), antioxidant capacity, and intestinal bioaccessibility. J. Agric. Food Chem, 58(6), 3399-3406.
View ArticleHuang, X. M.,. Zhang, Z. Q., & Duan, X. W. (2003). Effects of 1- methylcyclopropene on storage quality of hot pepper (Capsicum frutescens) at room temperature. China Vegetables, 1, 9-11.
Iqbal, Q., Amjad, M., Asi, M. R., Ariño, A., Ziaf, Z., Nawaz, A., & Ahmad, T. (2015). Stability of Capsaicinoids and Antioxidants in Dry Hot Peppers under Different Packaging and Storage Temperatures. Foods, 4(2),51-64.
View ArticleIlić, Z., Trajković, R., Perzelan, Y., Alkalai-Tuvia, S., & Fallik, E. (2012). Influence of 1-Methylcyclopropene (1-MCP) on Postharvest Storage Quality in Green Bell Pepper Fruit. Food and Bioprocess Technology, 5(7), 2758-2767.
View ArticleKalita, D., & Jayanty, S.S. (2014). Comparison of Polyphenol Content and Antioxidant Capacity of Colored Potato Tubers, Pomegranate and Blueberries. J. Food. Process.Technol, 5(8), 1-7.
View ArticleKevers, C., Falkowski, M., Tabart, J., Defraigne, J.O., Dommes, J., & Pincemail, J. (2007). Evolution of antioxidant capacity during storage of selected fruits and vegetables. J. Agric. Food. Chem, 55(21), 8596-8603.
View ArticleKumar, O.A., & Tata, S.S. (2009). Ascorbic acid contents in chilli peppers (Capscium L.). J. Nat Sci Biol, 1(1), 50-52.
View ArticleLim, C. S., Kang, S. M., Cho, J.L., & Gross, K.C. (2007). Bell pepper (Capsicum annuum L.) fruits are susceptible to chilling injury at the breaker stage of ripeness. Hortscience. 42 (7), 1659-1664.
View ArticleMahajan, B.V.C., Bhullar, K., & Dhillon, W. (2010). Effect of 1- methylcyclopropane (1-MCP) on storage life and quality of pear fruits. J. Food Sci. Technol. 47(3), 351-354.
View ArticleMahajan, B. V. C., Dhillon, W. S., Sidhu, M. K., Jindal, S. K., Dhaliwal, M. S., & Singh, S. P. (2016). Effect of packaging and storage environments on quality and shelf-life of bell pepper (Capsicum annuum). Indian Journal of Agricultural Sciences, 86(6), 738-742.
Manolopoulou, H., Lambrinos, G., & Xanthopoulos, G. (2012). Active Modified Atmosphere Packaging of Fresh-cut Bell Peppers: Effect on Quality Indices. J. Food. Research 1(3), 140-158.
View ArticleMarcus, F., Daood, H.G., Kapitany, J., & Biacs, P.A. (1999). Change in the carotenoid and antioxidant content of spice red pepper (paprika) as a function of ripening and some technological factors. J. Agri. Food. Chem,47(1), 100-107.
View ArticleNunes, M.CN., Delgado, A., & Emond, J.P. (2012). Quality curves for green bell pepper (Capsicum annuum L) stored at low and recommended relative humidity levels. Journal of the American Society for Horticultural Science, 127(5), 71-78.
View ArticleOz, A.T., & Ulukanli, Z. (2011). Effects of 1-methylcylopropene (1-MCP) and Modifed Atmosphere Packing (MAP) on postharvest browning and microbial growth of loquat fruit. J. Applied. Botany. Food Quality, 84(2), 125 - 133.
Palma, J.M.P., Sevilla, F., Jime ́nez, ́n., A., del R ́ıo, L. A., Corpas, F.J., de Morales, P.A., & Camejo, D.M. (2015). Physiology of pepper fruit and the metabolism of antioxidants: chloroplasts, mitochondria and peroxisomes. J. Ann Bot, 116(4), 627-636.
View ArticlePožrl, T., Žnidarčič, D., Kopjar, M., Hribar, J., & Simčič, M., (2010). Change of textural properties of tomatoes due to storage and storage temperatures. J. Food .Agric. Environ, 8(2), 292-296.
Ramchiary, N., Kehie, M., Brahma, V., Kumaria, S., & Tandon, P. (2013). Application of genetics and genomics towards Capsicum translational research. Plant Biotechnology Report, 1-23.
View ArticleSahoo, N., Bal, L., Pal, U., & Sahoo, D. (2014). A comparative study on the effect of packaging material and storage environment on shelf life of fresh bell-pepper. J. Food Measure, 8(3), 164-170.
View ArticleSharma, A., Woldetasaidik, K., & Workmen, T.S. (2013). Postharvest quality and shelf life of some hot pepper varieties. J. Food Sci.Technol ,50(5), 842-855.
View ArticleSingh, R., Giri, S.K., & Rao, K. (2014). Respiration rate model for mature green capsicum (Capsicum annum L.) under closed aerobic atmospheric conditions. J. Food Sci. Technol. 6 (2), 110-115.
View ArticleSingh, J.P., Kaur, A., Shevkani, K., & Singh, N. (2015). Influence of jambolan (Syzygium cumini) and xanthan gum incorporation on the physicochemical, antioxidant and sensory properties of gluten- free eggless rice muffins. Int .J. Food. Sci .Technol. 50,(5),1190-1197.
View ArticleSora, G.T.S., Haminiuk, C.W.I., Vieira da Silva, M., Zielinski, A.A.F., Gonçalves, G.A., Adelar Bracht, A., & Peralta, R.M. (2015). A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: An application of chemometrics. J. Food Sci. Technol, 52(12), 8086-8094.
View ArticleSun, T., Xu, Z., Wu, C.T., Janes, M., Prinyawiwatkul, W., & No, H.K. (2007). Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). Journal of Food Science, 72(2), S98-S102.
View ArticleSzwejda-Grzybowska, J.I., Kosson, R., & Grzegorzewska, M. (2016). The Effect of Short-term Storage and the Hot Water Treatment of Fresh-Cut Pepper Fruit cv. 'Blondy F1' and 'Yecla F1' on the Content of Bioactive Compounds and Antioxidant Properties. Journal of Horticultural Research, 24(2), 83-90.
View ArticleThakur, K.S., Jyoti, K., Kumar, S., & Gautum, S. (2017). Improvement of Postharvest Keeping Quality of Bell Pepper (Capsicum annum L.) Fruits Treated with Different Chemicals following Cold Storage. Int. J. Curr. Microbiol. App. Sci. 6(7), 2462-2475.
View ArticleTsegay, D., Tesfaye, B., Ibrahim, A.M., Yirga, H., & Bayleyegn, A. (2013). Effects of harvesting stage and storage duration on postharvest quality and shelf life of sweet bell pepper (Capsicum annuum L.) varieties under passive refrigeration system. International Journal for Biotechnology and Molecular Biology Research, 4(7), 98-104.
Watada, A.E. (1982). High-performance Liquid Chromatography Method for Determining Ascorbic Acid Content of Fresh Fruits and Vegetables. HortScience, 17(3), 334-335.
Yamaguchi, T., Katsuda, M., Oda, Y., Terao, J., Kanazawa, K., Oshima, S., Inakuma, T., ishiguro, Y., Takamura, H., & Matoba, T. (2003). Influence of polyphenoloxidase and ascorbate oxidase during cooking process on the free radical scavenging activity of vegetables. J. Food Sci. Technol. Res, 9 (1)79-83.
View ArticleZhang, D., & Hamauzu, Y. (2003). Phenolic compounds, ascorbic acid and antioxidant properties of green, red and yellow bell peppers. J. Food .Agric. Environ,1(2), 22-27.
