Soo Peng Koh
Email: karenkoh@mardi.gov.my or karenkoh999@gmail.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 6 ISSUE: 3
Page No: 391-405
Soo Peng Koh
Email: karenkoh@mardi.gov.my or karenkoh999@gmail.com
Soo Peng Koh*1, Yun Shin Sew2, Sarah Sabidi 1, Syahida Maarof 1, Shaiful Adzni Sharifudin1,
Rosmawati Abdullah1
1Food Science and Technology Research Centre, Malaysian Agricultural Research and Development Centre, Persiaran MARDI-UPM, 43400 Serdang, Selangor, Malaysia.
2Biotechnology and Nanotechnology Research Centre, Malaysian Agricultural Research and Development Centre, Persiaran MARDI-UPM, 43400 Serdang, Selangor, Malaysia.
Soo Peng Koh, Yun Shin Sew, Sarah Sabidi, Syahida Maarof, Shaiful Adzni Sharifudin, Rosmawati Abdullah, SCOBY papaya beverages induce gut microbiome interaction as potential antiobesity in weight management control supplement(2021) Journal of Food Science & Technology 6(3) :391-405
The prevalence of obese populace is one of the major health concerns worldwide and had caused an economic burden to government and increased consumption of medical resources. In this study, we have developed new fermented papaya beverages using selected symbiotic culture of bacteria and yeast (SCOBY) under controlled biofermentation process. Two-months treatment on high fat diet-induced obese mice with SCOBY papaya beverages showed a remarkable body weight loss of 21.3% (SCOBY papaya leaves) and 23.4% (SCOBY papaya pulp), significantly higher than commercial anti-obesity drug, Orlistat (11.3%). Further investigation on blood glucose profile of all mice groups revealed a healthy level of blood glucose. Haematology analysis of obese mice fed with SCOBY papaya beverages demonstrated no adverse effect. Findings from quantitative PCR (qPCR) technique have disclosed anti-obesity mechanism of SCOBY papaya beverages. The 16S rRNA metagenomics sequencing analysis confirmed that gut microbiome of high fat diet-induced mice treated with individual SCOBY papaya beverage showed an increase in beneficial gut microbe abundances, particularly Bifidobacterium, Faecalibaculum, Akkermansia, Lactobacillus and Bacteroides. Comprehensive evidence findings indicated great potential of SCOBY papaya beverages as a functional drink therapy in weight management control and assist in improving gut microbiome of obese mice to healthier state.
Keywords: Anti-obesity, Fermentation, Gut microbiota, Papaya leaves, Papaya pulp
The incidence of global epidemic in obesity has emerged as one of the major health issues as a consequence of excessive consumption of energy-dense diets and sedentary lifestyle. In certain case, obesity is partially contributed by the key factors like age, psychiatric disorder, genetic predisposition (Ghee 2016). The obesity is a complicated condition as it facilitates the development of various metabolic disorders such as diabetes, osteoarthritis, cardiovascular disease, hypertensive effect and etc. as reported by scientific findings (Grundy 2004; Zhu et al. 2019). This phenomenon has resulting major social health problems, particularly in Malaysia which has encountered dilemma in controlling populace obesity as Malaysian foods are mainly high in sugars and fats. The latest statistical data showed that Malaysia has experienced an alarming increase in the prevalence of obesity. Nearly half of its 30 million Malaysian populace are overweight (38.5%) and obese (13.3%), causing huge economic burden to the nation (New Straits Times 2018). With the rapid expansion of obesity epidemic worldwide, it is crucial to develop new effective anti-obesity therapeutic agent for prevention and control measures to tackle the global obesity scenario.
Development of health functional foods is a new emerging area that has been explored extensively as innovative approach to meet consumer demand for maintaining good health. Microbial fermentation is one of the promising processing strategies used to improve the functional value of food. There are many fermented foods known to possess health beneficial effects as a result of microbial activity which produces more organic acids and secondary metabolites. For example, vinegar and Kimchi are some of the nutritionally enhanced fermented foods that have been reported to have anti-obesity effects, supported by evidence collected from the in-vivo studies which confirm their influences on gut microbiota and gene expression (Han et al. 2015; Beh et al. 2017). Papaya (Carica papaya Linn.) is ranking in the top 10 nutritionally beneficial fruits with significant health profile because of its rich source of phytonutrient content and high antioxidant capacity (Tacio 2020). The therapeutic value of papaya leaves has been confirmed with many published scientific evidence to support its contribution role as a food remedy for diabetes treatment (Juárez-Rojop et al. 2014), anti-inflammatory effect (Owoyele et al. 2008), immunomodulatory effects (Otsuki et al. 2010), chemopreventive effects (Tan et al. 2014), antiplasmodial effect (Ahmad et al. 2011) and analgesic activity (Hasimun and Ernasari 2014). The effectiveness of fermented papaya pulp was reported to have favourable effects in modulating immunological, neuroprotective, anti-inflammatory, antioxidant activity and prevention of diabetic complication from oxidative damage (Aruoma et al. 2010; Marotta et al. 2012; Barbagallo et al. 2015; Raffaelli et al. 2015).
The overweight may lead to alteration of gut microbiota and lowered immune system leading to higher disease risks (Li et al. 2017). The effects of high fat diet on the modification of composition and function of host obesity linked gut microbiota have been investigated in which involved of changes in energy extraction, intestinal permeability and systemic inflammation (Mulders 2018). The alteration of microorganisms that colonize gastrointestinal tract has reduced the capability to generate obesity-suppressing short chain fatty acids (SCFA) (Chambers et al. 2015; Li et al. 2017; Mulders 2018). Our work aims to investigate the potential of SCOBY papaya beverages as new cost-effective anti-obesity therapy treatment by altering the gut microbiota profile as preventives measure in combating the obesity problem in a natural way. Various assays have been conducted to investigate the mechanism of action of SCOBY papaya beverages in reducing body weight. The safety aspects of prolonged consumption of SCOBY papaya beverage were also included in this current study.
2.1. Preparation of SCOBY papaya beverages
In Malaysian Agricultural Research and Development Institute (MARDI), we have developed a new controlled biofermentation process to produce SCOBY papaya beverage using Kombucha consortium strains which was specifically selected from MARDI’s Collection of Function Food Cultures. The ultimate target of using selected pure mixed cultures is to improve the biological functionality of papaya phytonutrients (leaves, PL; pulp, PP) and ensure the consistency and quality of SCOBY papaya beverages. Papaya leaves and pulp were chosen as substrate to produce SCOBY papaya beverages as these two raw materials are enriched with valuable phytonutrients that have been reported to contain multiple pharmacological properties.
The papaya (Carica papaya Linn.) with variety of Sekaki was purchased from a local papaya plantation in Temerloh, Pahang. A suspension of papaya pulp and leaves at the concentration of 5% was prepared and inoculated with 10% of selected Kombucha consortium strains (Dekkera sp. & Komagataiebacter sp.) with the colony count of at least 1×108 colony forming units/mL. Both papaya leaves and pulp were incubated at 30 °C for 4 days before subjected to centrifugation at 10,000 rpm for 5 min to remove substrate residue and the end product were named as SCOBY papaya leaves (PL) and SCOBY papaya pulp (PP), respectively. The SCOBY papaya beverages were pasteurised at 90°C for 30 min to stop the microbial growth. The carbohydrate and protein content of SCOBY PL and PP beverages were 5.8 g/100 mL, 0.3 g/100 mL and 9.6 g/100 mL, 0.4 g/100 mL, respectively. Both SCOBY PL and PP beverages did not contain any fat.
2.2. In vivo anti-obesity study
A total of 30 ICR mice were divided into 5 groups: a) normal diet (normal); b) high fat diet (HFD, C(-); c) HFD-Orlistat, C(+); d) HFD-SCOBY papaya pulp (PP); e) HFD-SCOBY papaya leaves (PL). All mice groups except for normal diet mice group, were fed with high fat diet (34.5% fat, D12491 purchased from Specialty Feeds, Australia) for 3 months to induce obesity (at least 20% body weight higher than normal diet mice) before treated with Orlistat (10 mg/kg), PP (2 mL/kg) & PL (2 mL/kg) for a period of 2 months with continually fed with high fat diet. The mice body weight, blood glucose profile was monitored and measured twice a month. The physical appearances of the mice such as sudden body weight drop, fur loss and reduce in mobility were observed. After 2 months treatment, mice were euthanized in CO2 chamber. The collected organs (kidney, liver, spleen & stomach) and adipose tissue were weighed before proceeded to histopathology analysis. All organs were fixed in neutral buffered 10% formalin solution and were embedded in paraffin, sectioned, stained with haematoxylin and eosin before examining individual organ tissue under microscope (Leica, Germany).
2.3. Determination of haematology, serum biochemistry and lipids profile
The blood was drawn afterwards via the brachial artery from all mice groups and stored in tubes containing anticoagulant EDTA-2K. The haemetology profile (Red blood cell, platelets, white blood cell, haemoglobin and haematocrit) were determined using automated haematology analyzer (Exigo Blood Haematology Analyzer, Sweden). Serum samples were obtained by centrifuging blood samples (Centrifuge S417R, Eppendorf, CA, USA) at 4,000 rpm for 10 min under chilled condition of 4 °C and were measured using a clinical chemistry autoanalyzer (DIRUI CS-300, China) to examine the liver function, kidney function and lipids profiles (alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), total protein, creatinine, urea readings, triglycerides, high density lipoprotein, low density lipoprotein and cholesterol level) for all treated HFD-induced obese mice and normal diet mice.
2.4. Quantification of short chain fatty acids via gas chromatography (GC)
Short chain fatty acids (SCFAs) were extracted from mice faecal samples and quantified using gas chromatography technique. A total of 0.3 g frozen faecal sample was smashed first using mortal and pastle before adding 3 mL distilled water with the adjusted pH to 2 using 10 M hydrochloric acid. The acidic solution was added onto tube containing Lysing Matrix E and subjected to homogenisation using FastPrep-24 homogenizer (MP Biomedicals, Germany) at the agitation rate of 10,000 rpm for 10 min to extract the SCFAs solution. The homogenate was transferred into new tubes and further centrifuged at 10,000 rpm for 5 min to remove unwanted residues before filtered using 0.22 µm syringe filter for GC injection. The SCFAs chromatographic analysis was carried out using Agilent 6890N GC system equipped with a flame ionization detector (FID). A Zebron ZB-Waxplus capillary column (30 m x 0.25 mm internal diameter x 0.25 µm film thickness) was used to separate the SCFAs peaks using gradient heating profile: The initial oven temperature was set for 50°C for 1 min before heating up the column to 200°C at 60°C/min and held for 1 min before further raised to 250°C with the heating rate of 20°C/min and then maintained at 250°C for 3 min. The nitrogen was used as carrier gas and the split ratio was set at 100:1 with the split flow of 99.4 mL/min. The injection port and FID temperature was set at 250°C. The flow rate of hydrogen, air and make up gas were maintained at 40, 350 and 30 mL/min, respectively with total run time of 10 min. The amount of SCFAs was quantified from external calibration curve of SCFAs standards (acetic acid, butyric acid and propionic acid) using HP Chemstation Plus software (Agilent, USA).
2.5. Quantitative real-time polymerase chain reaction (qPCR) analysis
The adipose tissue samples were collected from treated and control mice (three individual mice samples per group). Total RNAs were extracted from adipose tissue samples using RNeasy Lipid Tissue Mini kit (Qiagen, Germany). Complementary DNAs were synthesized using QuantiNova Reverse Transcriptase kit (Qiagen, Germany). Predesigned PrimeTime Assay Std Probes 5' FAM/ZEN/3' IBFQ were synthesized by Integrated DNA Technology, IDT, Singapore) as follows: Actin-b (Actb) (Mm.PT.39a.22214843.g), glyceraldehyde 3-phosphate dehydrogenase (GADPH) (Mm.PT.39a.1), hypoxanthine phosphoribosyl transferase-encoding gene (Hprt) (Mm.PT.39a.22214828), glucose transporter 4 (Slc2a4) (Mm.PT.58.9683859), sterol regulatory element binding protein-1 (SREBP-1) (Mm.PT.58.8508227), adiponectin receptor 1 (Adipor1) (Mm.PT.58.42620207), nitric oxide synthase 2 (NOS2) (Mm.PT.58.5680554), tumor necrosis factor alpha (TNF-α) (Mm.PT.58.12575861), transforming growth factor β1 (TGF-β1) (Mm.PT.58.11254750), matrix metalloproteinase-2 (MMP2) (Mm.PT.58.9606100), interleukin 6 (IL6) (Mm.PT.58.12506617) and C-C motif chemokine ligand 2 (CCL2) (Mm.PT.58.42151692). The qPCR assay was performed using synthesized PrimeTime Assay Std Probe 5' FAM/ZEN/3' IBFQ in a StepOnePlus real-time PCR system (Applied Biosystems, USA). The PCR cycling conditions used for sample analysis were as follows: 1 cycle of 95 °C (3 min) for DNA polymerase activation, 40 cycles of 95 °C (5 s) for denaturation, and 60 °C (30 s) for annealing and extension. The fluorescent dye ROX served as an internal reference for normalization of the FAM fluorescent signal. The fold expression value of each targeted gene in individual sample relative to normal control sample was calculated by normalizing individual expression value with the average expression value of a set of reference genes (Actb, GADPH and Hprt) using 2–[delta][delta]Ct method.
2.6. Gut microbiota analysis via 16S rRNA gene sequencing
The composition of gut microbiota of individual treated and control mice samples were evaluated via 16S rRNA sequencing on mice faecal samples. Bacterial genomic DNA was extracted from mice faecal samples of each group using NucleoSpin tissue mini kit (Macherey-Nagel, Germany) according to the manufacturer’s instructions. The 16S rRNA variable V3-V4 regions were amplified using 16S Amplicon PCR Forward Primer, 5’- CCTAYGGGRBGCASCAG-3’ and 16S Amplicon PCR Reverse Primer 5’-GGACTACNNGGGTATCTAAT-3’ and Phusion High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA, USA). Sequencing libraries were constructed using NEBNext® UltraTM DNA Library Prep Kit for Illumina ® according to the manufacturer’s instructions. Sequencing of 16S rRNA libraries was performed using lllumina HiSeq 2500 platform with 250 bp paired end reads generated. Paired-end reads were merged using FLASH (V1.2.7) (Magoč and Salzberg 2011) to generate raw tags before the raw tags were subjected to quality filtering to obtain clean tags using QIIME (V1.7.0) (Caporaso et al. 2010). To detect chimera sequences, the tags were compared with the reference data namely Gold database using UCHIME algorithm (Edgar et al. 2011) for obtaining the effective tags. Sequences analysis was performed by Uparse software (V7.0.1001) using all the effective tags (Edgar 2013). Sequences with ≥97% similarity were assigned to the same OTUs. Annotation of OTUs at each taxonomic rank was performed using Mothur software against the SSUrRNA database of SILVA Database (Wang et al. 2007). The evolutionary tree was generated with RAxML and viewed in ITOL (Letunic and Bork 2007).
In this study, the effectiveness of SCOBY papaya beverages to combat obesity was investigated using a high fat diet-induced obese mice model. The mice were fed with high fat diet for three months to induce obese condition. On the 4th month onwards, the selected obese mice were treated with SCOBY papaya beverages and a commercial drug (Orlistat) for 2 months intervention under continuous feeding with high fat diet. Negative obese mice group were continually fed with high fat diet without treatment while the normal diet mice which served as a control for comparison purpose were fed with standard pellet diet. Surprisingly, the obese mice group fed with SCOBY papaya beverages showed remarkable weight reductions when compared to commercial anti-obesity drug, Orlistat under short period of treatment. Even though obese mice received continuous high fat diet, treatments with SCOBY papaya beverages were efficiently reducing body weight of obese mice at a higher percentage (21.3% SCOBY papaya leaves, PL; 23.4% SCOBY papaya pulp, PP) compared to treatment using commercial anti-obesity drug (Orlistat), with a lower weight reduction of 11.3% (Fig. 1A). Further examination on mice blood glucose profile revealed no hypoglycaemia or hyperglycemia effect observed in all obese and normal diet mice with their blood glucose level maintained within the healthy levels (6 - 8 mmol/L) (Fig. 1B). Generally, the percentages of relative weight of kidney, liver, spleen, stomach and adipose tissue of all mice group were also examined (Fig. 1C). There were no swelling symptom or abnormal physical appearance observed in all mice organs after the two months high fat diets intervention. However, obese mice without treatment, C(-) were found to have higher percentage of adipose tissue (3.6% ±0.18) deposits when compared to other treated obese mice groups (within range of 1.32 – 1.48%), which was closer to normal diet mice group (1.37%±0.26). In order to examine the safety aspect of prolonged consumption of SCOBY papaya beverages and commercial drug, the histopathology assessments on mice’s various organs (kidney, liver, spleen and stomach) of treated high fat diet obese mice and control mice group were performed. No inflammation symptoms were observed indicating no toxicity effect of consuming SCOBY papaya beverages within two months treatment.
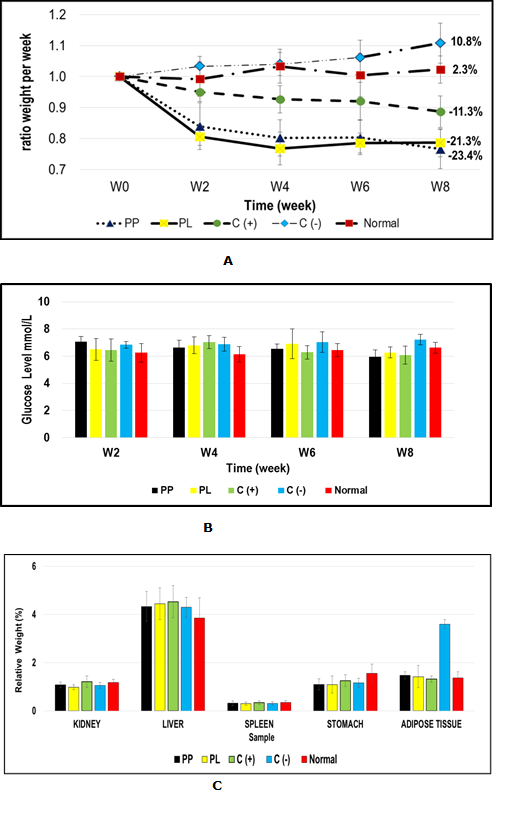
Figure 1 Compamiceive study between treated high fat diet obese mice and control mice group: (A) ratio body weight; (B) glucose level; (C) relative weight
Abbreviations: PL – SCOBY papaya leaves-treated high fat diet obese mice; PP – SCOBY papaya pulp treated high fat diet obese mice; C(+): Orlistat-treated high fat dietobese mice; C(-): High fat diet obese mice; Normal: normal control mice
The safe consumption of SCOBY papaya beverages was further supported by the healthy levels of white blood cells, lymphocytes, haemoglobins, haematocrits, red blood cells and platelets. The haematology profile within acceptable ranges suggested that SCOBY papaya beverages and Orlistat were safe for consumption as depicted in Fig. 2. Serum biochemistry analysis revealed that minor increase of alanine aminotransferase, total protein, creatinine and cholesterol in some of high fat diet-induced obese mice as summarized in Table 1. Although blood chemistry profile of some mice showed slight increases in readings, those mice were physically active with no sickness.
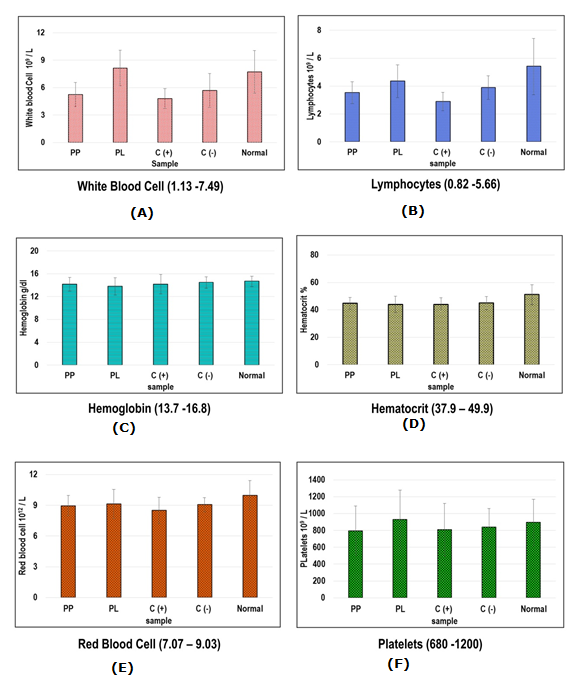
Figure 2 Haematology profile for treated high fat diet obese mice and control mice group: a) white blood cell; b) Lymphocytes; c) haemoglobin; d) haematocrit; e) red blood cell; f) platelets
Abbreviations: PL – SCOBY papaya leaves-treated high fat diet obese mice; PP – SCOBY papaya pulp treated high fat diet obese mice; C(+): Orlistat-treated high fat dietobese mice; C(-): High fat diet obese mice; Normal: normal control mice
Table 1. Liver & kidney function and lipid profile between treated high fat diet obese mice and control mice group
|
Sample |
Liver Function profile |
Kidney function Profile |
Lipids Profile |
||||||||
|
ALT (U/l) |
AST (U/l) |
ALP (U/l) |
Total Protein (g/l) |
Albumin (g/l) |
Urea (mmol/l) |
Creatinine (µmol/l) |
TG (mmol/l) |
HDL (mmol/l) |
LDL (mmol/l) |
CHOL (mmol/l) |
|
|
Normal |
48.40±5.10c |
134.57±29.82ab |
76.43±12.84c |
71.62±7.10b |
38.10±2.44a |
10.41±1.79a |
48.44±6.25c |
1.09±0.20a |
1.21±0.23b |
1.31±0.30c |
2.37±0.42d |
|
C (-) |
49.33±7.15c |
126.55±25.30ab |
71.57±15.00c |
71.52±8.47b |
36.98±2.65a |
5.72±0.69b |
53.00±2.83cb |
1.07±0.11ab |
1.99±0.38a |
2.91±0.71ab |
3.68±0.63c |
|
C (+) |
67.25±6.65b |
127.83±34.37ab |
72.30±19.12c |
77.12±6.30ab |
37.57±1.86a |
4.54±0.63cb |
54.40±2.59b |
1.24±0.31a |
2.12±0.83a |
2.57±0.34ab |
4.07±0.47bc |
|
PL |
47.60±1.52c |
143.50±34.84ab |
127.67±34.23a |
79.84±5.16ab |
38.35±3.31a |
4.30±0.64c |
61.17±2.40a |
0.76±0.19bc |
2.47±0.59a |
2.86±0.49ab |
4.97±0.72a |
|
PP |
83.75±8.62a |
119.29±11.21b |
104.18±21.99b |
76.40±6.14ab |
38.11±2.02a |
4.16±0.21c |
62.00±7.21a |
1.05±0.07ab |
2.07±0.30a |
2.37±0.46b |
4.09±0.65bc |
|
Normal range |
16 - 48 |
65 – 203 |
26 - 147 |
52 – 71 |
36 – 55 |
4.39 – 8.78 |
17.68 – 44.2 |
0.23-1.29 |
N.A |
N.A |
0.91-2.20 |
The SCFAs are important gut metabolites which are produced by gut microbiota in the gastrointestinal system. The SCFAs were extracted from mice faecal samples to investigate the changes of these important gut microbial metabolites presents in obese mice group treated with SCOBY papaya beverages in comparison with untreated obese mice. The acetic acid, propionic and butyric acid are the three major SCFAs that had been analysed from each mice group and the changes of each SCFAs contents were investigated between the initial and after 2 months treatment (Fig. 3). Relatively, obese mice treated with SCOBY papaya beverages showed higher increment in total SCFAs content (acetic acid, propionic and butyric acids) than untreated obese mice and normal control mice, particularly for obese mice treated with SCOBY papaya pulp (PP).
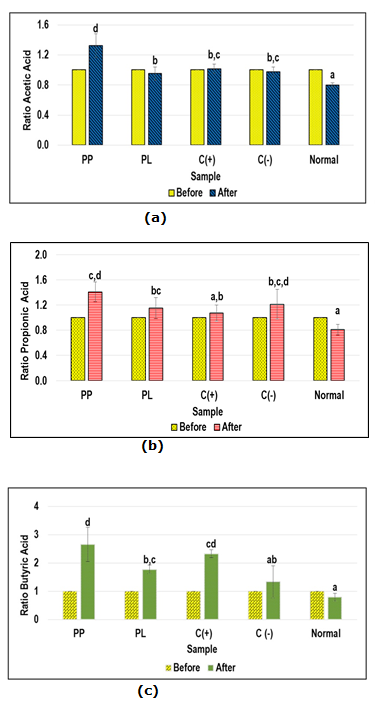
Figure 3 Short chain fatty acids profile for treated high fat diet obese mice and control mice group: a) ratio acetic acid; b) ratio propionic acid; c) butyric acid
Abbreviations: PL – SCOBY papaya leaves-treated high fat diet obese mice; PP – SCOBY papaya pulp treated high fat diet obese rat; C(+): Orlistat-treated high fat diet obese mice; C(-): High fat diet obese mice; Normal: normal control mice
Obesity is a metabolic disorder which causes low-grade chronic inflammation. There are changes in the body immune response during the development of obesity, as the circulating pro-inflammatory cytokines will be elevated and trigger inflammation. In order to evaluate the effectiveness of SCOBY papaya beverages in controlling obesity and body weight management, this study has performed qPCR to measure the expression of adipocyte genes that involved in glucose metabolism (Slc2a4), fat metabolism (Adipor1, SREBP-1, and MMP2), and inflammation processes (TNF-α, NOS2, TGF-β1, IL6 and CCL2) (Fig. 4). The short term consumption of SCOBY papaya beverages reduced the expression gene related to glucose transportation namely solute carrier family 2 (Slc2a4) significantly in HFD-induced obese mice compared to non-treated obese mice (Fig. 4A, P<0.01). The expression profile of adiponectin receptor 1 (Adipor1) showed similarity with Slc2a4, where significant reductions of the expression level in samples treated with SCOBY papaya beverages (Fig. 4B, P<0.01). Gene markers that associated with fat mass accumulation and adipogenesis such as sterol regulatory element binding protein 1 (SREBP-1) and MMP2 were down-regulated markedly in SCOBY papaya beverages (PL & PP) diet treated groups compared to non-treated obese mice (Fig. 4C and 4D, P<0.05). The high fat diet caused increases of inflammation gene marker expressions (TNF, IL6, TGF, CCL2 and NOS2) by 1.25- to 7-fold in obese mice relative to the normal control (Fig. 4E-4I). However, under SCOBY papaya beverages (PL & PP) diet interventions, the altered expression levels of these inflammatory genes in treated groups were restored to normal levels (Fig. 4E-4I, P<0.01).
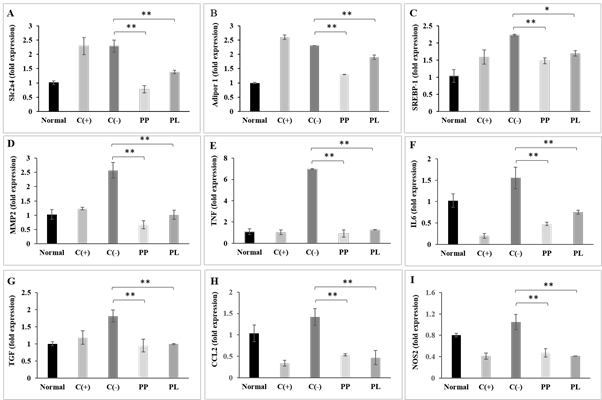
Figure 4 Expression of gene markers related to obesity. Gene expression of Slc2a4, Adipor1, SREBP-1, MMP2, TNF-α, IL6, TGF-β1, CCL2 and NOS2 in control groups (positive and negative controls) and fermented diet treated groups (PP and PL) relative to Normal. Results are presented as mean ± SEM (n=3). Significant differences between individual fermented treated and control group are marked with * (P<0.05) and ** (P<0.01).
Abbreviations: OTUs: operational taxonomy units; PL – SCOBY papaya leaves-treated high fat diet obese mice; PP – SCOBY papaya pulp treated high fat diet obese mice; C(+): Orlistat-treated high fat diet obese mice; C(-): High fat diet obese mice; Normal: normal control mice.
The composition of gut microbiome of individual control and treated mice group was analysed using 16S rRNA sequencing technique on the mice stool samples. Fig. 5A shows the relative abundance of gut microbe as demonstrated by 12 most abundant percent OTUs at the phylum level in each sample group. Results showed that there were reductions in the percentage of relative abundance of Firmicutes in SCOBY papaya pulp (PP) treated and positive control, C(+) groups compared to negative control, C(-) with approximately 9% and 11%, respectively (Fig. 5). For both SCOBY papaya beverages (PP and PL) treated groups, there were significant increments of Actinobacteria by approximately 6% compared to C(-) group. In addition, Verrucomicrobia was found to be increased after both SCOBY papaya beverages diet intervention, with higher increment in SCOBY papaya pulp, PP (4.9%) than in SCOBY papaya leaves, PL (1.6%) compared to high fat diet-induced obese mice group without treatment, C(-).
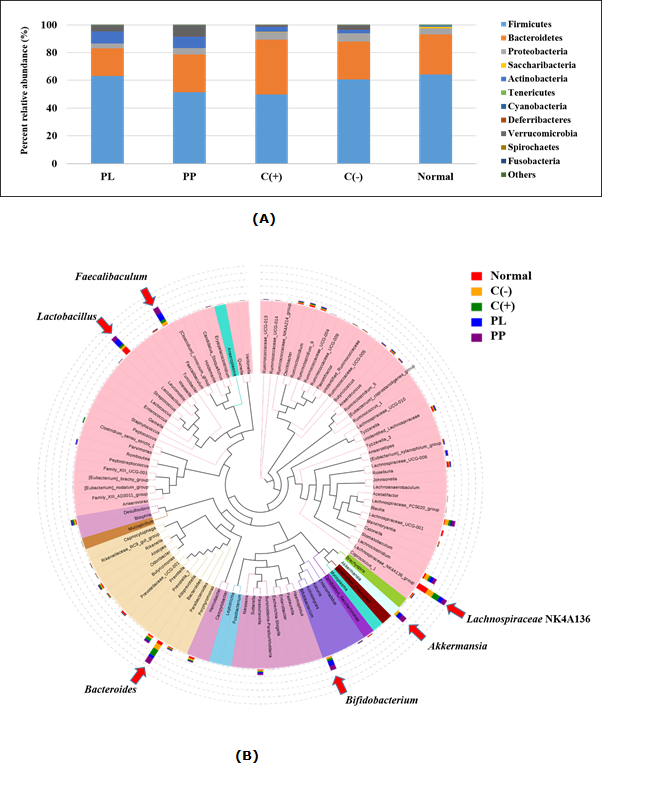
Figure 5 Gut microbiome analysis between treated high fat diet obese mice and control mice groups (A) relative abundance of gut microbiome (OTUs) (B) comparative study of evolutionary tree at the genus level
Abbreviations: OTUs: operational taxonomy units; PL – SCOBY papaya leaves-treated high fat diet obese mice; PP – SCOBY papaya pulp treated high fat diet obese mice; C(+): Orlistat-treated high fat diet obese mice; C(-): High fat diet obese mice; Normal: normal control mice
An evolutionary tree that demonstrating the distribution of gut microbes at the genus level across different sample groups is shown in Fig. 5B. The circulating bars are the indicators for overall abundance and magnitude of difference between controls and treated groups. There are a number of microbes that abundantly present particularly for treated groups are highlighted by red arrows such as Faecalibaculum, Lactobacillus, Bacteriodes, Bifidobacterium, Akkermansia and Lachnospiraceae NK4A136. Among these, Lactobacillus and Bifidobacterium were found to be increased markedly in SCOBY papaya leaves (PL) treated group whereas the Bacteriodes and Akkermansia were abundantly present in SCOBY papaya pulp (PP) treated group. Worth mentioning that the abundance of Faecalibaculum increased with a similar magnitude in both SCOBY papaya beverages (PL and PP) treated groups. In contrast, Lachnospiraceae NK4A136 reduced in abundance upon SCOBY papaya beverages diet interventions compared to all other groups.
Globally, current diet trend is not only covering the basic nutrient needed for daily requirements, but also includes functional metabolites that can prevent or reduce the risk of chronic diseases. The consumers demand for multiple healthy functional foods with the prophylactic potential has been increasing. One of the major innovative solutions to address consumer health is to eat better foods. For instance, fermented foods have gained great attention due to the presence of biologically active compounds with therapeutic potential and lesser side effects. In this study, we have developed functional fermented papaya beverages, known as SCOBY papaya beverages which were produced using selected pure mixed cultures of bacteria and yeast under controlled fermentation process to ensure the consistency and quality of SCOBY papaya beverages.
Our ultimate aim of producing SCOBY papaya beverages is to promote these beverages as effective food remedy solution for tackling the current obesity epidemic with little side effect. Long term consumption of high fat diet is well known to be the causal factor for the increased risk of obesity. In this study, SCOBY papaya beverage treatments were applied to high fat diet-induced obese mice for a duration time of two months on top of continually feeding with high fat diet. Orlistat, a popular commercial anti-obesity drug was used as positive control to evaluate the anti-obesity efficacy level. Surprisingly, it was found that both SCOBY papaya beverages (PP and PL) treated obese mice groups showed a remarkable body weight loss just after two weeks of treatment and achieved the percentage of body weight loss of 21.3% and 23.4% for PL and PP, respectively after 8 weeks of treatment. On the contrary, Orlistat which acts as lipase inhibitor to reduce fat absorption showed a lower efficiency in reducing body weight (11.3%). Most interestingly, it was found that obese mice treated with SCOBY papaya beverage have lower percentage of adipose tissue than obese mice without treatment. This phenomenon supports the efficacy of SCOBY papaya beverage in significant reduction of fat loss in obese mice. The presence of natural acetic acid (~1.5-1.6%) in SCOBY papaya beverages as part of microbial metabolites produced by Kombucha consortium strains is likely to be the primary contributing factor for significant body weight reduction. This was based on previous findings where the presence of sufficient acetic acid in some of natural fermented vinegar was suggested to have anti-obesity and anti-inflammatory effect, predominantly in increasing satiety and reducing lipid deposition (Kondo et al. 2009; Yamashita et al. 2016; Beh et al. 2017). Various analyses on blood glucose profile, haematology analysis, serum biochemistry profiling and histopathology examination were conducted to evaluate the safety aspect of SCOBY papaya beverages. Prolonged consumption of SCOBY papaya beverages did not cause any hyperglycemia or hypoglycaemia effect on blood glucose profile of treated obese mice groups. Examination of various organs like liver, kidney, stomach and spleen showed no swelling effect and any inflammatory symptoms. Therefore, the overall data collected have confirmed the safe consumption of SCOBY papaya beverages, suggesting these beverages as new therapeutic agent in the weight management control with no toxicity symptoms shown. Nevertheless, prolonged consumption of high fat diet in all mice group except normal diet mice group did experience minor increase in certain serum biochemistry profile such as alanine aminotransferase, total protein, creatinine and cholesterol levels. Particularly, higher cholesterol levels were observed all high fat diet-induced mice group was likely due to continuously consumption of high fat diet for a duration period of 5 months. This phenomenon showed that both SCOBY papaya beverages and Orlistat have no capability to reduce the cholesterol level in high fat diet-induced obese mice.
Novel research showed that gut microbiota is associated with obesity and metabolic disorders, where gut microbiota composition is different between obese and lean animal and human subjects. Long term intake of high fat diet is one of the identified contributing factors that can indirectly affect the gut intestinal microbiota by modifying the composition and function of microorganisms colonized in the gastrointestinal tract. Here, we investigated the changes of gut microbiota of high fat diet-induced obese mice under SCOBY papaya beverages interventions. The SCFAs which are also known as gut metabolites were evaluated with their suggested role as new key mediator in modulating the effect of diet and interaction on the host metabolism, indirectly impacting the pathophysiology of obesity and metabolic disorders. On the whole, the SCFAs contents of obese mice fed with SCOBY papaya beverages increased significantly (P<0.05) after two months treatment than untreated obese mice. The SCFAs also considered as an energy metabolite that are likely to increase body metabolic activity and act as a defensive system against inflammation and atherosclerosis (Ohira et al. 2017). Prolonged consumption of SCOBY papaya beverages by obese mice has shown significant changes in the gut microbiota composition in comparison to untreated obese and normal mice group. This could be due to obesity suppressing SCFAs produced in SCOBY papaya beverages (e.g. acetic acid, propionic acid and butyric acid) that enhanced the reduction of body weight. Similar scientific findings have been reported in other high fat diet-induced obese mice studies (Beh et al. 2017; Mulders 2018). Moreover, propionic acid has been associated with appetite regulation by enhancing the secretion of peptide YY and glucagon like peptide -1 (GLP-1), hence reduce energy intake and weight gain in overweight adults (Chambers et al. 2015).
The two months consumption of high fat diet had induced obesity in mice with significant metabolic changes as observed in negative control group. These changes were detected molecularly using qPCR technique, indicated by genes in adipocytes with functions involved in glucose transportation, fat tissue build up and obesity linked inflammatory cytokines were abnormally up-regulated their expressions. In a former study conducted by Shepherd et al. (1993) found that Slc2a4 which is also known as glucose transporter 4 is responsible for glucose intake of adipocytes where up-regulation of this gene could lead to increase in adipose tissue mass. This result was in agreement with increased expression level of adiponectin receptor 1 (Adipor1) that enhances AMPK activity which in turn up-regulates insulin-stimulated glucose and fatty acid uptake by adipose tissues in obese mice (Karbowska and Kochan 2006). The high fat diet could also enhance the expression levels of transcription factor SREBP-1 which regulate activity of genes related to cholesterol and fatty acid biosynthesis in adipose tissue (Porstmann et al. 2005). An essential proteinases for extracellular matrix remodelling namely MMP2 that responsible for accumulation of adipose tissue during obesity development (Chavey et al. 2003) exhibited approximately 2.5 fold up-regulated gene expression compared to untreated obese and normal mice. The inflammation process in adipocytes during the progress of obesity was clearly shown by increased expressions of a number of inflammatory gene markers. Those markers include pro-inflammatory cytokines such as TNF-α and IL6 as well as inflammatory chemokines like TGF-β1 and CCL2. Studies have shown that the expressions of these inflammatory cytokines and chemokines were initiated by the deposition of fat tissues (Xu et al. 2002; Fain et al. 2005; Park et al. 2005; Neeld and Olefsky 2006), subsequently the low-grade inflammation process of adipocytes stimulated and up-regulated NOS2 expression, which is involved in the development of insulin resistance (Perreault and Marette 2001; Sugita et al. 2005). However, following individual SCOBY papaya pulp (PP) and SCOBY papaya leaves (PL) treatment, the altered expression of the above-mentioned gene markers resulted from high fat diet were able to be restored to a normal expression level (P<0.05). These results suggest that both SCOBY papaya beverages (PP and PL) are effective in preventing the development of obesity related pathogenesis that induced by high fat diet.
Dietary intake is one of the primary factors that determine the composition of gut microbiota besides genetics, mode of delivery at birth and use of medications. The consumption of high fat diet has contributing to an irregular energy intake and energy balance, which directly will alter the composition of intestinal microbiota and leads to development of obesity. From the OTUs relative abundance analysis, the gut microbiota of fermented diet PP and PL treated groups were altered as compared to the non-treated control group. Clearly, the abundances of Actinobacteria and Verrucomicrobia increased markedly following individual PP and PL diet treatment, resulting in decreased abundances of major residing microbes such as Firmicutes, Bacteriodetes and Proteobacteria in comparison to negative and normal controls. These changes were in agreement with increases of Bifidobacterium and Akkermansia in both SCOBY papaya beverages (PP and PL) treated groups, which are the major genus belonging to phylum Actinobacteria and Verrucomicrobia as shown in the evolutionary tree. Bifidobacterium spp. was found to provide anti-obesity and lipid-lowering effects in high fat diet-induced obese (An et al. 2012), which could explain the weight loss upon consumption of PP or PL diet in our current study. Similarly, there were cases which showed higher abundance of Akkermansia in the faecal samples of those has successfully lost body weight among obese human (Louis et al. 2016). The role of Akkermansia spp. in the gut system has been related to obesity characteristics which include glucose metabolism regulation, intestinal immunity and strengthening the intestinal barrier (Naito et al. 2018).
Comparison between the two fermented papaya (PP & PL) diet treatments, Faecalibacterium and Bacteroides were found to be more abundantly present in SCOBY papaya pulp (PP) treated group whereas Lactobacillus in SCOBY papaya leaves (PL) treated group. It was reported that treatment using Faecalibacterium prausnitzii improved metabolic health and reduced inflammation of adipose tissue in high fat fed mice (Munukka et al. 2017). This finding is in agreement with the current qPCR analysis results where greater reductions of expression for genes encoding for pro-inflammatory cytokines (TNF and IL6) in adipocytes of PP-treated than PL-treated obese mice. In addition, Faecalibacterium prausnitzii is known to be a butyrate producer, which was likely to be the main contributor for a 2.5-fold increase of faecal butyrate in PP-treated obese mice as indicated in our SCFAs analysis results. Studies have shown that one of the health effects by butyrate compound is protecting against diet-induced obesity without causing hypophagia by acting as a major energy source for colonocytes (Chakraborti 2015). On the other hand, there were several studies reported that an anti-obesity effects exerted by Lactobacillus spp. which significantly reduced body weight by down-regulating the adipogenesis in high fat diet induced obese mice (Lee et al. 2013; Zhu et al. 2019; Choi et al. 2019; Choi et al. 2020).
By applying selected Kombucha consortium strains fermentation on papaya leaves and pulp, the presence of mixed microbial activity has transformed the papaya leaves and pulp into functional beverages that possess anti-obesity effect. Treatments using SCOBY papaya beverages, particularly SCOBY papaya pulp has resulted in significant increases of faecal SCFAs contents after the intervention, in agreement with increased abundances of SCFAs producing gut bacteria. These SCFAs are crucial to regulate fat metabolism by decreasing fat storage. Evidence findings collected from in vivo obese mice study confirmed the potential of SCOBY papaya beverages to alleviate obesity problem by altering lipid metabolism, reducing inflammation and enhanced the growth of gastrointestinal beneficial microbes. Compared to untreated obese mice, SCOBY papaya beverages treated obese mice showed greater abundances of beneficial microbiota. The overall results suggest that SCOBY papaya beverages are effective in restoring gut microbiota of obese subjects to a healthy state and ameliorate obesity related metabolic disorders. In conclusion, SCOBY papaya beverages represent a novel functional papaya beverage which offers a cost-effective food remedy solution, potentially become the next generation preventive and therapeutic solution for body weight management control and global obesity epidemic with less side effect.
This research work was supported by Horticulture Research Centre, Malaysian Agricultural Research and Development Institute (MARDI) and financially funded by the Malaysia Government Development Fund RMK 11 (P21003004050001).
Ethical approval
The animal experiments were approved by Animal Ethics Committee of MARDI (20170815/R/MAEC24) and the procedures were carried out in accordance with the approved guidelines.
Author contributions
Koh develops SCOBY papaya beverages and design the experiment; Sew and Sarah assists in qPCR and gut microbiome analysis and interpreted the results. Shaiful and Syahida carried out animal work and blood sample analysis. Rosmawati assists in the preparation of SCOBY papaya beverages.
Conflict of Interest
All authors declared no conflict of interest.
Ahmad N, Fazal H, Ayaz M, Abbasi BH, Mohammad I, Fazal L (2011) Dengue fever treatment with Carica papaya leaves extracts. Asian Pacific Journal of Tropical Biomedicine 1(4): 330-333 60055-5
View ArticleAn HM, Kim MJ, Moon JS, Kang JY, Lee DK, Lee KH, Shin HS, Ha NJ (2012) Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Federation of American Societies for Experimental Biology.
View ArticleAruoma OI, Hayashi Y, Marotta F, Mantello P, Rachmilewitz E, Montagnier L (2010) Applications and bioefficacy of the functional food supplement fermented papaya preparation. Toxicology 278(1): 6-16 PMid:20870007
View Article PubMed/NCBIBarbagallo M, Marotta F, Dominguez LJ (2015) Oxidative stress in patients with Alzheimer's disease: effect of extracts of fermented papaya powder. Mediators of Inflammation. PMid:25944987
View Article PubMed/NCBIBeh BK, Mohamad NE, Yeap SK, Ky H, Boo SY, Chua JYH, Tan SW, Ho WY, Sharifuddin SA, Long K (2017) Anti-obesity and anti-inflammatory effects of synthetic acetic acid vinegar and Nipa vinegar on high-fat-diet-induced obese mice. Scientific Reports 7(1): 1-9 PMid:28751642
View Article PubMed/NCBICaporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7(5): 335-336 PMid:20383131
View Article PubMed/NCBIChakraborti CK (2015) New-found link between microbiota and obesity. World Journal of Gastrointestinal Pathophysiology 6(4): 110 PMid:26600968
View Article PubMed/NCBIChambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS (2015) Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut Microbiota 64(11): 1744-1754 PMid:25500202
View Article PubMed/NCBIChavey C, Mari B, Monthouel MN, Bonnafous S, Anglard, P, Van Obberghen E, Tartare-Deckert S (2003) Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. Journal of Biological Chemistry 278(14): 11888-11896 PMid:12529376
View Article PubMed/NCBIChoi WJ, Dong HJ, Jeong HU, Jung HH, Kim YH, Kim TH (2019) Antiobesity effects of Lactobacillus plantarum LMT1-48 accompanied by inhibition of Enterobacter cloacae in the intestine of diet-induced obese mice. Journal of Medicinal Food 22(6): 560-566 PMid:31013456
View Article PubMed/NCBIChoi WJ, Dong HJ, Jeong HU, Ryu DW, Song SM, Kim YR, Jung HH, Kim TH, Kim YH. (2020). Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Scientific Reports 10(1): 1-9 PMid:31964951
View Article PubMed/NCBIEdgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16): 2194-2200 PMid:21700674
View Article PubMed/NCBIEdgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods 10(10): 996-998 PMid:23955772
View Article PubMed/NCBIFain J N, Tichansky DS, Madan AK (2005) Transforming growth factor β1 release by human adipose tissue is enhanced in obesity. Metabolism 54(11):1546-1551 PMid:16253647
View Article PubMed/NCBIGhee LK (2016) A review of adult obesity research in Malaysia. Medical Journal of Malaysia 71(1): 7
Grundy SM (2004) Obesity, metabolic syndrome, and cardiovascular disease. The Journal of Clinical Endocrinology & Metabolism 89(6): 2595-2600 PMid:15181029
View Article PubMed/NCBIHan K, Bose S, Wang JH, Kim BS, Kim MJ, Kim EJ, Kim H (2015) Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Molecular Nutrition & Food Research 59(5):1004-1008 PMid:25688926
View Article PubMed/NCBIHasimun P, Ernasari G (2014) Analgetic activity of papaya (Carica papaya L.) leaves extract. Procedia Chemistry 13:147-149
View ArticleJuárez-Rojop IE, Tovilla-Zárate CA, Aguilar-Domínguez DE, Roa-de la Fuente LF, Lobato-García CE, Blé-Castillo JL, López-Meraz L, Díaz-Zagoya JC, Bermúdez-Ocaña DY (2014) Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Revista Brasileira de Farmacognosia 24(3): 341-347
View ArticleKarbowska J, Kochan Z (2006) Role of adiponectin in the regulation of carbohydrate and lipid metabolism. Journal of Physiology and Pharmacology 57(6): 103-113
Kondo T, Kishi M, Fushimi T, Ugajin S, Kaga T. (2009). Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Bioscience, Biotechnology and Biochemistry 73(8): 1837-1843. PMid:19661687
View Article PubMed/NCBILee BH, Lo YH, Pan TM (2013). Anti-obesity activity of Lactobacillus fermented soy milk products. Journal of Functional Foods 5(2): 905-913.
View ArticleLetunic I, Bork P (2007). Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 23(1): 127-128. PMid:17050570
View Article PubMed/NCBILi X, Shimizu Y, Kimura I (2017) Gut microbial metabolite short-chain fatty acids and obesity. Bioscience of Microbiota, Food and Health 36(4): 135-140 PMid:29038768
View Article PubMed/NCBILouis S, Tappu RM, Damms-Machado A, Huson DH, Bischoff SC (2016) Characterization of the gut microbial community of obese patients following a weight-loss intervention using whole metagenome shotgun sequencing. PLoS One
View ArticleMagoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21): 2957-2963 PMid:21903629
View Article PubMed/NCBIMarotta, F, Celep GS, Cabeca A, Polimeni A (2012) Novel concepts on functional foods and nutrigenomics in healthy aging and chronic diseases: a review of fermented papaya preparation research progress. Functional Foods in Health and Disease 2(5): 120-136
View ArticleMulders R, De Git K, Schéle E, Dickson S, Sanz Y, Adan R (2018) Microbiota in obesity: interactions with enteroendocrine, immune and central nervous systems. Obesity Reviews 19(4): 435-451 PMid:29363272
View Article PubMed/NCBIMunukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, Hänninen A, Vuopio J, Huovinen P, Jalkanen S (2017) Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. The ISME Journal 11(7): 1667-1679. PMid:28375212
View Article PubMed/NCBINaito Y, Uchiyama K, Takagi T (2018) A next-generation beneficial microbe: Akkermansia muciniphila. Journal of Clinical Biochemistry and Nutrition 63(1): 33-35 PMid:30087541
View Article PubMed/NCBINeels JG, Olefsky JM (2006) Inflamed fat: what starts the fire? The Journal of Clinical Investigation 116(1): 33-35 PMid:16395402
View Article PubMed/NCBINew Straits Times. (2017). Malaysians most obese in region. (accessed on 7 June 2017).
View ArticleOhira H, Tsutsui W, Fujioka Y (2017) Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? Journal of Atherosclerosis and Thrombosis 24(7): 660-672 PMid:28552897
View Article PubMed/NCBIOtsuki N, Dang NH, Kumagai E, Kondo A, Iwata S, Morimoto C (2010) Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. Journal of Ethnopharmacology 127(3): 760-767 PMid:19961915
View Article PubMed/NCBIOwoyele BV, Adebukola OM, Funmilayo AA and Soladoye AO (2008) Anti-inflammatory activities of ethanolic extract of Carica papaya leaves. Inflammopharmacology 16(4): 168-173 PMid:18759075
View Article PubMed/NCBIPark HS, Park JY, Yu R (2005) Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Research and Clinical Practice 69(1): 29-35 PMid:15955385
View Article PubMed/NCBIPerreault M, Marette A (2001) Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nature Medicine 7(10): 1138-1143 PMid:11590438
View Article PubMed/NCBIPorstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A (2005) PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 24(43): 6465-6481 PMid:16007182
View Article PubMed/NCBIRaffaelli F, Nanetti L, Montecchiani G, Borroni F, Salvolini E, Faloia, E, Ferretti, G, Mazzanti, L, Vignini A (2015) In vitro effects of fermented papaya (Carica papaya, L.) on platelets obtained from patients with type 2 diabetes. Nutrition, Metabolism and Cardiovascular Diseases 25(2): 224-229 PMid:25511784
View Article PubMed/NCBIShepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB (1993) Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. Journal of Biological Chemistry 268(30): 22243-22246 41516-5
View ArticleSugita H, Fujimoto M, Yasukawa T, Shimizu N, Sugita M, Yasuhara S, Martyn J, Kaneki M (2005) Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. Journal of Biological Chemistry 280(14): 14203-14211 PMid:15805118
View Article PubMed/NCBITacio HD (2020) Top 10 healthiest fruits. (accessed on 13 July 2020)
View ArticleTan SA, Goya L, Ramanathan S, Sulaiman S F, Alam M, Navaratnam V (2014) Chemopreventive effects of standardized papaya leaf fraction on oxidatively stressed human liver cells. Food Research International 64: 387-395 PMid:30011665
View Article PubMed/NCBIWang Q, Garrity G M, Tiedje J M and Cole J R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology 73(16): 5261-5267 PMid:17586664
View Article PubMed/NCBIXu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS (2002) Altered tumor necrosis factor-α (TNF-α) processing in adipocytes and increased expression of transmembrane TNF-α in obesity. Diabetes 51(6): 1876-1883 PMid:12031976
View Article PubMed/NCBIYamashita H (2016) Biological function of acetic acid-improvement in obesity and glucose tolerance by acetic acid in type 2 diabetic rats. Critical Reviews in Food Science and Nutrition 56(1): S171-S175 PMid:26176799
View Article PubMed/NCBIZhu K, Tan F, Mu J, Yi R, Zhou X, Zhao X (2019) Anti-obesity effects of Lactobacillus fermentum CQPC05 isolated from Sichuan pickle in high-fat diet-induced obese mice through PPAR-α signaling pathway. Microorganisms 7(7): 194 PMid:31284674 PMCid:PMC6680547
View Article PubMed/NCBI