Shoulei Yan
Email: yanshoulei1225@ mail.hzau.edu.cn
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 6 ISSUE: 3
Page No: 406-416
Shoulei Yan
Email: yanshoulei1225@ mail.hzau.edu.cn
Huixia, Wang1, †, Yassin Haran 2, 3, †, Shoulei Yan* 2, 3ZhiyongFan1, Li Zhang1, Xugang, Wan1, Tingting, Yu1, Feng, Jiang1
† Co-first author
1. Hubei Provincial Institute for Food Supervision and Test, Wuhan. Hubei 430070, China.
2. College of Food Science and Technology, Huazhong Agricultural University, Wuhan. Hubei 430070.China.
3. Aquatic Vegetable Preservation and Processing Technology Engineering Center, Wuhan. Hubei 430070, China.
Huixia,Wang,Yassin Haran, Shoulei Yan, ZhiyongFan, Li Zhang, Xugang, Wan, Tingting, Yu, Feng, Jiang, Purification and Clean-up of 3-NPA from Sugarcane by using Molecularly Imprinted Polymer Solid Phase Extraction Coupled with HPLC (2021) Journal of Food Science & Technology 6(3):406-416
The agricultural product is in constant need of improved analytical techniques that can be used to control the manufacturing process and the food safety of the products. A new selective material based on molecularly imprinted polymers (MIPs) were prepared by bulk polymerization methods and used as solid-phase extraction (SPE) sorbent for sample enhancement of 3-nitropropanic acid (3-NPA) in sugarcane samples prior to high-performance liquid chromatography (HPLC). The objective of this work was to devolved techniques for the extraction, purification, clean up, and preconcentration of 3-NPA from sugarcane samples employing MIP for SPE. The obtained polymers were characterized using field emission scanning electron microscopy, Fourier transforms infrared and the adsorption capacity of SA-SMIP was studied. The developed MISPE method showed excellent linearity in the range of 40–70 mg L with coefficient of determination (r²) >0.9997 and good 3-NPA recoveries of >99% and limits of detection LOD and LOG were ranging from 0.19 mg L to 0.58 mg L, respectively. The method was successfully applied to the analysis of 3-NPA in selected sugarcane samples. MIP-SPE allows not only the analyte to be pre-concentrated but also removed the other compounds from the sample matrix. The MISPE technique has been successfully applied to purification, preconcentration, and clean-up of 3-nitropropionic acid in poisoning sugarcane.
Key words: Sugarcane, 3-nitropropionic acid (3-NPA), molecularly imprinted polymers (MIPs), Solid phase extraction, HPLC.
Past 41 years, 1972 2-3 month had occurred in northern China more than a cause of eating moldy sugarcane poisoning caused severe patients are mostly children and young people.3-Nitropropionic acid (3-NPA) is a white crystalline compound, mainly in mildew sugarcane. Ingestion of food containing 3-NPA often results in central nervous system damage (Wang et al., 2008), severe or even death or left to extrapyramidal lesions, mainly squealed. 3-nitropropane acid (3-NPA, C3H5NO4) is a mycotoxin produced by various species of fungus, including ArthriniumSP and Aspergillus SP that produce 3-NPA, which grows on sugarcane (Bhat et al., 2010), Human intoxication has occurred in 13 provinces in China when toxic to humans; the main organs affected are in the nervous system, such as liver, kidney, and lung (Ezekiel et al., 2012; Peraica et al., 1999). It can also cause a potent mitochondrial inhibitor. It is naturally present in leguminous plants used to feed animals and can poison grazing livestock, humans and experimental animals exposed to 3-NPA develop severe dystonia and decreased motor activity. At the cellular level, 3-NPA inhibits succinate dehydrogenase a key enzyme of oxidative energy production that is localized in the mitochondrial inner membrane and complex II of the respiratory chain, causing ATP levels in the brain to fall (Becker et al., 2017). Thus, a major factor in 3-NPA toxicity is cellular metabolic impairment due to mitochondrial stress. 3-NPA has been detected in several methods, was identified as a culture of Arthriniumsp isolated from samples of sugarcane that induced a poisoning outbreak. The structure it was confirmed by Thin Layer Chromatography (TLC), Ultraviolet spectrophotometry, infrared spectrometry, Gas chromatography (GC), HPLC analysis (Liu et al., 2016), proton and carbon nuclear magnetic spectrometry and mass spectrometry spectral analyses were used for identification.
Recently, a molecularly imprinted polymer (MIP hereinafter) proved to be useful materials in several areas of analytical chemistry. Today, MIP is a viable synthetic approach to design robust molecular recognition materials able to mimic natural recognition entities, such as antibodies and biological receptors (Vasapollo et al., 2011). Application of molecular imprinting has become attractive in many fields of chemistry, biology, and engineering, particularly as an affinity material for sensors. It is also known as artificial antibodies, having good physical and chemical stability, a structure-activity predetermination, specific recognition, and broad applicability. These days, it is widely used for the analysis of food-safety in different food items (Ashley et al., 2017), including separation and enrichment, detects pesticides, toxins, bacteria and other harmful substances. Molecular imprinting is a quickly developing technique for the preparation of polymers having specific molecular recognition properties. Solid-phase extraction (SPE) based on MIPs is a highly attractive and promising approach for matrix clean-up, enrichment and selective extraction of analytes in such a kind of complex samples thereby making the SPE the most popular technique currently available for rapid and selective sample preparation (Qiao et al., 2006).MIPs technique has high selectivity for purification and separation of the target compounds from the natural subsistence because the target compounds were used as a temple (Wang et al., 2008).
The principle of SPE is similar to that of liquid-liquid extraction (LLE), involving a partitioning of solutes between two phases (Huck & Bonn, 2000). The SPE could represent a suitable way to clean up and pre-concentrate samples (Baggiani et al., 2007), the classical SPE sorbents such as ion exchange, C18 column, and size-exclusion phases are lacking in selectivity towards target analytes. This technique is more rapid, simple, economical and the environment-friendly than the traditional liquid-liquid extraction. SPE procedure has many benefits, switch sample matrices to a form more compatible with chromatographic analysis, concentration analytes for increased sensitivity, remove interferences to simplify chromatography and improve quantitation, and protect the analytical column from contaminants (Harris, 2015). The most common applications for this technique including, pharmaceutical compounds and metabolites in biological fluids, drugs, environmental pollutants in drinking and wastewater, pesticides, antibiotics and mycotoxin in food (Gao et al., 2015). Selectivity is introduced during MIP synthesis in which a template molecule, designed to mimic the analyte, is dissolved in a solvent together with one or more functional and cross-linking monomers. Spontaneous complex formation between functional monomers and template occurs, the strength of which will be reflected in the selectivity of the MIP polymer. After polymerization, the template molecule is removed leaving behind vacant cavities or imprints that are satirically and chemically complementary to the template (Beltran et al., 2010). These cavities are then capable of binding a single target analyte or a class of chemically similar analytes present within a complex sample. In the recent years, MIPs have been developed to use as sorbents in SPE; in addition, MIPs offer more flexibility in analytical methods as they are stable to extremes PH, to an organic solvent, and temperature (Maier et al., 2004). Recently, the utilization of MISPE significantly has been increased in the food contaminant analysis. The potential value of MIP–SPE lies in the ability of selectively isolating specific compounds or their structural analogs from a complex matrix. The application of these synthetic polymers as sorbents allows not only pre-concentration and clean-up of the sample but also selective extraction of the target analyte (Wu et al., 2016), which is important, particularly, when the sample is complex and impurities can interfere with quantification (Saini & Kaur, 2013).
In this study, a new MIP was develop based on SA-MIP as a temple for use in MIP-SPE sample improvement of selected 3-NPA, named succinic acid (SA) alias for succinic acid, acrylamide, and 3-NPA structure is similar as shown in figure 1. To the best of our knowledge, no report has been published on MIP-SPE of 3-NPA.
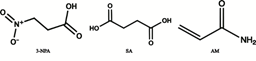
Fig 1. Chemical structure of 3-Nitropropionic acid (3-NPA), Succinic Acid (SA) and Acrylamide (AM).
2.1 Chemicals and Reagents
3-Nitropropionic acid ≥ 97 % (Used as internal standard), the structure of the analysis is presented in finger,and ethylene glycol methacrylate (EGDMA) 98% (used as a crosslinker), Methacrylate Acid (MAA) acrylamide (AM) as the functional monomers were acquired from Sigma-Aldrich Company. Azobisisobutyronitrile (AIBN) 98% and 3-Aminopropyltriethoxysilane (APTES) were purchased from Shanghai Reagent Company. Methanol and acetic acid, HPLC grade acetonitrile, were purchased from SINOPHARM Company. Stock solutions of 1000 mg/ L1 of 3-NPA prepared in acetonitrile. Working solutions were prepared daily by appropriate serial dilution of the stock solution with a solvent acetonitrile, all the chemicals and reagents were chemicals grade. The stock solution and working solutions were stored in the refrigerator at - 4 8C when not in use. The double-distilled deionized water of at least 18. MV.
2.2 Synthesis of MIP and NIP Polymers
The new MIPs was synthesis according to the previously described methods with some modification and additions (Kutner, 2018). The bulk polymerization was used for preparing the MIP and NIP by dissolved all the compounds in a suitable solvent, which can be as a porogen. Silica gel with 3mol / L hydrochloric acid soaked overnight to modify, active and remove a few inorganic impurities for increasing the number of hydroxyl groups on the surface of silica gel, it was modified and activated based on previous methods with minor modifications and used as the carrier (Du et al., 2014). Hydrochloric acid was washed with distilled water until the pH was neutral, dried at 120 ° C for 12 h, and placed in a desiccator for use. 10 g of activated silica gel and 100 mL of toluene was placed in a three-neck round-bottomed flask equipped with a reflux condenser and a vent tube. Under nitrogen, 4 ml of APTES and 1 ml of triethylamine was added dropwise and stirred at 95°C for 10h. The reacted silica gel was filtered, washed with toluene and methanol, and then vacuum-dried at 50 ° C for 12 h. First, the silica gel and APTES role in the silica gel surface grafting of the amide. 0.1 mmol of template molecule and a certain amount of AM dissolved in 3 mL of acetonitrile solution and mixed well by sonication for 30 min. 1.0 g of salinized silica gel, 2.0 mmoL of EGDMA and 20 mg of AIBN were added, followed by ultrasonic for 30 min. Under magnetic stirring, the reaction product was obtained by polymerization in the water bath at 60 ℃ for 24 hr. The reaction products were extracted using 300 ml of methanol / acetic acid (7: 3, the same below) for 48 h. The template molecules were removed and rinsed with 300 ml of methanol to remove the fine particles and residual acetic acid and dried at 50 ℃ for 12 h.
SMIP, succinic acid surface-imprinted polymer (SA-SMIP) and 3-nitropropionic acid surface-imprinted polymer (NA-SMIP) were prepared and stored in a desiccator. The preparation of non-surface molecularly imprinted polymer (SNIP) was conducted in the same method as SMIP except that no template molecule was added.
2.3 Characterization of Molecularly Imprinted Polymer (MIP) and Non-Imprinted Polymer (NIP)
The obtained particles MIP and NIP were mixed into KBr pellets and analyzed by using Fourier transform infrared (FTIR). And the surface structure and particle size of polymer microspheres were analyzed by scanning electron microscopy (SEM).
2.4 Preparation of Sugarcane Extraction
The sugarcanes were peeled and then 2 g of samples were transferred into 50 mL centrifuge tube, added 20.0 mL of acetonitrile sonicated for 20 min on the ultrasonic cleaner. And then added 2 mg of sodium chloride and shook for 10 min at 10,000 r/min centrifugation 10 min. The supernatant was measured. The concentration of the sample was 20 μg / mL, and the samples were passed through the column of MISPE, and the extraction was conducted under the optimal conditions of solid phase extraction. The final eluate was filtered using 0.22 μm and then injected into HPLC instrument. The recovery was calculated by HPLC (n = 3).
2.5 Preparation of molecularly imprinted polymer solid phase extraction (MISPE) cartridges for Clean un
100 mg amount of dry molecular imprinted polymer (MIP) was packed in 3ml of empty polypropylene SPE cartridges provided frit to secure the packing and outlet stopcocks. The column was connected to a vacuum manifold and washed with acetonitrile (ACN) and water and then dried under vacuum. NISPE cartridges were also prepared by using the same procedure.
2.6 Extraction and clean-up of 3-NPA using MISPE cartridge
The MIP columns (cartridge) were placed in the SPE vacuum manifold (12 port). And then the MISPE cartridges were previously conditioned by passing 5mL deionized water and followed by 5mL acetonitrile/water (9:1v/v) before loading the sample. After conditioning 2 mL of spiked sugarcane sample was loaded into SPE cartridge at a flow rate of 0.5mL/min. SPE cartilages were washed with1 mL of dichloromethane, followed by 1 mL of pure dichloromethane and mixture of acetonitrile/dichloromethane (95: 5 v/v). Then after the cartilages were dried by nitrogen gas supply at low flow. Finally eluted with 2mL acetonitrile at the flow rate of 0.5mL / min, and then the eluates were collected and filtrated through membrane syringe filter 0.22 µm size before injected into HPLC.
2.7 Instrument and analytical conditions
The chromatography analysis was performed using Waters e2695 Liquid chromatography system Agilent ZORBAX XDB C18 Chromatographic column (4.6 mm × 250 mm, 5 μm) was used for determination of 3-NPA extracts after passing the MISPE clean-up methods ;The mobile phase: A: pure water B: acetonitrile, gradient elution; flow rate: 0.4 mL / min; detection wavelength:210 nm; PDA detector at the room temperature, the sample injection volume was 20µL. Characterizations of the molecularly imprinted polymer were carried out using Fourier transformed infrared (FTIR) by using KBr disc were recorded at a wavelength 4 cm -1 in the range of 450-4000 cm -1 on spectroscopy model Nexus 470 FTIR (Nicolet, USA). SEM image was obtained by using Field emission scanning electron microscopy (FE-SEM) (Carl Zeiss, Germany).
2.8 Methods Validation
Parameter validation is important to ensure that the method and instrument used are appropriate for the determination of the analyte. To validate the method of the HPLC in this work, the calibration curve was constructed for 3-NPA.Limit of Detection (LOD) and Limit of Quantification (LOQ), recovery, Relative Standard Deviation (RSD) and precision were validated.
3.1 Characterization of MIP and NIP
Characterizations of the molecularly imprinted polymer were conducted using Fourier transformed infrared (FTIR) spectroscopy model Nexus 470 FTIR a Nicolet (USA). The characterization was performed to determine the functional groups in MIP before and after the washing stage and also in NIP by using the KBr pellet method. Small amounts of polymer and potassium bromide (KBr) (ratio 1:100) were mixed by using a ground mortar and pestle at room temperature. The mixture was placed in a mini-press at 100000 psi, and the screws were tightened to squeeze the KBr and polymer mixture into a thin, semi-transparent disk. The mini-press containing the disk was placed into the FTIR instrument, and the disk was scanned and recorded at a resolution 4 cm -1 in the range of 450-4000 cm -1 on a Nexus 470 FTIR spectrometer. Figure 2 shows the IR spectra of SNIP (a), SA-SMIP (b) and (3-NPA) -SMIP (c). The infrared spectra of SNIP and (3-NPA) (1330 ~ 400 cm-1) was slightly different from that of SA-SMIP, suggesting that there was a slight change in the molecular structure of SA-SMIP, probably due to the fact that SNIP was not added to the template molecule, the polymer structure formed during the polymerization process is different.
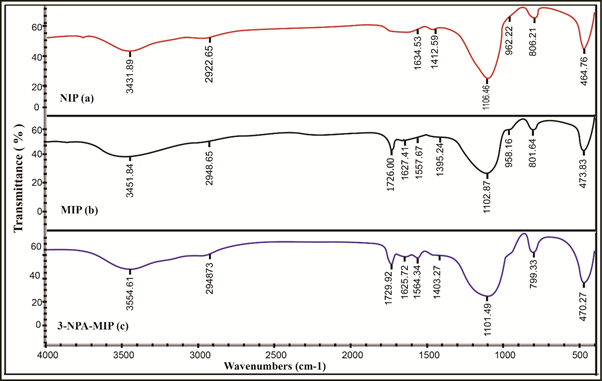
Fig 2. The IR spectra of SNIP (a), SA-SMIP (b) and 3-NPA-SMIP (c).
3.2 Field Emission Scanning Electron microscopy (FE-SEM)
Field emission scanning electron microscopy (FE-SEM) was used to determine the surface morphology and particle size image of the MIP and NIP. The surface morphology of the three polymers, SNIP (a), SA-SMIP (b) and (3-NPA) -SMIP (c), was observed by SEM image. Figure 3 shows that the three polymers are rounded, and the SA-SMIP surface exhibits more porous structure than SNIP. The difference of the surface morphology of the three polymers may be due to the specific binding sites between template molecules and functional monomers during the polymerization process. The images showed a rough MIP surface with an irregular order.
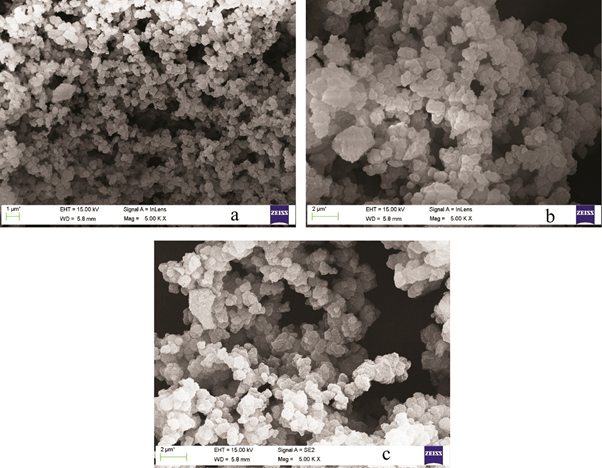
Fig 3. FE-SEM Micrographs of SNIP (a), SA-SMIP (b) and (3-NPA) -SMIP (c)
3.3 Optimization of MISPE Procedures
The extraction performance and the reusability of the MISPE column were studied according to the optimized extraction conditions. The optimization of MISPE was conducted by the following steps conditioning, washing solvent, loading volume, and elution type and elution solvent volume to achieve good sensitivity and precision of this method. The conditioning of the column was conducted by using 5 mL of acetonitrile followed by 5 mL of deionized water. Sugarcane samples spiked were loaded by different loading volumes was 1mL, 2 mL, 5mL, and 10mL into SPE cartridges containing MIPs as shown in figure 4.
Different concentration of acetonitrile in deionized water (9:1 v/v) were used as washing solvent of MISPE procedure. Pure dichloromethane and mixture of acetonitrile/dichloromethane (95: 5 v/v) were used for the optimization of eluting of MISPE. The volumes of eluting solvent were 2 mL of pure acetonitrile.
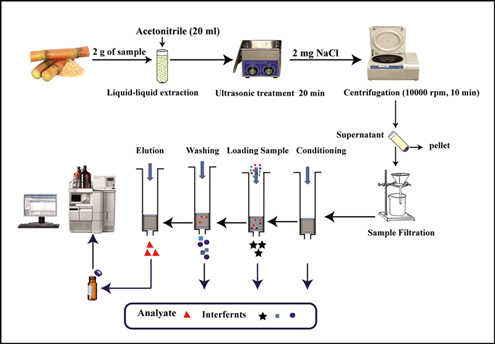
Fig 4. Experimental scheme of sugarcane sample preparation using MISPE methods.
3.4 Kinetic Analysis of 3 - Nitropropionic Acid Molecularly Imprinted Polymer Adsorption
The concentration of immobilized 3-NPA was 1 mmol/L. Adsorption amount of 3-NPA was determined by (3-NPA)-SMIP, SA-SMIP and SNIP at different concentrations for different time. The obtained adsorptive amount-time curve is shown in figure. 5-6. As the time goes by the adsorption amount gradually increases. Among them, SA-SMIP has the largest adsorption amount of 3-NPA and the smallest SNIP. In the first 3 h, the amount of adsorption increased rapidly with time, then gradually became flat, and then gradually reached the adsorption equilibrium. The maximum adsorption amount of 3-NPA for SA-SMIP was 114.78 μmol/g, and (3-NPA)- The maximum adsorption capacity of SMIP for 3-NPA was 86.26 μmol/g, and the maximum adsorption capacity of SNIP for 3-NPA was 51.61 μmol/g. The main reason that the amount of 3-NPA adsorbed by SA-SMIP is greater than that of (3-NPA)-SMIP may be because the 3-NPA in the polymer leaks out during the adsorption process, resulting in a smaller saturated adsorption capacity.
The binding isotherms for 3-NPA at room temperature (3-NPA) -SMIP, SA-SMIP, and SNIP are shown in figure 5 a and b. The adsorption amount per unit mass of SMIP increased with the increase of 3-NPA concentration in the range of studied concentration, and the adsorption capacity of SA-SMIP and NPA-SMIP was significantly larger than that of SNIP to be 3-NPA, indicating that 3-NPA binding sites were present in SA-SMIP and NPA-SMIP.
Scatchard model was used to evaluate the binding properties of molecularly imprinted polymers. The linear equations of (3-NPA) -SMIP, SA-SMIP and SNIP were obtained by Scatchard analysis of the data in the following formula: Q / C = -0.3451Q + 101.4 (R² = 0.9705); Q / C = -0.2637Q + 127.74 (R² = 0.9661) and Q / C = -0.3112Q + 51.875 (R² = 0.8424), the slope and intercept of the linear equation the adsorption parameters of 3-NPA on (3-NPA) -SMIP, SA-SMIP and SNIP are shown in Table 1. As indicated in figure 5 c that the correlation coefficients of the 3-NPA and 3-NPA-3-NPA-SA-SMIP to the 3-NPA were all greater than 0.96, indicating the presence of the 3-NPA in the microspheres of the imprinted polymer 3-NPA binding site, and the specificity of adsorption is strong, while the correlation coefficient of SNIP data is 0.84, indicating that the adsorption characteristic of 3-NPA is not obvious.
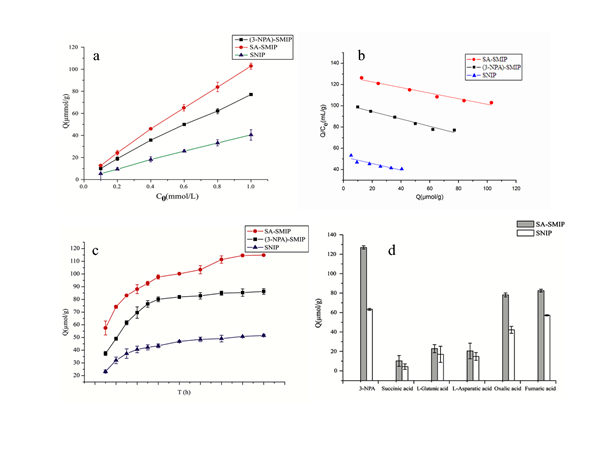
Fig 5. (a) Effect of adsorption time on adsorption capacity by (3-NPA)-SMIP, SA-SMIP and SNIP to 3-NPA. (b) Isotherm of 3-NPA adsorption on (3-NPA)-SMIP, SA-SMIP and SNIP. (c) Scatchard plot of 3-NPA adsorption on (3-NPA)-SMIP, SA-SMIP and SNIP. (d) Binding selectivity test of 3-NPA and structurally related compounds on SA-SMIP and SNIP.
Table 1. The adsorption parameters of 3-NPA on (3-NPA)-SMIP, SA-SMIP and SNIP
|
Polymer |
Qmax (μmol/g) |
KD(mol/L) |
|
(3-NPA)-SMIP |
293.91 |
2.90× 10-3 |
|
SA-MIP |
483.12 |
3.79× 10-3 |
|
SNIP |
166.69 |
3.21× 10-3 |
3.5 Specific adsorption analysis of 3-NPA molecularly imprinted polymers
L-aspartic acid, L-glutamic acid, fumaric acid, oxalic acid and succinic acid were used as templates to compare the adsorption capacity of the imprinted polymer. The results show that SA-SMIP has a good adsorption property to 3-NPA, and the adsorption of 3-NPA structural analog's succinic acid, L-aspartic acid, and L-glutamic acid is shown in figure 5 d. While the adsorption of oxalic acid and succinic acid is relatively high, probably due to the slight difference in its structure, indicating that the specific adsorption selectivity of the polymer is better.
3.6 Binding selectivity test of 3-NPA and structurally related compounds on SA-SMIP and SNIP
In this study, the binding energies of monomers and template molecules were optimized by Hartree-Fock (HF) method and 6-31G (d) basis set by computer simulation. The best functional monomers were selected from three functional monomers MAA, AM, and 2-VP and the best reaction ratio is 1: 3, and the binding energy is -49.03 KJ • mol-1.
Three kinds of molecularly imprinted polymers were synthesized by the method of surface molecular imprinting, using succinic acid as the template molecule. The results showed that the polymer had higher selectivity to 3-NPA and the adsorption capacity of SA-SMIP to 3-NPA was the highest, reaching 114.78 μmoL / g, followed by (3-NPA) -SMIP, in the concentration of 1 mmol/L 3-NPA solution, polymer. The results of specific adsorption experiments showed that the SA-SMIP had a good adsorption property to 3-NPA, which was superior to the adsorption of 3-NPA similarities.
3.7 Application to Sugarcane Samples
MISPE method was developed for selective sample clean up were investigated through the analysis of selected sugarcane samples. The molecular imprint polymer (MIP) was used as solid phase extraction (SPE) absorbent to selectively extract 3-NPA from the sugarcane sample. Sugarcane first was extracted by traditional Liquid-Liquid Extraction (LLE) and using optimized MISPE procedure. It found the method suitable clean-up and purification of 3-NPA in sugarcane samples. During the extraction process, the adsorption of 3-NPA in the polymer is lost due to seepage. The recovery percentage and the relative standard deviation (RSD) of the spiked samples for curve and sugarcane samples were calculated. The performance of the analytical of MISPE was validated through the determination of linearity, sensitivity, LOD and LOQ by using the optimized MISPE conditions prior to real sample analysis.
The concentration range of 3-NPA (before passed through the MISPE column and after passes) was found to be 66.8, 42.1 respectively. Both the analytes showed good linearity with a coefficient of determination (r²) = 0.9997showed in figure 6 a. the recovery ranged from 99.87 to 101.6. The LOD and LOQ were calculated as signal noise (S/N) at S/N ratio of 3:1 and 10:1, respectively. The LOD and LOQ of 3-NPA were found to be 0.19 and 0.58 respectively. This result indicated that MISPE showed better selectivity, chromatography, and then higher recovery showed in figure 6 b. The highest recoveries obtained with 3-NPA standard and 3-NPA MISPE were found to be >99%with Relative Standard Deviation (RSD) of the method was 0.69%.
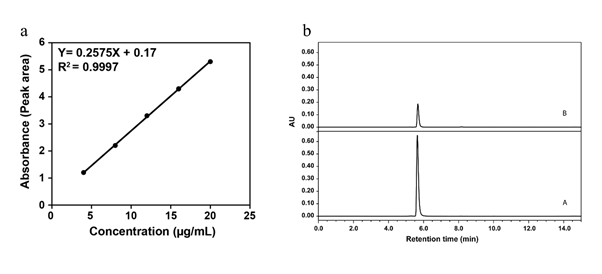
Fig 6. [a] NMR Spectrum of 3-NPA extracted from the sugarcane. [b] Chromatogram (a) 3-NPA before clean up and chromatogram b 3-NPA after clean up passed through MISPE and elution with ACN solution.
The molecularly printed polymers (MIPs) were successfully prepared by traditional bulk polymerization techniques using acetonitrile as template, silica gel as carrier, succinic acid (SA) was chosen as alternative template, MAA, AM as functional monomers, ethylene glycol dimethacrylate (EGDMA), azobisisobutyronitrile (AIBN), and functional monomer, cross-linker, initiator and porogen, respectively. The MISPE of the analytes from sugarcane samples was performed in an off-line mode. The results showed that the prepared polymer had high selectivity and adsorption capacity to 3-NPAin the concentration of 1 mmol/L 3-NPA solution, SA-SMIP showed the best adsorption capacity to 3-NPA which reach to 114.78 μmol/g, the second is (3-NPA)-SMIP, both are much higher than the blank polymer. Specific adsorption experiment results showed that the SA-SMIP had desired adsorption properties to 3-NPA.good adsorption properties to 3-NPA.selctive binding of the specific template molecules is a key feature of MIPs technique. The MISPE column firstly was applied to pre-concentrate, clean up and purify 3-NPA in sugarcane samples. The molecularly imprinted polymer greatly improves the selectivity of solid phase extraction coupled with HPLC and simplifies sample pre-treatment.
Ashley, J., Shahbazi, M. A., Kant, K., Chidambara, V. A., Wolff, A., Bang, D. D., & Sun, Y. (2017). Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosensors and Bioelectronics, 91(January), 606-615. PMid:28103516
View Article PubMed/NCBIBaggiani, C., Baravalle, P., Giraudi, G., & Tozzi, C. (2007). Molecularly imprinted solid-phase extraction method for the high-performance liquid chromatographic analysis of fungicide pyrimethanil in wine. Journal of Chromatography A, 1141(2), 158-164. PMid:17178127
View Article PubMed/NCBIBecker, T., Pasteels, J., Weigel, C., Dahse, H. M., Voigt, K., & Boland, W. (2017). A tale of four kingdoms-isoxazolin-5-one- and 3-nitropropanoic acid-derived natural products. Natural Product Reports, 34(4), 343-360. PMid:28271107
View Article PubMed/NCBIBeltran, A., Borrull, F., Marcé, R. M., & Cormack, P. A. G. (2010). Molecularly-imprinted polymers: Useful sorbents for selective extractions. TrAC - Trends in Analytical Chemistry, 29(11), 1363-1375.
View ArticleBhat, R., Rai, R. V., & Karim, A. A. (2010). Mycotoxins in Food and Feed: Present Status and Future Concerns. Comprehensive Reviews in Food Science and Food Safety, 9(1), 57-81. PMid:33467806
View Article PubMed/NCBIDu, W., Zhou, H., Luo, Z., Zheng, P., Guo, P., Chang, R., Chang, C., & Fu, Q. (2014). Selective determination of penicillin G from tap water and milk samples using surface molecularly imprinted polymers as solid-phase extraction sorbent. Molecular Imprinting, 2(1), 18-29.
View ArticleEzekiel, C. N., Sulyok, M., Warth, B., Odebode, A. C., & Krska, R. (2012). Natural occurrence of mycotoxins in peanut cake from Nigeria. Food Control, 27(2), 338-342.
View ArticleGao, Y., Hu, Y., & Yao, K. (2015). Surface molecularly imprinted polymers for solid-phase extraction of (-)-epigallocatechin gallate from toothpaste. Frontiers of Chemical Science and Engineering, 9(4), 467-478.
View ArticleHarris, C. (2015). President / Editor Comment from the President. 79(2), 67-118.
Huck, C. W., & Bonn, G. K. (2000). Recent developments in polymer-based sorbents for solid-phase extraction. Journal of Chromatography A, 885(1-2), 51-72. 00333-2
View ArticleKutner, W. and P. S. S. (2018). Molecularly Imprinted Polymers for Analytical Chemistry Applications. Royal Society of Chemistry, Vol. 28.
View ArticleLiu, H., Liu, G., Li, K., Chen, Y., & Jiang, J. (2016). Determination of 3-nitropropionic acid in poisoning samples by ultra-performance liquid chromatography-tandem mass spectrometry. Journal of Hygiene Research, 45(1), 56.
Maier, N. M., Buttinger, G., Welhartizki, S., Gavioli, E., & Lindner, W. (2004). Molecularly imprinted polymer-assisted sample clean-up of ochratoxin a from red wine: Merits and limitations. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 804(1), 103-111. PMid:15093164
View Article PubMed/NCBIPeraica, M., Radić, B., Lucić, A., & Pavlović, M. (1999). Toxic effects of mycotoxins in humans. Bulletin of the World Health Organization, 77(9), 754-766.
Qiao, F., Sun, H., Yan, H., & Row, K. H. (2006). Molecularly imprinted polymers for solid phase extraction. Chromatographia, 64(11-12), 625-634.
View ArticleSaini, S. S., & Kaur, A. (2013). Molecularly Imprinted Polymers for the Detection of Food Toxins: A Minireview. Advances in Nanoparticles, 02(01), 60-65.
View ArticleVasapollo, G., Sole, R. Del, Mergola, L., Lazzoi, M. R., Scardino, A., Scorrano, S., & Mele, G. (2011). Molecularly imprinted polymers: Present and future prospective. International Journal of Molecular Sciences, 12(9), 5908-5945. PMid:22016636
View Article PubMed/NCBIWang, T., Zhang, L., Jiang, L., & He, N. (2008). Neurotoxicological effects of 3-nitropropionic acid on the neonatal rat. NeuroToxicology, 29(6), 1023-1029. PMid:18775747
View Article PubMed/NCBIWu, N., Luo, Z., Ge, Y., Guo, P., Du, K., Tang, W., Du, W., Zeng, A., Chang, C., & Fu, Q. (2016). A novel surface molecularly imprinted polymer as the solid-phase extraction adsorbent for the selective determination of ampicillin sodium in milk and blood samples. Journal of Pharmaceutical Analysis, 6(3), 157-164. PMid:29403976
View Article PubMed/NCBI