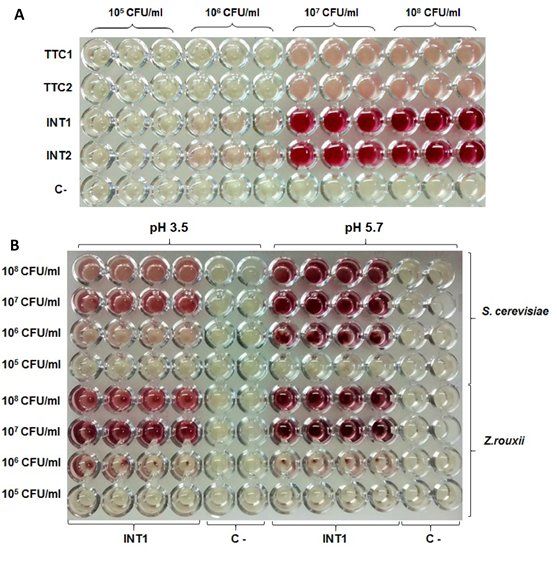
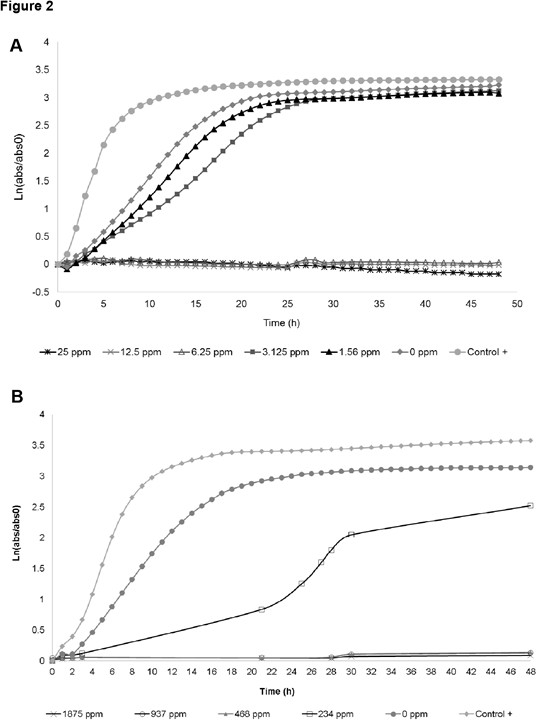
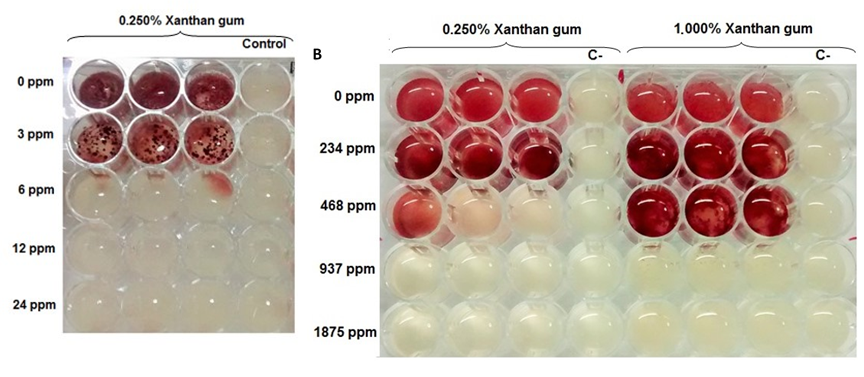
Carmen Adriana Campos
Departamento de Industrias, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Int. Güiraldes 2160, Ciudad Universitaria (1428), Buenos Aires, Argentina. Tel./fax:+5411 45763366.
E-mail address: carmen@di.fcen.uba.ar