Mr. Sajjad Esmaeili
E-mail: sajad.smaeili@kums.ac.ir
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 5 ISSUE: 3
Page No: 146-161
Mr. Sajjad Esmaeili
E-mail: sajad.smaeili@kums.ac.ir
Soodabeh Shafieea, Maryam Amoushahib, Yazdan Rahmatic, Hamzeh Baratia,d, Mohabbat Ansarie,
Sajjad Esmaeilif *
aDr. Barati Medical Diagnostic Laboratory, Gachsaran, Iran
b Department of Agricultural Science, Payam Nor University, Iran
c Department of Medical Genetics, Iran University of Medical Sciences, Tehran, Iran
d Department of Biology, Sciences & Research Branch, Islamic Azad University, Tehran, Iran
e Nano Drug Delivery Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
f Medical Biology Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
Sajjad Esmaeili, Anti-Diabetic Potential of Anti-Oxidant Compounds "Riboflavin, Flavin Mononucleotide and Flavin Adenine Dinucleotide" with High Therapeutic Potency through Inhibition of α-Amylase and α-Glucosidase Enzymes at Sub-Micromolar Concentrations(2020)Journal of Food Science & Technology 5(3) pp:146-161
Diabetes is a group of metabolic disorders characterized by a high blood sugar level over a prolonged period of time. Inhibition of carbohydrate hydrolyzing enzymes leads to decrease in the absorption of glucose which is considered as one of the effective managements of diabetes mellitus. Vegetable, fruit, milk, and fish are good sources of riboflavin (RF) and vitamin B5 with versatile health benefits. The well-adapted structural features of these compounds for the inhibition/activation of enzymes include several available hydrogen bond (H-bond) acceptors and donors, flexible backbone, and hydrophobic nature. The substrates of α-amylase (α-Amy) and α-glucosidase (α-Glu), known as key absorbing enzymes, have functional groups (OH groups) resembling RF and vitamin B5. Therefore, the present study was conducted to evaluate the inhibitory properties of fliavin, RF, flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD) and vitamin B5 against α-Amy and α-Glu. The median inhibition concentration (IC50) values for α-Glu in the presence of RF, FMN and FAD were determined 136.7±4.8, 235.4±5.2 and 78.9±3.4, respectively. The IC50 values α-Amy in the presence of RF, FMN and FAD were determined 165.6±7.1, 253.6±5.5 and 131.2±6.3, respectively. Moreover, the Ki values of RF were calculated as 14.2±1.8 and 18.6±1.7 µM for α-Glu and α-Amy in a mixed-mode. In addition, to communicate with the active site of α-Glu and α-Amy respectively, RF presented a binding energy of -8.1 and -7.3 kcal/mol, FMN -7.1 and -6.8, and FAD -10.3 and -7.8 kcal/mol. These antioxidant inhibitors may be potential anti-diabetic drugs, not only to reduce glycemic index, but also to limit the activity of the major reactive oxygen species (ROS) producing pathways.
Key words: Riboflavin, hydrolyzing enzymes, enzyme inhibition, hyperglycemia Introduction
Diabetes mellitus (DM) as a chronic disease includes the characteristics of abnormal insulin resistance and glucose tolerance [1]. There are some complications with DM including metabolic syndrome, heart disease, renal function recession, and blindness [2]. One of the most important metabolic complications in type-2 DM (T2D) is hyperglycemia which is associated with the development of nephropathy, retinopathy, neuropathy, and heart and blood vessel disease [3]. Preventing hydrolyzing of carbohydrates is a novel treatment method and alternative medicine for treating the T2D by a delay in the digestion of carbohydrates ultimately reducing the rate of glucose absorption [4, 5]. α-Glu and α-Amy are hydrolyzing enzymes which secreted and placed in the small intestine and they are responsible for hydrolyzing dietary carbohydrates.
Acarbose (ACR) is the most common drug used as an α-Amy/α-Glu inhibitor for T2D. However, it has been indicated that this drug has numerous adverse impacts including abdominal distention, flatulence, diarrhoea, and pneumatosis cystoides intestinalis [6-8]. Fermentation of undigested carbohydrates by colonic bacteria is considered as one of the most important reasons for these side-effects. On the other hand, diabetes and hyperglycemia lead to an increase in producing free radicals and oxidative stress [9, 10]. Extremely high levels of free radical’s lead to biomolecular damages to cellular proteins, nucleic acids, membrane lipids, and ultimately tissue injuries and cell death. However, this drug did not have anti-oxidant activity. Hence, the discovery and development of new antidiabetic drugs from natural plant sources with high therapeutic potency, less adverse effects and anti-oxidant properties is at the center of intense research effort.
It is known that vitamins contain an extensive array of bioactive materials with diverse health benefits. Vitamins are organic compounds with a different and relatively simple chemical structure that is different from carbohydrates, proteins or lipids existed in natural foods in quite low concentrations [11]. Vitamins are vital to maintaining normal growth and health and they should essentially be supplied in the diet since they are not synthesized by the body [12]. Their deficiency leads to pathological circumstances (avitaminosis). Based on their solubility features of vitamins, they are classified into water and lipid-soluble. The lipid-soluble vitamins (A, D, E, and K) exist in the food’s fatty constituent. Generally, by excessive ingestion of lipid-soluble vitamins, they are stored in various tissues, mostly in the liver, and are removed quite gradually. Adverse effects reproduced by the intake of these vitamins in higher amounts than normal needs within a long time [13]. On the other hand, water-soluble vitamins comprise all constituents of the vitamin C and B complex. Generally, they are not stored and must be delivered daily. Mainly, toxic impacts are not produced by their high doses since the excess is simply removed through the urine. From the nutritional viewpoint, the separation of vitamins on the basis of their solubility is reasonable. Vitamins in the same group normally exist together in foods, and various vitamins with similar solubility are generally included in their deficiency [14].
Riboflavin (B2) is a constituent of the flavin mononucleotide (FMN) and enzyme cofactors flavin adenine dinucleotide (FAD). FAD and FMN function in various reactions including reduction and oxidation such as decarboxylation reactions, electron transport, glutathione reduction, fatty acid oxidation, and etc. [15]. In most of the reactions transferring the pairs of electrons are included in the creation of double bonds in fatty acid desaturating reactions or the succinate oxidizing in the Krebs cycle [16]. Riboflavin abundantly exists in foods with animal origin rather than vegetables. The most important source of riboflavin is milk and dairy products as well as meat, kidney, liver, and egg yolk. It is also existent in tomatoes, spinach, broccoli, carrots, legumes, and asparagus. However, riboflavin is poorly found in grains, fruits, and even whole. In eggs and milk, a key quantity of the vitamin is free; in other foods, it is as FAD and FMN bound to proteins [17].
FAD is the coenzyme of some flavoprotein enzymes with the function as oxidation reduction catalysts within biological systems [18]. The FAD is a cofactor for cytochrome-b5 reductase that is the enzyme maintaining haemoglobin in its functional reduced mode, and for glutathione reductase, an enzyme also protecting erythrocytes from oxidative damage [19, 20]. According to a report in a Kentucky mountain saddle horse gelding and a Spanish mustang mare, an abnormal riboflavin kinase reaction leads to an erythrocyte FAD deficiency as the initial reaction in conversion of riboflavin to FAD. Clinicopathologic alterations involve 26%-46% persistent methemoglobinemia, eccentrocytosis, a slightly normal or reduced hematocrit, and erythroid hyperplasia in the bone marrow [21, 22].
Vitamin B5 or pantothenic acid is a coenzyme A component and phosphopantetheine itself as a coenzyme A component. Phosphopantetheine is also a prosthetic group for peptidyl carrier proteins, acyl carrier protein, as well as aryl carrier proteins [23]. Pantothenic acid is the key component of coenzyme A (CoA), important for transporting the fats into the cells and energy-producing mitochondria. CoA has a key role in the metabolism of lipids and carbohydrates. With no CoA, fats will not be able to metabolize into energy. Coenzyme A acts in creating thioesters with the carboxyl groups of fatty acids as well as other organic acids [24]. Whole-grain cereals, eggs, legumes, yogurt, and meat are the sources of pantothenic acid and derivatives. Pantothenic acid is abundantly found in the liver, heart, kidney, egg yolk, chicken and bovine meat, sweet potatoes, peas, cabbage, whole grains, broccoli, legumes, yeast, peanuts, and fungi [25].
Since the functional groups (OH group) of RF, PA, FAD, and FMN (Fig. 1) are similar to ACR and miglitol as main synthetic drugs in inhibition of α-Amy/α-Glu, thus, it proposed that impacts of inhibition of these enzymes by these compounds on glucose concentration can consider as one of the most important advantages of these compounds. Therefore, in this study, the inhibitory activities of these compounds have been investigated against the α-Glu and α-Amy for their anti-hyperglycemia potential. This work will contribute towards the understanding of anti-hyperglycemia activity of RF and their analogues, specifically towards the management and prevention of T2D.
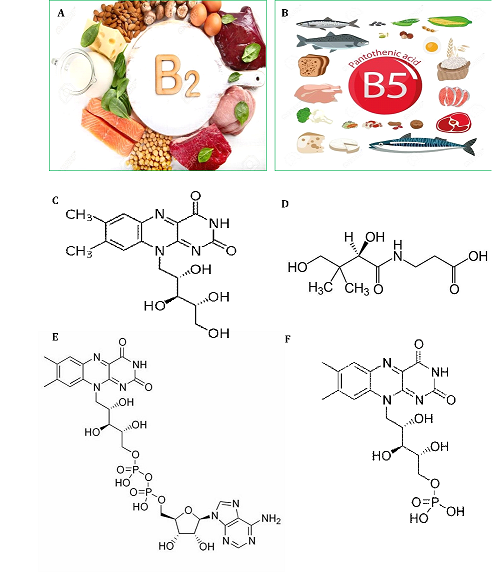
Fig 1. Natural resources of RF (vitamin B2) and Vitamin B5 (A-B). Chemical structures of RF (C), vitamin B5 (D), FAD (E) and FMN (F).
2.1. Chemicals and maintenance of animals
Our compounds, P-nitrophenyl alpha-d-glucopyranoside (p-NPG), ACR and 3,5-dinitrosalicylic acid (DNS) were the antibiotics procured from the Sigma Aldrich Chemical Company. Ten mM of a sodium hydroxide solution was used to obtain the stock solutions, which were then diluted in a phosphate buffer.
2.2. Preparing rat intestinal acetone powder
A formerly-suggested technique was utilized with minor changes in the core process to gather intestinal acetone powder from the rats [26, 27]. The rats’ intestines were sectioned into smaller parts that were rinsed utilizing thirty ml of cold NaCl (0.9%). The temperature for the operations was 4 ◦C [28]. The intestines were homogenized using Potter–Elvehjem homogenizer in two vol. of five mM of phosphate buffer with pH 7.0. After an hour, ten vol. of the acetone solution cooled down to −20 ◦C was inserted into the homogenates. Shaking the mixture, it was then centrifuged for thirty minutes at 1500×g followed by ten minutes. The precipitates were also washed utilizing 12 vol. of cold acetone, then the mixture was centrifuged at 1500×g for thirty minutes. Overnight the precipitate was dried at 4 ◦C and kept at −20 ◦C prior to usage [28].
2.3. Rat α-Glu inhibition assay
The rat enzyme inhibition assay was conducted based on our former investigations with main alterations. pNPG was used as the substrate in the enzyme assay. Then, glucose and pnitrophenol (pNP) were created by breaking down via the α-Glu enzyme. Within former investigations, through recording the absorbance at 405 nm, quantifying the yellow product attained on the basis of p-nitrophenol (pNP) formation was performed [26, 27, 29]. Considering the similarity between the absorption point of our compounds and pNP, it is not possible to use this wavelength. Conversely, the glucose is also created in addition to pNP formation, hence, we assessed the value of glucose created by glucose oxidase kit. By dissolving the intestinal acetone powder in ice cold phosphate buffer (pH 7.0,10 mM), the α-Glu activity was measured, and the solution was sonicated at 4 ◦C for 25 s. The suspension was then centrifuged with 10,500×g for 75 min at 4 ◦C, and the resultant supernatant was dialyzed for 24 h against the same buffer. The reaction mixture including rat α-Glu and different concentrations of our compounds were pre-incubated at 37◦C for 10 min, prior to adding pNPG. The glucose value was then monitored for 30 min at 500 nm. In this work, the AC, pharmacological inhibitor was utilized as a positive control while repeating all the tests for at least 3 times. The absorbance increase was then followed at 405 nm for thirty minutes. All the tests were repeated for at least 3 times.
2.4. α-Amy assay
α-Amy inhibitory activities related to the medicines were examined based on measuring glucose levels. α-Amy was incubated for ten minutes at 25 ◦C with the compounds and ACR. The test was carried out in 20 mM of a sodium phosphate buffer with a 6.9 pH. Then, after adding the starch solution (1% soluble starch), the reaction mixture was incubated for five minutes. The reaction was stopped followed by the addition of four hundred μl of the DNS reagent and boiling in a water bath for five minutes. After incubation of the solution and maintaining it at room temperature for fifteen minutes, 5 ml of water was introduced. By measuring the absorbance of the samples at 540 nm [30], it was compared with the experiment control absorbance. The findings were regarded as the criterion for the α-Amy inhibition percentage. The tests were repeated for three times.
2.5. Determining the inhibitory constant and inhibition fashion
To calculate the inhibitory constant and determine the inhibition mode, α-Glu activities were assayed at different concentrations of p-NPG in the occurrence of an increasing concentration of ACR (0, 0.25, 0.5, 1 µM) and RF, i.e. 0, 7, 14 and 30 µM. Moreover, α-Amy activities were assessed in various concentrations of starch (as substrate) by increasing the concentration of RF, i.e. 0, 8, 20 and 50 µM, for determining the inhibition fashion and the inhibitory constant. The primary velocity, V0, was also calculated as the variations’ slope in absorbance at 540 nm in the catalytic reaction linear phase. For the competitive inhibition, the Lineweaver-Burk equation is presented as [31]:
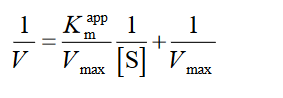 (1)
(1)
and the secondary plot is made as:
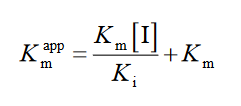 (2)
(2)
where [I] indicates the inhibitor concentration, [S] is the substrate concentration, the apparent Michaelis-Menten constant, Ki shows the inhibition constant and Km represents the Michaelis-Menten constant, V is the enzyme reaction rate by existence and nonexistence of the inhibitors [31].
2.8. Molecular docking
2.8.1. Homology modeling and model verifying
The protein sequence and homology for Baker’s yeast a-Glu (MAL12) were modeled using the technique provided by Imran [32, 33]. Using SWISSMODEL a search that was conducted and proteins were identified in the Protein Data Bank (PDB) with high sequence similarity.
The 72%-identical α-D glucose bound isomaltase with a similarity of 85% related to Saccharomyces cerevisiae (PDB ID: 3A4A) was exposed to homology modeling and sequence alignment using the automated homology modeling pipeline SWISS-MODEL controlled by the Swiss Institute of Bioinformatics [34]. PROCHECK was utilized to verify the quality of the established homology model.
2.8.2. Docking investigations
Docking studies were performed through the homology model of α-Glu and the α-Amy’s crystal structure with PDB ID:1PIF [35]. Based on Fig. 1, the compounds’ molecular structure depicted via ACD/LAB was traced by changing it to the three-dimensional conformation utilizing Avogadro 1.2.0. For minimizing energy and optimizing ultimate geometry, the steepest-descend algorithm in Avogadro was utilized. Adding Gastiger charges and activating the rotational bonds through the MGL tools package, the drugs were docked into α-Amy/α-Glu via Auto Dock Vina. A box with 30*30*30-nm in the directions of X, Y, and Z were chosen in the search space by adjusting its center on the proteins’ active sites.
2.9. MD simulations
The ligand-protein’s complex structure with the least energy determined by the docking process was utilized as the initial geometry for the MD procedure. Gromos53a6 force-field, GROMACS toolkit-5.1 (www.gromacs.org) was used to perform the MD simulation [36]. TET’s topology parameters and topology file were attained from the PRODRG server, and the charges were reallocated through the data related to the similar atom kinds in gromos 53a6 fore field [37]. The, the complexes were introduced into a periodic cubic box with 11×11×11-nm dimensions and a prolonged simple point charge (SPC/E) model of water utilized for dissolution [38]. Calcium (6 for α-Amy-RF system) and sodium ions (2 for free α-Amy, 8 for α-Glu-RF systems and 16 for free α-Glu) and were sufficiently added to the system for neutralizing. Within the system, 3D periodic boundary circumstances were also utilized. The energy was minimized through the steepest-descent technique for releasing undesired contacts in all the MD simulations, after 100 ps equilibration in NVT and NPT ensembles [39, 40]. A constant temperature of 300 K was obtained using velocity-rescale thermostat, and a constant pressure of 1 bar was attained by a Parrinello–Rahman barostat [41]. All the bonds were limited using he LINCS algorithm [42]. The long-range interactions were investigated using a 10 Å cut-off distance and the particle mesh Ewald technique [43]. The Verlet cut off scheme was utilized for a neighbour search, while updating the adjacent lists every 10 stages [44]. Ultimately, 50-ns MD simulations were performed using the leapfrog algorithm.
3. 1. Determining the α-Glu/α-Amy inhibition potency based on IC50 values
To measure the inhibitor strength, the half maximal inhibitory concentration (IC50) value is normally utilized. Based on Table. 1, the α-Glu activity was dose-dependently reduced while existing all the compounds. It is observed that the median inhibition concentration (IC50) values were calculated 136.7±4.8, 78.9±3.4, and 235.4±5.2 for α-Glu similar to existing RF, FAD and FMN, respectively. In the current work, the ACR median IC50 values were obtained for α-Glu as 11.9 ± 0.6 µM. As anticipated, ACR intensely suppressed the αGlu activity as its standard inhibitor. α-Amy is regarded as the key enzyme for breaking down the starch to more simple sugars, i.e. dextrin, maltotriose, glucose, and maltose. αAmy inhibitors called starch blockers have a key role as obstacles to the dietary starch absorption. Breaking down starch as a complex carbohydrate primarily needs digestive enzymes, such as amylase, and it needs secondary enzymes for its absorption. Thus, starch was used in the present study as the α-Amy substrate. Based on the findings, it is indicated that all the compounds are able to prevent the α-Amy activity in a dose-reliant mode. The obtained IC50 values were 165.6±7.1, 131.2±6.3, and 253.6±5.5 µM respectively by existing RF, FAD and FMN, respectively. Moreover, it was found that ACR can intensely inhibit the αAmy activity with an IC50 value 19.9 ± 0.6 µM in comparison to the study compounds.
Table 1. IC50 values for α-Glu and α-Amy in the presence of different concentrations of compounds. Production of glucose was monitored by absorbance at 405 nm and 540 nm for α-Glu and α-Amy, respectively.
|
Compounds |
IC50 values for α-Glu (µM) |
|
IC50 values for α-Amy (µM) |
|
ACR |
11.9 ± 0.6 |
|
19.9 ± 0.6 |
|
Flavin |
>1000 |
|
>1000 |
|
RF |
136.7±4.8 |
|
165.6±7.1 |
|
FMN |
235.4±5.2 |
|
253.6±5.5 |
|
FAD |
78.9±3.4 |
|
131.2±6.3 |
|
B5 |
>1000 |
|
>1000 |
3.2. Determining the inhibition mode and inhibition constant, Ki
To recognize the kinetic parameters and describe the inhibition activity of RF, Kinetic assessments were performed utilizing the Lineweaver–Burk double reciprocal plot. A straight line is mainly yielded by the plot of 1/V versus 1/[S] with an intercept of 1/Vmax and a slope of Km/Vmax. A pattern of line is obtained by overlapping the double reciprocal lines for an enzyme reaction at various constant concentrations of inhibitor as the feature of a specific kind of inhibitor. Ki is determined using the graph of the of the Lineweaver–Burk plot’s slope against [I], namely the secondary plot, which is attained from the plot’s xintercept. Based on the Lineweaver–Burk plot (Fig. 2A and 2C), it is appropriately possible to explain the α-Glu inhibition kinetics of ACR and RF by the competitive-state inhibition. In general, the competitive inhibitions are classified into three kinds of hyperbolic, linear, and parabolic, which can be recognized by their secondary plots’ shape. Considering the linear form of both the primary (Lineweaver–Burk) and secondary plots of the α-Glu inhibition by RF (Fig. 2B and 2D), the observed inhibition can be linear competitive. Thus, the substrate (p-NPG) and inhibitor (RF) are capable of competing for binding to the same active site. Km was plotted versus different concentrations of the drugs (secondary plot) (Fig. 2B and 2D) to determine the Ki of ACR as 0.4±0.06 µM in the low micro molar range, and RF as 14.2±1.8 µM against α-Glu. The inhibition mode and constant of α-Amy were determined in the present study by existing RF. The α-Amy inhibition kinetics of RF was described well by the mixed-state inhibition based on Fig. 3A and the Lineweaver–Burk plot. Drawing the secondary plot yields the Ki of RF as 18.6±1.7 µM in the low micro molar range (Fig. 3).
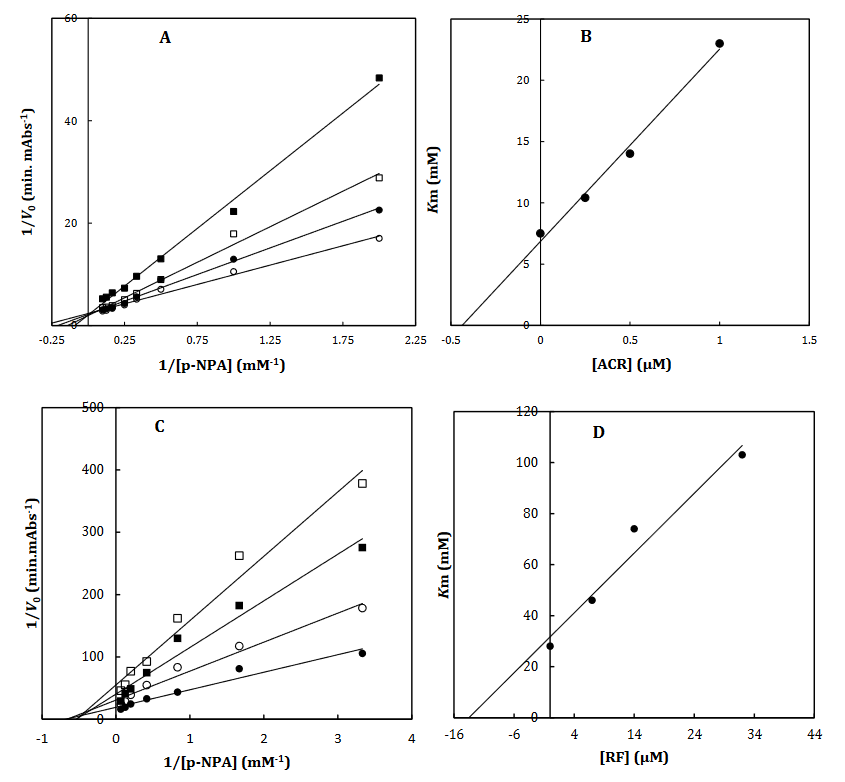
Fig 2. The Lineweaver-Burk plot of α-Glu in the absence (●) and the presence of 0.25 (○), 0.5 () and 1 (□) μM of acarbose (A) and in the absence (●) and the presence of 7 (○), 14 () and 30 (□) μM of RF (C). The value of the inhibitory constant was determined from the negative value of the x-intercept of the plot. Secondary plot for inhibition of α-Glu with ACR (B) and RF (D). Data shown are representative example of three independent experiments and standard deviations were approximately within 5% of the experimental values.
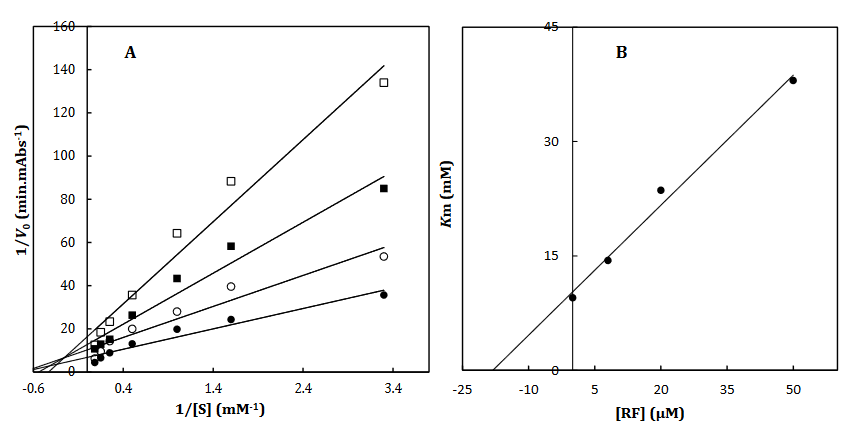
Fig 3. The Lineweaver-Burk plot of α-amylase in the absence (●) and the presence of 8 (○), 20 () and 50 (□) μM of RF (A). The value of the inhibitory constant was determined from the negative value of the x-intercept of the plot. Secondary plot for inhibition of α-Amy with RF (B). Data shown are representative example of three independent experiments and standard deviations were approximately within 5% of the experimental values.
To better comprehend the enzyme-inhibitory interactions mechanism, the molecular docking method was employed. This technique was also utilized for predicting the location of the drugs in the α-Amy/α-Glu active site. The In-silico docking indicated that tentatively decided hindrance data were accompanied to the docking outcomes supporting the legitimacy of the used in-silico methods. Since RF provided the least binding energies while interacting with α-Glu and α-Amy, it was considered to conduct the molecular dynamic simulation in subsequent phase. Performing the MD simulations, the amino acids were investigated included in the binding of RF to α-Amy/α-Glu, and more exact models were obtained for the ligand interaction with these enzymes in fairly actual/natural circumstances.
Considering the binding energies of -7.3, -7.8, -6.8 and -7.4 kcal/mol respectively obtained by RF, FAD, FMN, and ACR as the commercial antidiabetic’s agents, combined with the active site of α-Amy. Fig. 4 represents the graphical cooperation between the drugs and the α-Amy’s active site, and their connecting potential to the α-Amy’s nucleophilic active site.
Based on the virtual docking results, G306, D300, N352, H305, K200, G304, D197, E233, Y62, W59, H299, Y151,Q63, Y163, L165, H201, W59, R195 and I235 residues were found as the robust amino acids for interacting with the drugs. Moreover, the interaction elements’ dynamics was examined through MD simulations. The framework equilibration and basic solidness are normally evaluated through the root-mean-square deviation (RMSD). In Fig. 5A, the plots of the RMSD values are represented for α-Amy alone and the protein spine in the α-Amy-RF complex between 0 and 50 ns. As observed in Fig. 5A, after 33000 ps of the simulation, the RMSD values of α-Amy-RF frameworks and α-Amy alone achieved a relative equilibration, then they fluctuated nearby the normal values. Based on this compelling evidence, the reproduction time adequacy and the system reaching the relentless state are suggested. Based on Fig. 5B, by determining the RMSF values for the α-Amy-RF complex and α -Amy alone, the alterations in the protein residual fluctuations surrounding their normal qualities were analyzed. The findings indicated minor variations in most of the residues of α-Amy in the α-Amy-RF complex, indicating the incidence of a stable connotation.
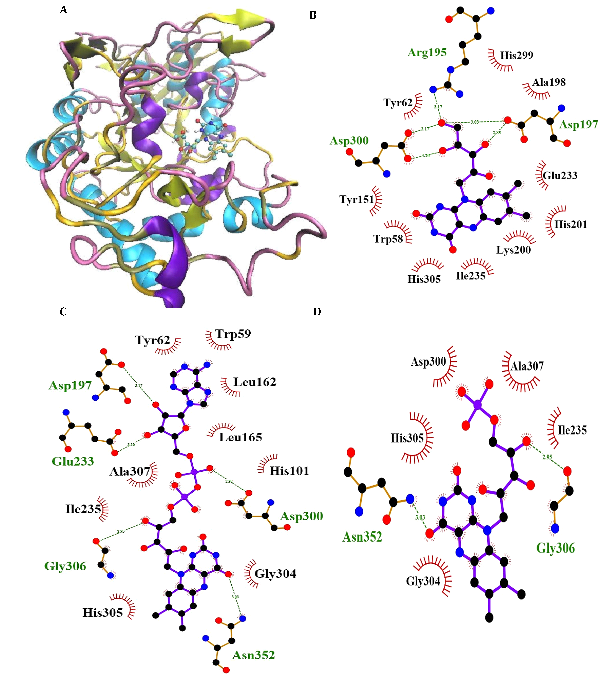
Fig. 4. Molecular docking of α-Amy. Two dimensional diagram shows interactions between binding site of αGlu and RF (B), FAD (C) and FMN (D). (For interpretation of references to color in this figure legend, the reader is referred to the web version of this article).
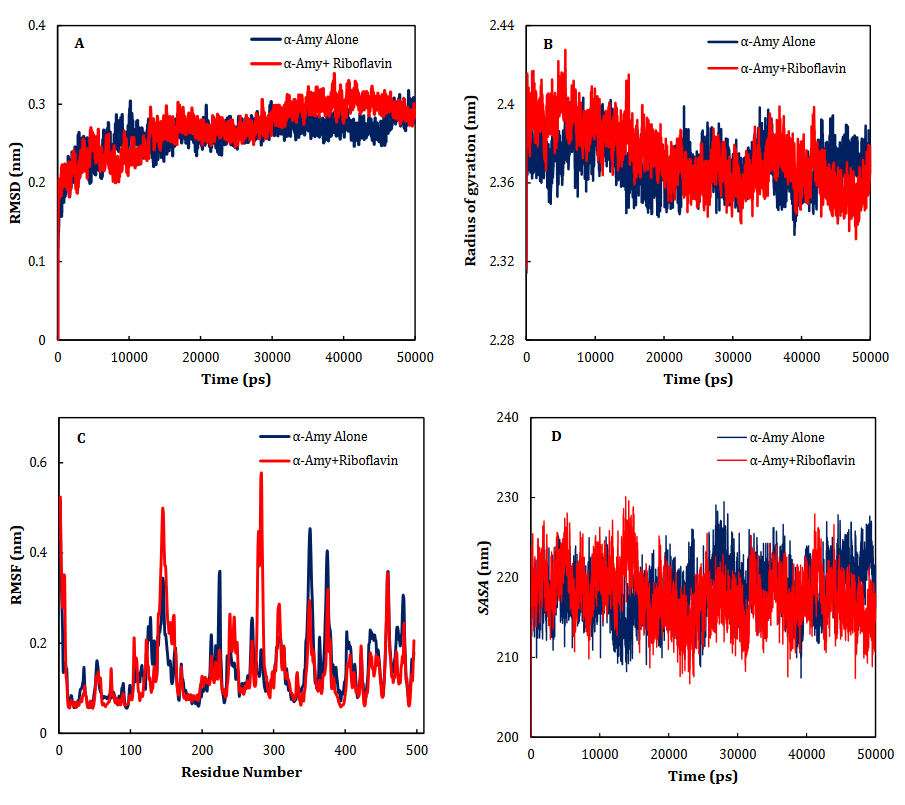
Fig. 5. Time dependencies of the RMSDs (nm) of the α-Amy alone (blue) and α-Amy-RF (red) systems (A). Time dependencies of radius of gyration from starting structure for the α-Amy alone (blue) and α-Amy-RF (red) systems in the 50-ns MD simulation (B). The RMSF and SASA of the α-Amy alone (blue) and α-Amy-RF (red) systems (C-D).
α-Glu is another digestive carbohydrate enzyme playing a vital (pivotal) role in development of diabetes. By performing docking and molecular dynamic simulations, the experimental results were verified to identify amino acids in the α-Glu active site included in communication with the inhibitors (the medicines and ACR) and to attain data regarding the components of the complex framework. Considering the inaccessibility of the PDB structure of α-Glu for eukaryotic organisms including rats and humans, α-Glu of S. cerevisiae was attained utilizing the homology modeling. Based on Fig. 6, N241, H439, P309, D408, F157, K155, S15, H239, F177, H279, R312, N412, S384, and R439 residues seem to be essential amino acids in interactions with these drugs. The residues have a significant role in maintaining the inhibitors, however, they bind with the enzyme’s active site. There is a complete consistency between the existing docking and findings for AC entirely and the detailed results previously gained by the authors. RF, FAD, FMN and ACR as the commercial anti-diabetic agents, respectively have the binding affinity energies of -8.1, -10.3, -7.1 and 7.9 kcal/mol for the interaction with the active site of α-Glu. Based on Fig. 7A, for the molecular elements, the RMSD values of α-Glu-RF frameworks and α-Glu alone reached equilibration followed by 28000 ps of the reenactment time flocculate around the normal value. To verify the protein compactness in the complex with RF, the RMSF findings (Fig. 7.B) can be obtained.
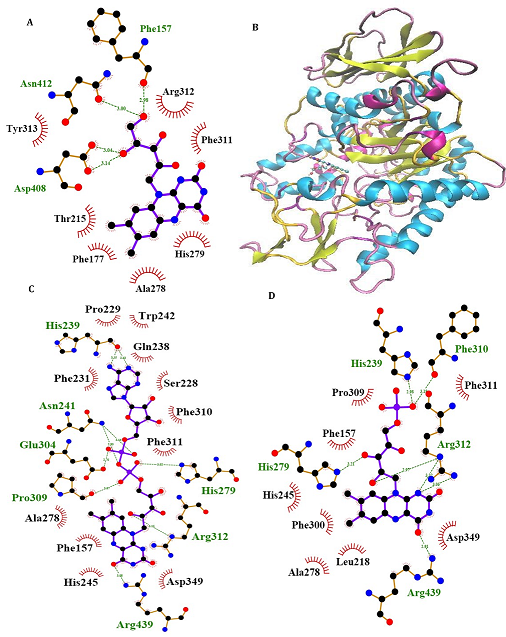
Fig. 6. Molecular docking of α-Glu. Two dimensional diagram shows interactions between binding site of αGlu and RF (A), FAD (C) and FMN (D). (For interpretation of references to color in this figure legend, the reader is referred to the web version of this article).
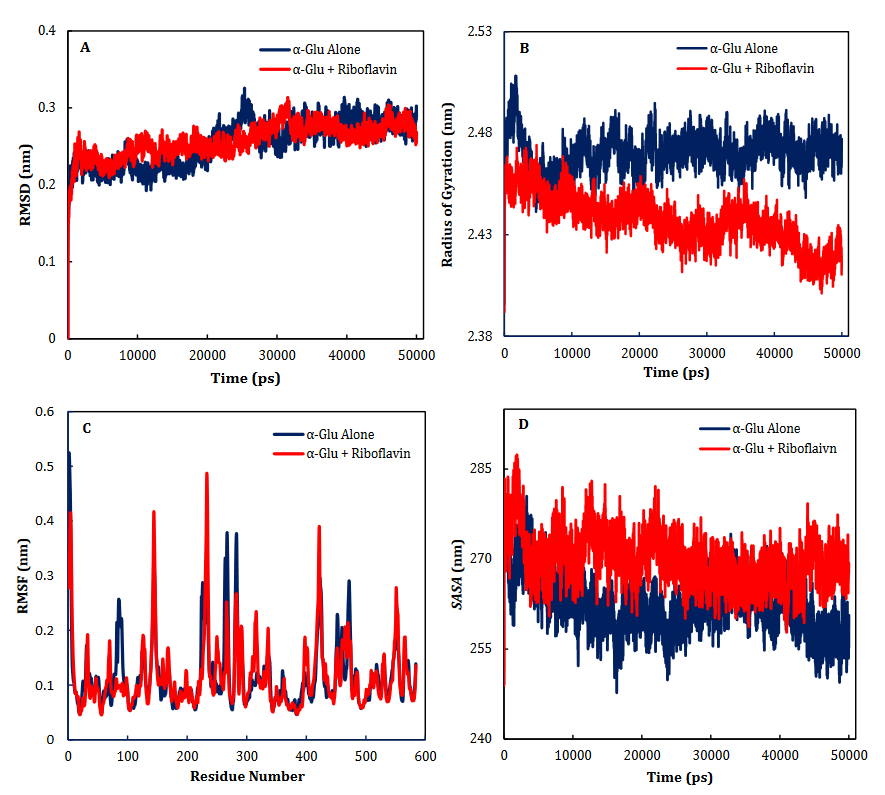
Fig. 7. Time dependencies of the RMSDs (nm) of the α-Glu alone (blue) and α- Glu-RF (red) systems (A). Time dependencies of radius of gyration from starting structure for the α-Glu alone (blue) and α- Glu-RF (red) systems in the 50-ns MD simulation (B). The RMSF and SASA of the α-Glu alone (blue) and α- Glu-RF (red) systems (C-D).
4.1. Hyperglycemia
Hyperglycemia is an abnormal increase in blood glucose level and an etiology of T2D; a disease triggered by insulin resistance [45, 46]. According to numerous studies, a key treatment of T2D is to reduce the post-prandial hyperglycemia by delaying the absorption of glucose via inhibiting the enzymes, α-Glu and α-Amy, in the digestive tract [47-49]. The glucose absorption rate is delayed by enzyme inhibitors by prevention of carbohydrate digestion and accordingly dampening the increase in the postprandial plasma glucose. The starch digestive enzymes inhibition by synthetic agents like acarbose, is a key clinical approach to control the postprandial glycemia [50-53]. Though acarbose , which is confirmed by the Food and Drug Administration, decreases blood glucose levels, it is reported that this inhibitor causes critical side effects like abdominal distention, flatulence, diarrhoea and pneumatosis cystoides intestinalis and the lack suitable antioxidant activity, while enzyme inhibitors with plant basis are potentially safer [54]. Therefore, it is an urgent task to search for natural and non-toxic α-Amy/α-Glu inhibitors without any adverse or unwanted secondary effects, high therapeutic potency, and suitable anti-oxidant activity.
According to enzymatic and chemical assessments, enzymatic hydrolysis by α-Amy results in cleave the linkage within the two glucose units [55]. It is extensively identified that the existence of two carboxyl-containing amino acids is required for the two-step mechanism to retain glycoside hydrolases. One acts as an acid/base catalyst and the other has the role of a nucleophile in charge of creating the glycosyl-enzyme intermediate [56]. Numerous works reported that both Glu233 and Asp197 may be needed for producing the â-linked glycosyl-enzyme intermediate. McCarter and Withers confirmed that Asp197 has the role of the catalytic nucleophile. As stated in former study, Glu233 that is close to the chloride ion, should act as the catalytic acid/base [57]. It was reported that α-Amy inhibition by flavonoids strongly depends on the position and nature of the substituents.
For instance, it was shown that the existence of –OH groups at different locations and the C=C double bond have different inhibitory impacts [56, 58]. As mentioned, RF has various functional groups like NH2, OH, N, NH, CO2, C=O and C=C, capable of interacting with the active site of enzymes utilizing hydrogen bond donors/acceptors. Therefore, since RF has anti-oxidant activity and the flexible backbone, numerous existing hydrogen bond donors/acceptors and aromatic rings well-suited structural characteristic for inhibition of α-Amy/α-Glu enzymes, in this study we investigated anti-diabetic effects RF. In addition, though the synthesis and discovery of innovative α-Amy/α-Glu inhibitors are vital in their own right, it is similarly vital to assess their particular mechanism of inhibition versus enzymes included in carbohydrate digestion. Therefore, in this study not only we investigated effects RF against α-Amy/α-Glu activity, but also we selected different analogs with at least an identical part for finding the most important parts in inhibition these enzymes. They differ both in their location of attachment and number of these functional groups and in their rings.
As shown in Table. 1, flavin cannot inhibit α-Amy and α-Glu activity, while α-Amy and α-Glu activity suppressed in the presence of RF with IC50 less than 200 µM. Since, ribitol molecule is only different between flavin and RF, thus, with comparing IC50 of flavin and RF against α-Amy and α-Glu activity we found that ribitol molecule plays the most important role in inhibition these enzymes. Also, we selected FMN that has a phosphate group attached to ribitol for comparing with RF. Our results showed that RF inhibited α-Amy and α-Glu activity with IC50 less than FMN. Therefore, we can conclude that the minor difference in the activity of these analogs can be caused by the difference functional groups on the ribitol part so that we can concluded that phosphate groups neither have impact or less impact on α-Amy/α-Glu inhibitory in comparison with hydroxyl groups. In addition, for investigating the importance of hydroxyl groups and hydrogen acceptor/donor bonds we chosen compounds with more hydroxyl and amine groups. Accordingly, in this study we selected FAD that has five hydroxyl and one amine groups. As shown in Table. 1, FAD suppressed αAmy and α-Glu activity with IC50 less than RF and FMN. Since, by incrementing the number of hydroxyl and amine groups we observed an increase in α-Amy and α-Glu inhibitory, it was concluded that the number of hydroxyl and amine groups have an great impact on αAmy/α-Glu inhibitory.
According to Fig. 7, our docking data implied that Asp300, Asp197, Gln63, Glu233, Phe282 Phe144, and Trp59 play vital roles at active site of α-Amy interacting with these compounds.
Furthermore, Arg312, Arg439, Lys155, His239 His245 and His279 exists at the entry of the pocket and into the active site of α-Glu that are positively charged at experimental pH 7.2 and interacted with the hydroxyl groups of the compounds. Moreover, aspartate, asparagine, phenylalanine, and tryptophan amino acids like Asn412, Glu276, Phe157, Asp408, Phe177, Phe158, Phe310, Phe313, Phe300, and Trp430 at active site, are both electron acceptors and donors with negatively charged at natural pH. In compared to the docking and experimental results on α-Glu and α-Amy together indicated that α-Glu is inhibited by these compounds with less IC50 and more potency. Comparison of amino acids included at active sites of α-Glu and αAmy implied that further Asp, Arg, Glu, His, Lys amino acids exist with positive charges at the α-Glu active site that may influence the better interactions with these compounds’ hydroxyl groups. Furthermore, it is normally supposed that carbohydrate mimics with nitrogen such as ACR and miglitol were protonated in the active site and function as α-Glu and α-Amy inhibitors due to their potential to imitate the shape and charge of the transition state supposed for the enzymatic glycoside hydrolysis[59, 60].
Heart failure is a serious and common comorbidity of diabetes and oxidative stress is related to the chronic diabetic complication’s pathogenesis such as cardiomyopathy. Therefore, the capability of antioxidants in inhibiting injury increased the probability of novel therapeutic trends for diabetic heart diseases. The blood sugar can be potentially lowered by some alternative and complementary medications, and most of the patients consider these materials as more natural than synthetic drugs. Vitamins are essential micronutrients with antioxidant potential probably providing a complementary therapy for patients with various illnesses [61]. In one of the studies, Guoguang Wang et al. have demonstrated that RF has the cardioprotective effect in diabetic rats [62]. In addition, several studies have demonstrated that vitamin B2 has been recognized as a potential antitumorigenic, antiflammator nutrient [63-65]. Accordingly, since in previous studies it has been demonstrated that oral administration of RF is safe even in high doses and also they exhibited various biological activities like anti-inflammatory, antioxidant, neuroprotective and anti-cancer [66], cardioprotective effects, thus, they can be suggested as new α-Amy and α-Glu inhibitors for treatment of diabetes.
4.2. Hypoglycemia
So far in this article, we have discussed the advantages of these compounds, but we must explain about lack using of these compounds because they may potentiate the hypoglycemia effects of non-diabetic and anti-diabetic drugs. Today, numerous medicines are utilized in different ways to reduce and control blood glucose levels thus treating T1D and T2D. Most of these drugs are able to provide hypoglycemia by interfering with the glucose metabolism, decreasing the insulin clearance and stimulation of the insulin release [67]. From a medical viewpoint, hypoglycemia denotes for a serious condition causing the body not to be able to meet its energy needs. In case dramatic drop in blood glucose levels, there will not be adequate energy for the body to properly function. Hypoglycemia can impose a danger for normal functionality of the brain, considering the dependence of the brain on the circulating glucose as its main energy source [68]. Severe hypoglycemia is associated with the incremented morbidity and even mortality in some cases in T1D as well as T2D. Since T2D is more usual than T1D by a factor of ten, severe hypoglycemia in patients with T2D is more problematic clinically [69].
Drugs are regarded as the main cause of hypoglycemia, particularly in diabetics [70]. Even in carefully management of diabetes, the utilized drugs can lead to medication-induced low blood sugar [71]. Furthermore, this condition is established in non-diabetics after taking the medicines for treatment of diabetes [72]. Though hypoglycemia is a well-defined adverse impact of anti-diabetic agents, various groups of pharmaceutical non-diabetic agents utilized daily in clinics can also contribute to the severe hypoglycemia. In Table 2, the absolute risk of emerging hypoglycemia related to commonly-utilized glucose-reducing agents for diabetes management is provided [67, 73]. In definite cases, non-diabetes related drugs lead to the less blood sugar (Table 3) [74-76].
|
Table 2. Risk of Hypoglycemia With Commonly Used Glucose-Lowering Agents [67] |
||
|
Drug Class |
Drug |
Mechanism of Glucose-Lowering Effects |
|
α-Glucosidase inhibitors |
Acarbose Miglitol |
Delay the process of digestion and absorption of carbohydrates in the small intestine |
|
Amylinomimetic |
Pramlintide |
Acts centrally to slow gastric emptying, suppress postprandial glucagon secretion, and decrease food intake |
|
Biguanides |
Metformin |
Decreases hepatic glucose production and intestinal absorption of glucose, improves insulin sensitivity of cells |
|
DPP-4 inhibitors |
Sitagliptin Saxagliptin |
Inhibit glucagon release
|
|
sulfonylureas |
Glimepiride Glipizide Glyburide |
Increase insulin release from the beta cells in the pancreas
|
|
Meglitinides |
Nateglinide Repaglinide |
Help the insulin-producing beta cells in the pancreas to release insulin |
|
Glucagon-like peptide-1 |
Exenatide Liraglutide |
Encourages the release of insulin from the pancreas, and holds back glucagon release |
|
Thiazolidinediones |
Pioglitazone Rosiglitazone |
Improve the patient’s sensitivity to insulin by reducing circulating fatty acid concentrations and lipid availability in liver and muscle |
|
Table 3. Non-Diabetes Drugs Associated with Hypoglycemia [67, 77] |
||
|
Drug Class |
Drug |
Mechanism of Glucose-Lowering Effects |
|
ACE inhibitors |
Benazepril
Enalapril Lisinopril Perindopril Ramipril
Captopril Fosinopril Moexipril Quinapril Trandolapril |
Indirectly increases insulin sensitivity by increasing circulating kinins, which leads to vasodilatation in the muscles and increased glucoseuptake in muscle tissue
|
|
β-Blockers |
Noncardioselective: Levobunolol Metipranolol Nadolol
Propranolol
Sotalol Timolol
Cardioselective:
Acebutolol
Atenolol Betaxolol Bisoprolol Esmolol Nebivolol Metoprolol |
Inhibits glycogenolysis; attenuates signs and symptoms
|
|
Chloramphenicol |
|
May inhibit the metabolism of SUs |
|
Chloroquine |
|
Unknown |
|
Clofibrate |
|
Enhances the effect of SUs |
|
Disopyramide |
|
Unknown; appears to result from endogenous insulin secretion |
|
Ethanol |
|
Impairs gluconeogenesis; increases insulin secretion |
The absence of pantothenic acid was experimentally created in different species. In young people, growth retardation is the initial symbol. Also, disorders in the nervous, reproductive, and immune systems have been reported. It is revealed by fatigue, restlessness, hypotension, headache, insomnia, motor incoordination, marked tachycardia during exercise, anorexia, hyperactive tendon reflexes, burning sensation in feet and hands, loss of touch sense, and reduced antibody production [78-80]. Riboflavin deficiency, causes cheilosis (lip fissuring and chapping), a scaly rash on scrotum or vulva, sore tongue, photosensitivity, night cataracts, blindness, mild anemia, migraines, and fatique/depression [17, 81]. In some cases, patients are located in compulsory situation of multi-pharmacological therapeutic procedures with various drugs. Accordingly, since in this study have found that RF can inhibit α-Amy and α-Glu at micromolar concentrations, thus consumption this drug with diabetic and non-diabetics drugs that can lead to hypoglycemia should be reconsidered by specialist.
In this work, we assessed the interactions between some RF, FAD and FMN with α-Amy/α-Glu enzymes in-vitro. According to the findings, these compounds can be considered as αAmy/α-Glu inhibitors. Comparing IC50 and Ki values of these compounds with ACR (as a commercial anti-diabetic drug), it was understood that these compounds can bind to the active sites of α-Amy and α-Glu enzymes almost slightly weaker than ACR could through mixed-mode inhibition. Furthermore, the experimental observations were confirmed by the docking studies. Thus, these hydrolyzing enzymes inhibitions can be considered a benefit of these compounds (or a side effect). These antioxidant inhibitors may be potential anti-diabetic drugs, not only to reduce glycemic index, but also to limit the activity of the major reactive oxygen species (ROS) producing pathways. Furthermore, as stated earlier considering the fact that insulin-induced hypoglycemia may take place in DM and other diseases, therefore, it is necessary to take caution in using of these compounds under certain hypoglycemic circumstances for patients and to reconsider by specialists.
