Hae Young Chung
Tel.: + 82 51 510 2814; fax: + 82 51 518 2821
Email: hyjung@pusan.ac.kr
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 4
Page No: 777-787
Hae Young Chung
Tel.: + 82 51 510 2814; fax: + 82 51 518 2821
Email: hyjung@pusan.ac.kr
Hee Jin Jung1,2,3, Dae Hyun Kim1,2,3, EunJin Bang1,2, Sugyeong Ha1,2, Hae Young Chung1,2,3*
1College of Pharmacy, Pusan National University, Busan 46241, Republic of Korea.
2Aging Tissue Bank, College of Pharmacy, Pusan National University, Busan 46241, Republic of Korea.
3Longevity life Science and Technology Institutes, Pusan National University, Busan 46241, Republic of Korea.
Hongzhuan Xuan H(hongzhuanxuan@163.com)
Luciana Bordin(luciana.bordin@unipd.it)
Haw-Wen Chen(chenhw@mail.cmu.edu.tw)
Mosad A Ghareeb(m.ghareeb@tbri.gov.eg)
Hae Young Chung, Anti-inflammatory and antioxidant activities of piperine on t-BHP-induced in Ac2F cells(2019) SDRP Journal of Food Science & Technology 4(4)
Background: In this study, to investigate whether piperine, an alkaloid from Pipper longum, could potentially exerts its effect for the suppression of inflammation and oxidative stress, we examined the modulatory effects of piperine in tert-butylhydroperoxide (t-BHP)-induced Ac2F rat liver cells.
Methods: Anti-inflammatory mechanism of piperine in Ac2F cells were examined by performing western blotting.
Results: The piperine exhibited remarkable reduction of intracellular reactive species (RS) levels in t-BHP- and SIN-1-induced Ac2F cells. In addition, piperine inhibited t-BHP-induced activation of nuclear factor kappa B (NF-κB) by suppressing the degradation of inhibitor-κB proteins (IκBα) and translocation of p65 from the cytosol to the nucleus, further indicating piperine’s inhibitory effects on nitric oxide synthase (iNOS), cyclooxyganse-2 (COX-2) expressions. As a consequence, piperine modulated through inhibition of ERK, JNK, and p38 MAPKs signal transduction pathway in cells. Moreover, piperine pretreatment also regulated the protein expression of antioxidant enzymes such as manganese-dependent superoxide dismutase (MnSOD) and catalase.
Conclusion: These results indicated that piperine, a major component of black pepper might be a potential anti-inflammatory agent by modulating RS-induced NF-κB activation through the MAPKs signaling pathway and possess the anti-oxidative property. Therefore piperine can be considered as a useful therapeutic and preventive approach for the treatment of inflammation and oxidative stress-related diseases.
Keywords: piperine, anti-inflammatory, reactive species, t-BHP, Ac2F cells
Inflammation progresses by complex interactions between mediators and inflammatory cells that activate the immune system to remove stimulant, inhibit infection and accelerate healing of tissue damage [1]. Chronic inflammation is associated with the pathogenesis of many diseases, including arthritis, cancer, stroke, and cardiovascular diseases [2]. Additionally, cumulative evidence shows that reactive species (RS) generated from oxidative stress are considered to be important components of inflammation [3, 4].
Nuclear factor-κB (NF-κB) plays a pivotal role in the early stages of the immune and inflammatory responses by regulating expression of inflammatory mediators, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2). In unstimulated cells, NF-κB dimers are bound to inhibitor of κB (IκB), which maintains NF-κB in the cytoplasm, thus preventing its translocation to the nucleus and its transcriptional activity. However, t-BHP (tert-butyl hydroperoxide)-induced activation of NF-κB involves phosphorylation of IκBα kinase (IKK), which phosphorylates IκBα protein, leading to ubiquitination and degradation of IκBα and translocation of NF-κB into the nucleus [5, 6]. Activation of NF-κB is also regulated by cellular kinases such as mitogen-activated protein kinases (MAPKs) [7]. MAPKs, extracellular signal regulated kinase 1/2 (ERK1/2), c-Jun NH2-terminal kinase (JNK), and p38 MAPK are involved in the transcriptional regulation of pro-inflammatory genes, including iNOS and COX-2, via NF-κB activation [8]. Understanding the underlying molecular mechanisms involved in these pathways is an important step in response to prevent deleterious effects of pro-inflammatory mediators. In contrast, antioxidant mediators play an important role in the cellular defense system against oxidative stress from pro-inflammatory factors such as t-BHP [9]. Manganese-dependent superoxide dismutase (MnSOD) is one of the most important antioxidant enzymes essential for reducing mitochondrial oxidative stress [10], on the other hand, t-BHP inhibit the most sensitive antioxidant enzyme in response to inflammation [11].
Piperine, one of the active components of black pepper (Piper nigrum) and long pepper (Pipper longum) (Figure 1), is commonly used as a spice in human diets, and it is also used as an effective remedy for gonorrhea, menstrual pain, tuberculosis, sleeping problems, respiratory tract infections, chronic gut-related pain and arthritic conditions in several Asian countries and Pacific islands [12]. Piperine has been shown to possess several biological activities, including antioxidant [13, 14], anti-diabetic [15], anti-photoprotective [16], anti-inflammatory [17-24] anti-thyroid [19], anti-platelet aggregation [25], anti-obesity [26], immunomodulatory [27, 28], and anti-tumor [29, 30]. In spite of many previous studies, the mechanism by which piperine inhibits t-BHP-induced inflammation and oxidative stress has not been studied so far. Therefore, the objective of this study was to investigate the protective properties of piperine against t-BHP-induced inflammation, and to determine the underlying molecular mechanisms of anti-inflammatory action and anti-oxidative stress.
Figure 1. Chemical structure of piperine
2.1. Materials
Piperine (≥ 97%), t-BHP, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox) and 3-morpholinosydnonimine hydrochloride (SIN-1) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 2′,7′-Dichlorofluorescein diacetate (DCFH–DA) was obtained from Molecular Probes (Eugene, OR, USA). Polyvinylidene fluoride (PVDF) membrane was obtained from Millipore Corp. (Billelica, MA, Germany) and the enhanced chemiluminescence (ECL) detection system was obtained from Amersham Life Sciences, Inc. (Buckinghamshire, UK). Antibodies targeted toward p65, p-p65, p-IκBα, IκBα, p-p38, p-ERK1/2, p-JNK, catalase, MnSOD, COX-2, iNOS, TFIIB, and b-actin were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All other chemicals were of the highest purity available from either Sigma Chemical Co. (St. Louis, MO) or Junsei Chemical Co. (Tokyo, Japan).
2.2. Cell culture and treatment with piperine
Donryu rat hepatocytes (Ac2F cells) were obtained from ATCC (American Type Culture Collection, Manassas, VA, USA). The cells were cultured in Dulbecco’s Modified Eagle Media (DMEM, Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Hyclone), 100 mg/mL penicillin-streptomycin, and 0.25 mg/mL amphotericin B in an atmosphere of 5% CO2. Piperine was dissolved in 100% DMSO and added directly to culture media before the addition of t-BHP. The final concentration of DMSO did not exceed 0.1%. For all experiments, cells were plated in 100 mm culture dishes and cultures at 70–80% confluence were used for chemical exposure. After a 24 h attachment period, media were replaced with serum free media and cells were preincubated for 2 h with piperine followed by treatment with t-BHP. Working solutions of t-BHP were made in PBS immediately before use.
2.3. Cell viability assay
Cell viability was determined using an EZ-Cytox assay kit. briefly, Ac2F cells were seeded in 96-wells at (1 ´ 104 cells/well) and allowed to attach at 37°C for 24 h. Media were then replaced with fresh DMEM containing piperine (up to 10 mM) and incubated for 24 h. After incubation, 10 mL of EZ-Cytox solution were added to each well, and cells were incubated for an additional 2–4 h. The absorbance of each well was measured at 450 nm using ELISA reader (Promega, Madison, WI, USA). Cell viabilities were calculated as percentages of the viabilities of untreated controls. All determinations were performed in triplicate then averaged.
2.4. Measurement of intracellular RS accumulation
Intracellular oxidants were evaluated using the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH–DA). This molecule is cleaved intracellularly by nonspecific esterase to 2′,7′-dichlorofluorescin (DCFH), which then forms the fluorescent compound 2′,7′-dichlorofluorescein (DCF) upon oxidation by RS [31]. To determine the extent of intracellular RS scavenging activity, Ac2F cells (2 × 104 cells/well) were seeded in 96-well black bottom-clear plates. After 24 h, the cells were treated with piperine (1-10 mM) for 1 h and then exposed to t-BHP (100 mM) or SIN-1 (10 mM) for 30 min to induce RS production, Cells were subsequently incubated with DCFH-DA (40 mM) for 30 min. The resultant fluorescence intensities were measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm with a fluorescence microplate reader (TECAN, Salzburg, Austria).
2.5. Preparation of cytosolic and nuclear fractions
Nuclear and cytosolic extracts were prepared according to Deng et al. (2001) [32]. Ac2F cells were plated in 60 mm dishes (2 ´ 105 cells/mL), treated with piperine, stimulated with t-BHP (100 mM) for 5 h, washed once with PBS, scraped into 1 mL of cold PBS, and centrifuged at 8,000 ´ g at 4°C for 5 min. The pellets were suspended in 10 mM Tris (pH 8.0) with 1.5 mM MgCl2, 1 mM DTT, 0.1% NP-40, and protease and phosphatase inhibitors and incubated on ice for 15 min. Nuclei were separated from cytosol by centrifugation at 10,000 ´ g at 4°C for 15 min. The cytosolic supernatants were removed and the precipitated pellets were suspended in 10 mM Tris (pH 8.0), with 50 mM KCl, 100 mM NaCl, and protease and phosphatase inhibitors and incubated on ice for 1 h. They were then they were centrifuged at 12,000 rpm at 4°C for another 30 min.
2.6. Measurement of proteins by western blotting
Western blotting was performed as described previously [11]. The cells were harvested, washed twice with ice-cold PBS and lysed for 30 min on ice, vortexing every 5 min. Lysates were centrifuged at 12,000 ´ g for 30 min to remove insoluble material. Equal amounts of protein were separated on SDS-PAGE gels. The separated proteins were subsequently transferred onto PVDF by electro blotting. The membranes were blocked in a 5% non-fat milk solution in TBS with 0.5% Tween-20 and incubated with primary antibodies overnight at 4°C as indicated. Membranes were washed and incubated for 2 h at room temperature with HRP-linked secondary antibodies. Pre-stained blue protein markers (Bio-Rad, Hercules, CA) were used for molecular-weight determination.
2.7. Statistical analysis
All experiments reported in this study were performed independently at least three times and data (expressed as mean ± S.E.M.) from a representative experiment are shown. Statistical significance was assessed by the one-way analysis of variance (ANOVA) for differences within treatments followed by the Bonferroni posttest. * p < 0.05 was considered significant.
3.1. Protective effects of piperine against t-BHP-induced cytotoxicity in Ac2F cells
To investigate the protective effects against t-BHP of piperine, Ac2F cells were treated with various concentrations of t-BHP (0–300 mM). As shown in Figure 2a, t-BHP caused a dose-dependent decrease in Ac2F cell viability. Particularly at 100 mM, cell viability was significantly reduced up to 60.2% compared to untreated cells. Therefore, this concentration of t-BHP was selected to induce cell death in subsequent experiments. Before determining whether piperine has anti-inflammatory activity, the cytotoxicity of piperine in Ac2F cells was determined by EZ-Cytox assay. Ac2F cells were incubated for 24 h and then pretreated with piperine (up to 10 mM). As shown in Figure 2b, piperine did not induce cytotoxic effects in Ac2F cells up to 10 mM. To test the extent of the protective action of piperine against t-BHP-induced cytotoxicity, Ac2F cells were incubated with different concentrations (2, 5 or 10 mM) of piperine for 24 h t-BHP significantly reduced cell viability, whereas pretreatment with piperine dose-dependently inhibited cell death by t-BHP (Figure 2c). These concentrations were therefore used in subsequent piperine experiments.
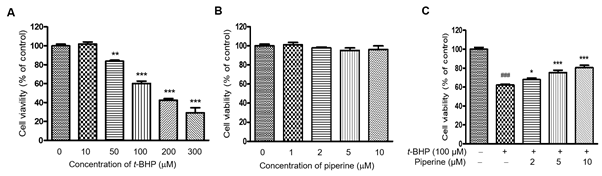
Figure 2. Effect of piperine on cell viability and t-BHP-induced cytotoxicity in Ac2F cells. Cells (1´105 cells/well) were incubated with different concentrations of t-BHP for 5 h (A). Cells were preincubated using various concentrations (up to 10 mM) of piperine for 24 h (B) and then incubated with 100 mM t-BHP for another 5 h (C). Cell viability was determined using the EZ-Cytox assay and expressed as the percentage of absorbance values relative to the control group. Data shown represent mean ± S.E.M. of triplicate experiments. One-factor ANOVA: ###p < 0.001 versus vehicle treated controls; * p < 0.05, ** p < 0.01, and *** p < 0.001 versus 100 mM t-BHP-induced cells.
3.2. Inhibitory effect of piperine against oxidative stress induced RS production
t-BHP stimulates oxidative stress in cells to produce ROS [33, 34]. To determine whether piperine has a protective effect on t-BHP-induced Ac2F cells, cells were pretreated with non-toxic doses for 24 h. As shown in Figure 3a, the level of increased intracellular ROS as a result of t-BHP treatment was significantly reduced by treatment with piperine in a dose-dependent manner. SIN-1, a metabolite of the vasodilator molsidomine, is used as RNS inducer [8]. The effect of piperine on the production of RS in SIN-1-induced Ac2F cells is shown in Figure 3b. Cells treated with SIN-1 increased fluorescence intensity compared to unstimulated cells. Pretreatment with different concentrations of piperine significantly inhibited RS production in a dose-dependent manner in SIN-1-induced Ac2F cells. Thus, piperine strongly scavenges RS production in t-BHP and SIN-1-induced Ac2F cells, indicating that piperine possessed anti-oxidative potential by suppressing RS production.
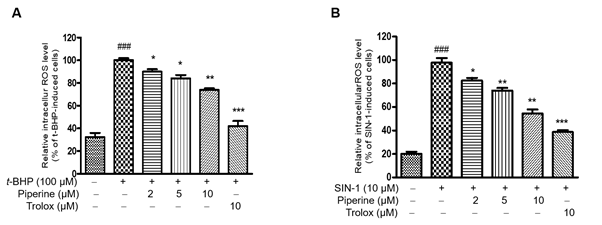
Figure 3. Effect of piperine against oxidative stress. Level of intracellular ROS in t-BHP-induced Ac2F cells. Cells were pretreated with the indicated concentrations (2, 5, or 10 mM) of piperine for 2 h and further treated with t-BHP (100 mM) for 30 min (A). Level of intracellular RNS in SIN-1-induced Ac2F cells. Cells were pretreated with the indicated concentrations (2, 5, or 10 mM) of piperine for 2 h and further treated with SIN-1 (10 mM) for 30 min (B). RS production was evaluated using a DCFH-DA (40 mM) assay to detect RS. Data are represented as mean ± S.E.M. of triplicate experiments. One-factor ANOVA: ###p < 0.001 versus vehicle treated controls; *p < 0.05, ** p < 0.01 and ***p < 0.001 versus 100 μM t-BHP-treated cells.
3.3. Modulatory effects of piperine against t-BHP-induced NF-κB transcriptional activation via inhibition of IκB-α degradation
NF-κB is one of the major transcription factors that expresses and regulates the expression of iNOS, COX-2, and inflammatory mediators [35]. We next investigated the effects of piperine on the translocation of NF-κB by Western blot. Our results showed that t-BHP induced nuclear phosphorylation of NF-κB p65, and pretreatment with piperine significantly down-regulated phosphorylated of p65 (Figure 4a). We also confirmed that the phosphorylation of IκBα was suppressed by pretreatment with 2 or 10 mM piperine in a dose-dependent manner (Figure 4b). Correspondingly, total of IκBα was reduced by t-BHP and restored by piperine. Although the concentration of p65 was decreased in the cytoplasm and increased in nucleus after t-BHP-induction, pretreatment with piperine reversed these trends in a dose-dependent manner. Thus, piperine potently modulates t-BHP-induced NF-κB activation in Ac2F cells.
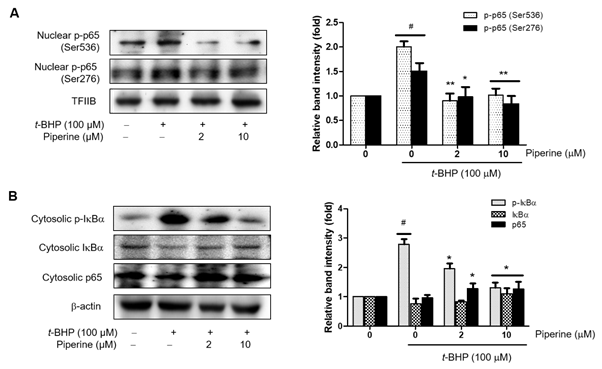
Figure 4. Effects of piperine on t-BHP-induced NF-κB activation in Ac2F cells. Cells were grown to 80% confluence in DMEM and changed to serum-free media. Pre-treatment with piperine (2 or 10 μM) for 2 h and treatment with of 100 μM t-BHP for 5 h. (A) Western blot was performed to detect p-p65 protein levels in the nuclear fraction. Levels were normalized to transcription factor IIB (TFIIB). (B) Western blot was performed to detect p-IκBα, IκBα, and p-65 protein in the cytosol fraction. Levels were normalized to β-actin. Values are the relative optical intensity of each band normalized as a percentage of the untreated control. One-factor ANOVA: # p < 0.05 versus vehicle treated controls; * p < 0.05 and ** p < 0.01 versus 100 μM t-BHP-treated cells.
3.4. Modulatory effects of piperine against t-BHP-induced phosphorylation of ERK1/2, JNK, and p38 MAPKs
MAPKs, including ERK1/2, p38, and JNK regulates the expression of iNOS, COX-2, and proinflammatory enzymes. Phosphorylation of MAPKs is known to be modulate by t-BHP-induced oxidative stress [36]. Thus, we investigated the effects of piperine on the activation of intracellular signaling kinases, including the family of MAPKs, in t-BHP-induced Ac2F cells. As shown in Figure 5, t-BHP-induced increased MAPK phosphorylation however, pre-treatment with piperine (2 or 10 µM) down-stream t-BHP-induced phosphorylation of ERK1/2, JNK and p38 in dose-dependent manner. These results indicate that piperine attenuates t-BHP activation in the MAPK signaling pathway including ERK, JNK, and p38.
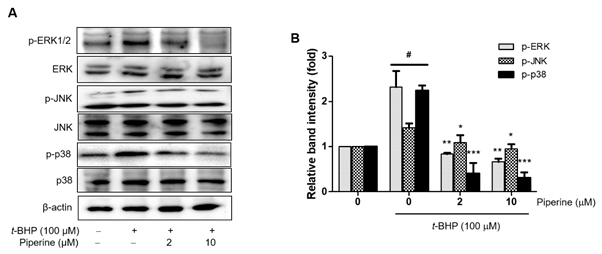
Figure 5. Effects of piperine on t-BHP-induced phosphorylation of MAPKs in Ac2F cells. Cells were grown to 80% confluence in DMEM and changed to serum-free media. Pre-treatment with piperine (2 or 10 µM) for 2 h and treatment with 100 µM t-BHP for 5 h. Western blot was performed to detect p-ERK1/2, p-JNK, p-p38, and the total form of each phosphor-form levels in whole cell lysate. Levels were normalized to β-actin. Values are the relative optical intensity of each band normalized as a percentage of the untreated control. One-factor ANOVA: # p < 0.05 versus vehicle-treated controls; * p < 0.05, ** p < 0.01, and *** p < 0.001 versus 100 µM t-BHP-treated cells.
3.5. Modulation of pro-inflammatory genes by piperine
In order to determine whether the expression of t-BHP-induced NF-kB-dependent pro-inflammatory genes was suppressed by piperine, we analyzed expression of COX-2 and iNOS by Western blot. As shown in Figure 6, exposure of Ac2F cells to t-BHP induced significant induction of iNOS and COX-2 proteins, while piperine down-regulated t-BHP-induced iNOS and COX-2 expression in a dose-dependent manner. These results indicate that piperine modulates iNOS and COX-2 expression in t-BHP-induced Ac2F cells by suppressing the NF-kB signaling pathway.
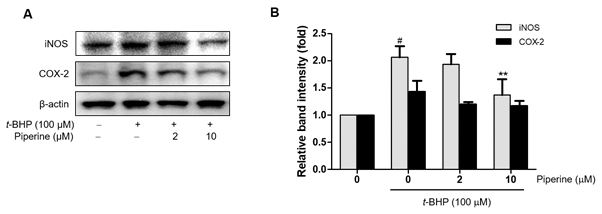
Figure 6. Effects of piperine on t-BHP-induced NF-κB-dependent pro-inflammatory genes expression in Ac2F cells. Cells were grown to 80% confluence in DMEM and changed to serum-free media. Pre-treatment with piperine (2 or 10 µM) for 2 h and treatment with 100 µM t-BHP for 5 h. Western blot was performed to detect iNOS and COX-2 protein levels in whole cell lysate. Levels were normalized to β-actin. Values are the relative optical intensity of each band normalized as a percentage of the untreated control. One-factor ANOVA: #p < 0.05 versus vehicle-treated controls; *p < 0.05 and ** p < 0.01 versus 100 µM t-BHP-treated cells.
3.6. Modulation of antioxidant enzyme expression by piperine
Antioxidant mediators also play an important role in regulating the inflammatory reaction [37]. MnSOD and catalase are two major antioxidant enzymes that protect against oxidative stress by metabolizing RS [11]. Therefore, in order to investigate whether piperine has the ability to upregulate the expression of antioxidant enzymes such as catalase and MnSOD, Ac2F cells were pretreated with piperine for 2 h and subsequently co-incubated with t-BHP for an additional 5 h. As shown in Figure 7, the expression of MnSOD and catalase was decreased by oxidative stress; conversely, piperine increased expression of both catalase and MnSOD. These results suggest that piperine exhibits antioxidant activity through increasing of the protein expression levels of antioxidant enzymes in hepatocytes.
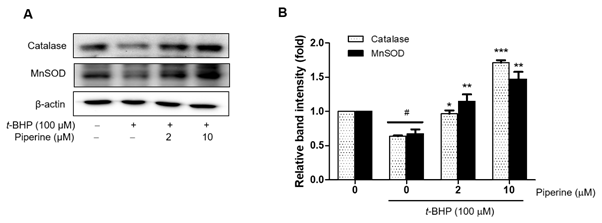
Figure 7. Effects of piperine on t-BHP-induced antioxidant enzyme gene expression in Ac2F cells. Cells were grown to 80% confluence in DMEM and changed to serum-free media. Pre-treatment with piperine (2 or 10 µM) for 2 h and treatment with 100 µM t-BHP for 5 h. Western blot was performed to detect catalase and MnSOD protein levels in whole cell lysate. Levels were normalized to β-actin. Values are the relative optical intensity of each band normalized as a percentage of the untreated control. One-factor ANOVA: # p < 0.05 versus vehicle-treated controls; * p < 0.05, ** p < 0.01, and *** p < 0.001 versus 100 µM t-BHP-treated cells.
In this study, we investigated antioxidant and anti-inflammatory potential of piperine, one of the active constituents of black pepper [12, 38], in t-BHP-induced inflammation. To further comprehend the molecular mechanisms of piperine-mediated anti-inflammation and antioxidant effects, we demonstrated the effects of piperine on NF-κB and MAPK signaling via RS radical scavenging in Ac2F cells. Our results indicate that piperine effectively inhibits the pro-inflammatory genes, NF-κB and MAPKs, as well as up-regulates radical scavenging activity by increasing antioxidant enzymes including, catalase and MnSOD. These results indicate that piperine can be further studied to develop therapeutics for the prevention of inflammatory disease due to significant prevention of oxidative stress.
Piper species have been shown to be effective as anti-inflammatory [39, 40]. In particular, anti-inflammatory activity of piperine has been reported in rats, with several experimental models, such as carrageenan-induced rat paw edema, cotton pellet granuloma and cotton-oil-induced granuloma pouch [17], but the mechanism of action remains unknown in t-BHP-induced rat liver cells through the prevention of oxidative stress. Though several of beneficial effects of piperine have been reported, to the best of our knowledge, this is the first report establishing that piperine exerts an anti-inflammatory and antioxidant effects.
Oxidative stress is characterized by overwhelming cellular antioxidant defenses in the increased production of RS [41]. Increased RS levels result in the oxidation of many biomolecules including lipids, carbohydrates, proteins and DNA [42]. These evidences support the involvement of oxidative stress in the initiation and progression of various inflammatory diseases. According to our study, piperine significantly reduces RS generation an oxidative stress-induced condition. Therefore, inhibition of RS generated oxidative stress by piperine is likely attributed to down regulation of pro-inflammatory responses and induction of antioxidant enzymes, including MnSOD and catalase. To confirm the mechanisms by which piperine inhibits NF-kB activity, we tested the effect of piperine on NF-kB signaling. Inactive NF-kB is retained in the cytoplasm with IkBa, and t-BHP activates NF-kB via triggering IkBa degradation. Once activated, NF-kB subunit p65 dissociates from its inhibitory protein IkBa and may trigger the transcription of specific target genes such as iNOS and COX-2 [43]. In the present study, we demonstrated that piperine significantly inhibits t-BHP-induced phosphorylation of IkBa, IkBa degradation and the subsequent reduction of nuclear p65 in a dose-dependent manner. These results suggest that piperine inhibits NF-kB activation by inhibiting IkBa phosphorylation and the translocation of the p65 subunit of NF-kB from the cytosol to the nucleus in t-BHP-induced Ac2F cells.
MAPKs, including ERK1/2, p38, and JNK, are activated by extracellular stimuli and control a variety cellular responses, such as inflammatory cytokines, mitosis, differentiation and cell survival/apoptosis. In addition, the MAPK pathway is associated with NF-κB activation, where inhibition of MAPKs inhibits NF-κB expression [44]. We found that piperine suppresses phosphorylation of ERK1/2, JNK and p38 in t-BHP-induced Ac2F cells. Within the MAPKs pathway, phosphorylation of ERK1/2 was the most significantly decreased signal by concentration-dependent manner. Based on our findings, piperine suppresses MAPKs activation, resulting in NF-κB inactivation in t-BHP-induced Ac2F cells. In our study, the anti-inflammatory properties of piperine were mediated by the down-regulation of NF-κB activation in Ac2F cells. Thus, modulation of NF-κB activation is an effective approach to treat inflammation-related diseases. Our results indicate that piperine inhibits t-BHP-induced phosphorylation of NF-κB and IκBα in a dose-dependent manner, and these findings suggest that piperine negatively regulates protein expression of iNOS and COX-2 through inactivation of NF-κB in t-BHP-induced Ac2F cells. Nevertheless, the precise mechanism involved in the regulation of inflammation by piperine in Ac2F cells is still unclear.
MnSOD and catalase are major antioxidant enzymes that play an important role in the antioxidant protection mechanisms to protect the cells from radical-mediated damage. Downregulation of antioxidant enzyme has been reported in relation to chemical/oxidative stress, where the antioxidant system stabilizes the generated free radicals [45, 46]. Accordingly, in the present investigation we observed a decrease in antioxidant levels in t-BHP-induced Ac2F cells that was recovered with piperine pretreatment. These results demonstrate that the antioxidant activity of piperine is due to induction of the antioxidant enzymes, including MnSOD and catalase. In summary, piperine has been shown to reduce RS through induction of antioxidant enzymes including catalase and MnSOD, and expression of inflammatory-promoting enzymes, such as iNOS and COX-2, as well as inhibition of MAPKs, IκBα phosphorylation and p65 nuclear translocation in t-BHP-induced Ac2F cells (Figure 8).
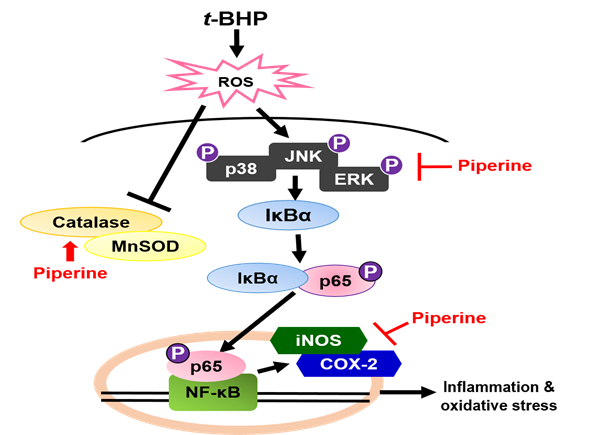
Figure 8. Possible mechanism of piperine on anti-inflammation and antioxidant. COX-2, cyclooxygenase-2; ERK, extracellular-signal-regulated kinase; iNOS, inducible nitric oxide synthase; IkBα, inhibitor κ B-alpha; JNK, c-Jun N-terminal kinase; MnSOD, manganese-dependent superoxide dismutase.
The results of our study show that piperine protects t-BHP-induced Ac2F cells against oxidative damage, which is due to the regulation of RS production via inactivation of the NF-kB and MAPK signaling pathways. With our findings, we expect that piperine possesses great potential as a therapeutic agent for prevention of inflammatory diseases caused by oxidative stress.
This work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ006522132013)" Rural Development Administration, Republic of Korea. This study was supported by the Korean National Research Foundation (NRF) funded by the Korean government (No. 2015M3A9B8029074).
Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, et al. Inflammatory mechanisms in the lung. Journal of inflammation research. 2009;2:1-11. Epub 2009/01/01.
Garrido G, Gonzalez D, Delporte C, Backhouse N, Quintero G, Nunez-Selles AJ, et al. Analgesic and anti-inflammatory effects of Mangifera indica L. extract (Vimang). Phytotherapy research : PTR. 2001;15(1):18-21. Epub 2001/02/17. 15:1<18::AID-PTR676>3.0.CO;2-R
View ArticleConforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, et al. The protective ability of Mediterranean dietary plants against the oxidative damage: The role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chemistry. 2009;112(3):587-94.
View ArticleMelagraki G, Afantitis A, Igglessi-Markopoulou O, Detsi A, Koufaki M, Kontogiorgis C, et al. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. European journal of medicinal chemistry. 2009;44(7):3020-6. Epub 2009/02/24. doi: 10.1016/j.ejmech.2008.12.027. PMid:19232783
View Article PubMed/NCBIMercurio F, Murray BW, Shevchenko A, Bennett BL, Young DB, Li JW, et al. IkappaB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Molecular and cellular biology. 1999;19(2):1526-38. Epub 1999/01/16. doi: 10.1128/mcb.19.2.1526. PMid:9891086
View Article PubMed/NCBIZandi E, Karin M. Bridging the gap: composition, regulation, and physiological function of the IkappaB kinase complex. Molecular and cellular biology. 1999;19(7):4547-51. Epub 1999/06/22. doi: 10.1128/mcb.19.7.4547. PMid:10373503
View Article PubMed/NCBIVanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, et al. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. The Journal of biological chemistry. 1998;273(6):3285-90. Epub 1998/03/07. doi: 10.1074/jbc.273.6.3285. PMid:9452444
View Article PubMed/NCBIKim JM, Lee EK, Park G, Kim MK, Yokozawa T, Yu BP, et al. Morin modulates the oxidative stress-induced NF-kappaB pathway through its anti-oxidant activity. Free radical research. 2010;44(4):454-61. Epub 2010/03/02. doi: 10.3109/10715761003610737. PMid:20187708
View Article PubMed/NCBIYu JY, Ha JY, Kim KM, Jung YS, Jung JC, Oh S. Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules (Basel, Switzerland). 2015;20(7):13041-54. Epub 2015/07/25. doi: 10.3390/molecules200713041. PMid:26205049
View Article PubMed/NCBIBecuwe P, Ennen M, Klotz R, Barbieux C, Grandemange S. Manganese superoxide dismutase in breast cancer: from molecular mechanisms of gene regulation to biological and clinical significance. Free radical biology & medicine. 2014;77:139-51. Epub 2014/09/17. doi: 10.1016/j.freeradbiomed.2014.08.026. PMid:25224035
View Article PubMed/NCBIKim DH, Park CH, Park D, Choi YJ, Park MH, Chung KW, et al. Ginsenoside Rc modulates Akt/FoxO1 pathways and suppresses oxidative stress. Archives of pharmacal research. 2014;37(6):813-20. Epub 2013/08/07. doi: 10.1007/s12272-013-0223-2. PMid:23918648
View Article PubMed/NCBISrinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Critical reviews in food science and nutrition. 2007;47(8):735-48. Epub 2007/11/08. doi: 10.1080/10408390601062054. PMid:17987447
View Article PubMed/NCBISethiya NK, Shah P, Rajpara A, Nagar PA, Mishra SH. Antioxidant and hepatoprotective effects of mixed micellar lipid formulation of phyllanthin and piperine in carbon tetrachloride-induced liver injury in rodents. Food & function. 2015;6(11):3593-603. Epub 2015/09/04. doi: 10.1039/c5fo00947b. PMid:26333006
View Article PubMed/NCBIMittal R, Gupta RL. In vitro antioxidant activity of piperine. Methods and findings in experimental and clinical pharmacology. 2000;22(5):271-4. Epub 2000/10/14. PMid:11031726
View Article PubMed/NCBIArcaro CA, Gutierres VO, Assis RP, Moreira TF, Costa PI, Baviera AM, et al. Piperine, a natural bioenhancer, nullifies the antidiabetic and antioxidant activities of curcumin in streptozotocin-diabetic rats. PloS one. 2014;9(12):e113993. Epub 2014/12/04. doi: 10.1371/journal.pone.0113993. PMid:25469699 PMCid:PMC4254914
View Article PubMed/NCBIVerma A, Kushwaha HN, Srivastava AK, Srivastava S, Jamal N, Srivastava K, et al. Piperine attenuates UV-R induced cell damage in human keratinocytes via NF-kB, Bax/Bcl-2 pathway: An application for photoprotection. Journal of photochemistry and photobiology B, Biology. 2017;172:139-48. Epub 2017/05/28. doi: 10.1016/j.jphotobiol.2017.05.018. PMid:28550736
View Article PubMed/NCBIMujumdar AM, Dhuley JN, Deshmukh VK, Raman PH, Naik SR. Anti-inflammatory activity of piperine. Japanese journal of medical science & biology. 1990;43(3):95-100. Epub 1990/06/01. PMid:2283727
View Article PubMed/NCBIPradeep CR, Kuttan G. Piperine is a potent inhibitor of nuclear factor-kappaB (NF-kappaB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. International immunopharmacology. 2004;4(14):1795-803. Epub 2004/11/09. doi: 10.1016/j.intimp.2004.08.005. PMid:15531295
View Article PubMed/NCBIBang JS, Oh DH, Choi HM, Sur BJ, Lim SJ, Kim JY, et al. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis research & therapy. 2009;11(2):R49. Epub 2009/03/31. doi: 10.1186/ar2662. PMid:19327174
View Article PubMed/NCBIVaibhav K, Shrivastava P, Javed H, Khan A, Ahmed ME, Tabassum R, et al. Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-kappaB in middle cerebral artery occlusion rat model. Molecular and cellular biochemistry. 2012;367(1-2):73-84. Epub 2012/06/07. doi: 10.1007/s11010-012-1321-z. PMid:22669728
View Article PubMed/NCBIKim HG, Han EH, Jang WS, Choi JH, Khanal T, Park BH, et al. Piperine inhibits PMA-induced cyclooxygenase-2 expression through downregulating NF-kappaB, C/EBP and AP-1 signaling pathways in murine macrophages. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2012;50(7):2342-8. Epub 2012/05/01. doi: 10.1016/j.fct.2012.04.024. PMid:22542552
View Article PubMed/NCBIYing X, Chen X, Cheng S, Shen Y, Peng L, Xu HZ. Piperine inhibits IL-beta induced expression of inflammatory mediators in human osteoarthritis chondrocyte. International immunopharmacology. 2013;17(2):293-9. Epub 2013/07/11. doi: 10.1016/j.intimp.2013.06.025. PMid:23838114
View Article PubMed/NCBIZhai WJ, Zhang ZB, Xu NN, Guo YF, Qiu C, Li CY, et al. Piperine Plays an Anti-Inflammatory Role in Staphylococcus aureus Endometritis by Inhibiting Activation of NF-kappaB and MAPK Pathways in Mice. Evidence-based complementary and alternative medicine : eCAM. 2016;2016:8597208. Epub 2016/06/14. doi: 10.1155/2016/8597208. PMid:27293467
View Article PubMed/NCBILu Y, Liu J, Li H, Gu L. Piperine Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via Modulating NF-kappaB Signaling Pathways. Inflammation. 2016;39(1):303-8. Epub 2015/09/28. doi: 10.1007/s10753-015-0250-x. PMid:26410851
View Article PubMed/NCBIIwashita M, Saito M, Yamaguchi Y, Takagaki R, Nakahata N. Inhibitory effect of ethanol extract of Piper longum L. on rabbit platelet aggregation through antagonizing thromboxane A2 receptor. Biological & pharmaceutical bulletin. 2007;30(7):1221-5. Epub 2007/07/03. PMid:17603157
View Article PubMed/NCBIPark UH, Jeong HS, Jo EY, Park T, Yoon SK, Kim EJ, et al. Piperine, a component of black pepper, inhibits adipogenesis by antagonizing PPARgamma activity in 3T3-L1 cells. Journal of agricultural and food chemistry. 2012;60(15):3853-60. Epub 2012/04/03. doi: 10.1021/jf204514a. PMid:22463744
View Article PubMed/NCBIBae GS, Kim JJ, Park KC, Koo BS, Jo IJ, Choi SB, et al. Piperine inhibits lipopolysaccharide-induced maturation of bone-marrow-derived dendritic cells through inhibition of ERK and JNK activation. Phytotherapy research : PTR. 2012;26(12):1893-7. Epub 2012/03/21. doi: 10.1002/ptr.4649. PMid:22430952
View Article PubMed/NCBIChuchawankul S, Khorana N, Poovorawan Y. Piperine inhibits cytokine production by human peripheral blood mononuclear cells. Genetics and molecular research : GMR. 2012;11(1):617-27. Epub 2012/04/27. doi: 10.4238/2012.March.14.5. PMid:22535397
View Article PubMed/NCBISunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. Journal of ethnopharmacology. 2004;90(2-3):339-46. Epub 2004/03/12. doi: 10.1016/j.jep.2003.10.016. PMid:15013199
View Article PubMed/NCBIGunasekaran V, Elangovan K, Niranjali Devaraj S. Targeting hepatocellular carcinoma with piperine by radical-mediated mitochondrial pathway of apoptosis: An in vitro and in vivo study. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2017;105:106-18. Epub 2017/03/28. doi: 10.1016/j.fct.2017.03.029. PMid:28341137
View Article PubMed/NCBILebel CP, Bondy SC. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochemistry international. 1990;17(3):435-40. Epub 1990/01/01. 90025-O
View ArticleDeng L, Lin-Lee YC, Claret FX, Kuo MT. 2-acetylaminofluorene up-regulates rat mdr1b expression through generating reactive oxygen species that activate NF-kappa B pathway. The Journal of biological chemistry. 2001;276(1):413-20. Epub 2000/10/06. doi: 10.1074/jbc.M004551200. PMid:11020383
View Article PubMed/NCBIHwang JM, Tseng TH, Hsieh YS, Chou FP, Wang CJ, Chu CY. Inhibitory effect of atractylon on tert-butyl hydroperoxide induced DNA damage and hepatic toxicity in rat hepatocytes. Archives of toxicology. 1996;70(10):640-4. Epub 1996/01/01. PMid:8870957
View Article PubMed/NCBIOsseni RA, Debbasch C, Christen MO, Rat P, Warnet JM. Tacrine-induced Reactive Oxygen Species in a Human Liver Cell Line: The Role of Anethole Dithiolethione as a Scavenger. Toxicology in vitro : an international journal published in association with BIBRA. 1999;13(4-5):683-8. Epub 1999/08/01. 00050-8
View ArticleFan GW, Zhang Y, Jiang X, Zhu Y, Wang B, Su L, et al. Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-kappaB-dependent pathways. Inflammation. 2013;36(6):1584-91. Epub 2013/07/31. doi: 10.1007/s10753-013-9703-2. PMid:23892998
View Article PubMed/NCBIWhiteman M, Spencer JP, Zhu YZ, Armstrong JS, Schantz JT. Peroxynitrite-modified collagen-II induces p38/ERK and NF-kappaB-dependent synthesis of prostaglandin E2 and nitric oxide in chondrogenically differentiated mesenchymal progenitor cells. Osteoarthritis and cartilage. 2006;14(5):460-70. Epub 2006/01/24. doi: 10.1016/j.joca.2005.11.002. PMid:16427328
View Article PubMed/NCBIMo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxidants & redox signaling. 2014;20(4):574-88. Epub 2013/07/24. doi: 10.1089/ars.2012.5116. PMid:23875776
View Article PubMed/NCBIEe GC, Lim CM, Lim CK, Rahmani M, Shaari K, Bong CF. Alkaloids from Piper sarmentosum and Piper nigrum. Natural product research. 2009;23(15):1416-23. Epub 2009/10/08. doi: 10.1080/14786410902757998. PMid:19809914
View Article PubMed/NCBIGupta SK, Bansal P, Bhardwaj RK, Velpandian T. Comparative anti-nociceptive, anti-inflammatory and toxicity profile of nimesulide vs nimesulide and piperine combination. Pharmacological research. 2000;41(6):657-62. Epub 2000/05/19. doi: 10.1006/phrs.1999.0640. PMid:10816335
View Article PubMed/NCBINgo QM, Tran PT, Tran MH, Kim JA, Rho SS, Lim CH, et al. Alkaloids from Piper nigrum Exhibit Antiinflammatory Activity via Activating the Nrf2/HO-1 Pathway. Phytotherapy research : PTR. 2017;31(4):663-70. Epub 2017/02/12. doi: 10.1002/ptr.5780. PMid:28185326
View Article PubMed/NCBIBetteridge DJ. What is oxidative stress? Metabolism: clinical and experimental. 2000;49(2 Suppl 1):3-8. Epub 2000/02/29. 80077-3
View ArticleHalliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401(1):1-11. Epub 2006/12/08. doi: 10.1042/bj20061131. PMid:17150040
View Article PubMed/NCBIYuan F, Chen J, Sun PP, Guan S, Xu J. Wedelolactone inhibits LPS-induced pro-inflammation via NF-kappaB pathway in RAW 264.7 cells. Journal of biomedical science. 2013;20:84. Epub 2013/11/02. doi: 10.1186/1423-0127-20-84. PMid:24176090
View Article PubMed/NCBIWaterfield MR, Zhang M, Norman LP, Sun SC. NF-kappaB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Molecular cell. 2003;11(3):685-94. Epub 2003/04/02. 00070-4
View ArticleTiwari M, Kakkar P. Plant derived antioxidants - Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicology in vitro : an international journal published in association with BIBRA. 2009;23(2):295-301. Epub 2009/01/13. doi: 10.1016/j.tiv.2008.12.014. PMid:19135518
View Article PubMed/NCBIKandikattu HK, M.P V, Pal A, Khanum F. Phytochemical analysis and exercise enhancing effects of hydroalcoholic extract of Celastrus paniculatus Willd2014. 217-24 p.
View Article