Njoya Moyouwou Amadou
Email: njoya_amadou@yahoo.fr / njoyaamadou5@gmail.com ;
Telephone: 00(237) 677860978 / 656481791
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 5 ISSUE: 3
Page No: 136-145
Njoya Moyouwou Amadou
Email: njoya_amadou@yahoo.fr / njoyaamadou5@gmail.com ;
Telephone: 00(237) 677860978 / 656481791
NJOYA MOYOUWOU Amadou *1, SONCHIEU Jean2, NAIN Caroline WAINGEH1, SEMI Aphonsius YAM3
1Food Technology and Post-Harvest Laboratory, IRAD-Bambui; P.O. Box 51 Bamenda, Cameroon
2Department of Social Economy and Family Management, Higher Technical Teacher Training College, University of Bamenda, PO. Box 39 Bamenda, Cameroon
3Nutrition Laboratory, IRAD-Mankon; P.O. Box 125 Bamenda
Njoya et al., Assessment of physicochemical, microbial and sensory properties of whey wine coloured with beet roots and carrots (2020) Journal of Food Science & Technology 5(3) pp:136-145
The physicochemical, microbial and sensory properties of beet roots and carrots coloured whey wine were investigated in the present study. Four whey wine samples A, B, C and D were prepared by addition of 0%; 10% (V/V) of beet roots juice; 5% (V/V) of carrots juice and 5% (V/V) of beet roots juice and 10% (V/V) of carrots juice, respectively. During primary fermentation (07 days), sugar content, alcohol content, pH and titratable acidity were measured daily. After 21 days maturation, the wine samples were subjected to physicochemical analysis (pH; titratable acidity; dry matter, sugar, alcohol and ash contents), microbial analysis (total bacteria, total coliforms, E. coli and yeasts and moulds counts) and sensory evaluation (colour, clarity, Aroma, taste, alcohol burn, after taste and overall acceptability). From the results, parameters studied were not affected by colouring during primary fermentation of whey wine. Colouring did not affect (p˃0.05) pH; titratable acidity; dry matter, sugar and alcohol contents of whey wine. Carrots juice decreased the ash content. The yeasts and moulds count reduced (p˂0.05) with beet roots juice alone or in association to carrots juice. There was no significant (p˃0.05) effect of colouring on colour, clarity, smell/aroma, taste and alcohol burn of whey wine. At 10%, carrots juice increased (p˂0.05) after taste appreciation and overall acceptability; had high (p˂0.05) after taste and similar (p˃0.05) appreciation concerning clarity, alcohol burn and overall acceptability compared to commercial red wine. Hence, whey wine coloured with carrots juice at 10% (V/V) could be recommended.
Keywords: whey whine, beet roots, carrots, properties
Research Highlights
¨ Valorisation of waste products from dairy processing
¨ Development of new food product and contribution in wine diversification
¨ Reduction of environmental pollution
Whey is the by-product from cheese or casein production and represents the watery portion obtained after milk coagulation or curd forming. It can be defined as the yellowish-greenish liquid that remains after casein coagulation by enzymes or acids (Tratnik and Božanić, 2012). It is mostly constituted of milk water soluble components (mineral, vitamins, lactose and protein) with lactose being the main constituent. In fact, whey is a nutritionally valuable coproduct with numerous potentially beneficial effects on human health and may be considered as functional food for various groups of consumers, targeting in the first line athletes, but also children or elderly individuals (Barukčić, 2018).
Whey is an excellent source of functional proteins and peptides, vitamins, minerals and lactose, a sugar with low glycemic index (Barukčić, 2018). The most abundant minerals in whey are sodium, potassium and calcium which are present in form of salts such as chlorides, phosphates, citrates and sulphates (Barukčić, 2013). It contains almost all the B-complex vitamins (water soluble vitamins) of milk. Whey proteins, although present in small quantity, possess high protein efficiency ratio (3.6), net protein utilisation (95) and biological value (104); also, compared to all other protein sources available, they are next to egg protein in terms of nutritive value (Chavan et al., 2015). In addition, the biological value of whey proteins is approximately 15% higher comparatively to egg proteins which were regarded as referent considering essential amino acid concentration (Tratnik and Božanić, 2012). Whey proteins is a source of α-lactalbumin, β-lactoglobulin, bovine serum albumin, caseinomacropeptides, immunoglobulins, lactoferrin, lysozyme and lactoperoxidase which are often associated with health promoting properties (Kumar et al., 2008; Chavan et al., 2015), such as enhancing immunity, positive effect on nervous system, hypocholesterolemic and anti-stress activities, anticancer properties and adhesive effect against pathogenic properties, as well as antiviral, antimicrobial (iron binding properties) and antihypertensive properties (Chatterton et al., 2006; Darewicz et al., 2014; Chavan et al., 2015).
Despite its nutritive value and positive effects on human health, whey was removed along with sewage, which posed a threat to the ecosystem due to the organic compounds it contained (Wesołowska-Trojanowska and Targoński, 2014). In fact, the main problem of whey is associated to its strong polluting power due to its high values of Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (Chatzipaschali and Stamatis, 2012). Therefore, use of whey as wastage (waste water) or as fertilizer is strictly prohibited (Barukčić, 2018). In addition, whey is mostly used today as animal feed for pigs, sheep, dogs and other animal. However, its use can have a positive impact not only on the consumers health but also on the finances of many companies, by reducing the costs of raw materials, and thus production costs (Królczyk et al., 2016). Thus, in order to valorise whey, new opportunities have been introduced like the production of dehydrated products such as whey powder, whey protein concentrates and isolates. Also, beverages has been developed from whey including alcoholic beverage such as beer, liqueurs or wine (Chavan et al., 2016). The use of colorants during wine production could represent a strategy to increase the attractiveness of consumers’ and accordingly increases its consumption. Use of natural food colorants such as beet roots and carrot should be more benefits considering their nutritional and health benefits.
Carrot (Daucus carota L.) is a vegetable commonly known to play a great role in improving vision. They have an important role in nutrition due to their high dietary value and good storage attributes (Leja et al., 2015; Umar et al., 2015). In addition, it is rich in beta carotene, ascorbic acid, tocopherol and classified as vitaminized food (Hashimoto and Nagayama, 2004). Carotenoids and anthocyanins are the major antioxidant pigments found in carrots and are used as food colorants. The four types of phytochemicals found in carrots, namely phenolics, carotenoids, polyacetylenes, and ascorbic acid aid in the risk reduction of cancer and cardiovascular diseases due to their antioxidant, anti-inflammatory, plasma lipid modification, and anti-tumour properties (Ahmad et al., 2019).
Beet root (Beta vulgaris L.) is fulfill with the sources as antioxidants and nutrients, including vitamins A, B1, B2, B6 and C; calcium, magnesium, sodium, cupper, phosphorus, iron, potassium and betalains (Mathlouthi, 2001, Neha et al., 2018). Betalains are responsible for the bright red colour in beet roots (Gokhale and Lele, 2011) and are used as natural colorants in food processing. Its ingestion can be considered as a factor in cancer prevention; in addition, it acts as antimicrobial, antiviral and, participates for the prevention and treatment of hypertension and cardiovascular diseases due to the presence of betalains, mainly betacyanins and betaxanthins (Neha et al., 2018).
Hence, the objective of the present study was to investigate on the physicochemical, microbial and sensory properties of whey wine coloured with beet roots and carrots.
2.1. Whey collection
Whey was collected hygienically and immediately from the cheese production after curd separation by draining using a cheese cloth. The milk coagulation of curd forming was done by addition of cheese starter culture (1%) and rennet solution (0.03%).
2.2. Preparation of beet roots and carrots juice
The carrot and beet root vegetables were purchased from the Bamenda Food Market in the North-West Region of Cameroon and brought to the Food Technology and Post-Harvest Laboratory of the Regional Centre of IRAD-Bambui, North-West Region of Cameroon. The root vegetables were washed several times with tap water (Potable water). Then, they were peeled or scrapped, rewashed using tap water and sliced into small sizes using a kitchen sharp knife. The pieces obtained were blend using a heavy duty blender for about 10 minutes without water addition. The pulp (paste) achieved was then squeezed using a muslin cloth and, the juice extracted was pasteurised at 75 °C for 10-15 seconds, cooled rapidly at room temperature and kept in the refrigerator (4-6 °C) for further uses.
2.3. Preparation of lemon juice
The lemon juice was obtained by washing, slicing in two parts and squeezing of the lemon fruit. The juice was then filtered using a muslin cloth and stored in the freezer prior to utilisation.
2.4. Preparation of whey wine coloured with beet roots and carrot
The whey collected was deproteneised by heating at 85 °C for 5 minutes and the clots (coagulated whey proteins) separated by filtration using a muslin cloth. The juice (carrots and beet roots juices) previously prepared was added to the deproteneised whey and the mixture well homogenised before chaptalization to 24-25 °Brix using table sugar. Then, 50 ml of lemon juice (acidity corrector) and 100 ppm of potassium metabisulfite were introduced and the mixture well homogenised and allowed for 24 hours. Yeast was then inoculated and the inoculated whey allowed for seven days in a dark room for primary fermentation to occur. After primary fermentation, the fermented whey or whey wine was decanted and filtered using a muslin cloth and then pasteurised at 60-65 °C for 30 min and rapidly cooled at room temperature. It was then stored away from sunlight (in the dark room) for 21 days at room temperature for maturation (ageing). The mature wine was decanted, filtered using a muslin cloth, pasteurised at 60-65 °C for 30 minutes, packaged in sterilised glass bottles in presence of 0.08% (W/V) of potassium metabisulfite and sealed.
Four wine samples were prepared according to the quantity of root vegetable juice added:
2.5. Physicochemical analysis
The physicochemical analysis of each sample was done in duplicate. The pH and sugar content (°Brix) were obtained by using respectively a manual pH meter (Hand held pH meter) and the refractometer. The dry matter (DM), Titratable Acidity (TA) and ash were determined according to the standard Association of Official Analytical Chemists methods (AOAC, 1990). The alcohol content was obtained by using the Jacobson’s alcohol equation (Jacobson, 2006) as follow:
Alcohol (%) = 0.592 (Si-Sf)
Si = Initial sugar content (°Brix) Sf = Final sugar content (°Brix).
The pH, sugar content and alcohol content were also evaluated daily during the primary fermentation.
2.6. Microbial analysis
Microbial analysis of wine samples consisted of determination of total bacterial count using nutrient agar, total coliforms count using MacConkey agar, E. coli count using EMB agar and yeasts and moulds using SDA agar. 10 ml of sample were measured and added to 90 ml of peptone water 0.1% (W/V) firstly sterilised to obtain diluted sample solution 10-1. 1 ml of the obtained solution was transferred to a tube containing 9 ml of peptone water and mixed well to obtain diluted sample solution 10-2. Subsequent serial dilutions up to diluted sample solution 10-6 were made. Culturing was done according to pour plate method by mixing 1 ml of appropriate dilution with 15-20 ml of the culture media.
2.7. Sensory evaluation
Sensory evaluation was carried out on whey wine samples in addition of one commercial red wine sample. It was done using a five-point hedonic scale with the following as categories: Excellent=5; Very Good=4; Good=3; Fair=2 and Poor=1. An untrained panel made of 16 persons was constituted and consisted of researchers, technicians and students on internship of the centre of IRAD-Bambui. The wine samples were served chilled (12 to 15°C) and simultaneously. The following attributes were assessed: colour, clarity, aroma, taste, alcohol burn, and after taste in addition to overall acceptability.
2.8. Data analysis
Data collected were expressed as Mean ± SD and subjected to analysis of variance (one-way ANOVA) using the Statgraphics Plus, version 5.0 statistical package. Means obtained were separated using the Fischer test (LSD) at 95% confidence level.
3.1. Sugar, alcohol, pH and titratable acidity change during primary fermentation of beet roots and carrots coloured whey wine
Sugar content
The sugar content decreased during the primary fermentation (figure 1). The fermentation showed a rapid sugar content reduction at the beginning up to day 3. After, the decrease observed was gradual and finally, between day 7 and day 8, the sugar content remained steady indicating the end of fermentation. Maragatham and Panneerselvam (2011), Iyang et al (2015) and Okemini and Dilim (2017) who worked respectively on papaya wine, Cola agentea wine and tiger nuts wine also obtained a decreasing of total sugars during fermentation, which should be as result of utilisation of sugars in alcohol production. The result observed is also in accordance with that of Sharma et al. (2018) who observed similar trend with total soluble solids (TSS) mainly made of sugar during production of pumpkin based wine due to greater availability of sugar and less ethanol in the medium. Nevertheless, as the alcohol content increases, the content of TSS decreases (Maragatham and Paneerselvam, 2008) and implicitly that of sugars.
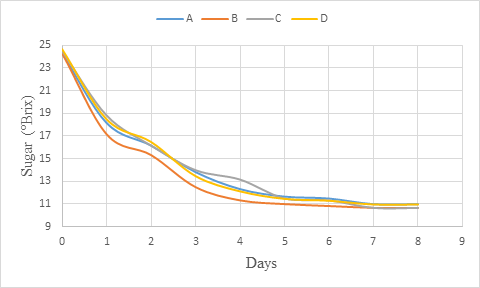
Figure 1: sugar (°Brix) change during primary fermentation of beet roots and carrots coloured wine
Alcohol content
The alcohol of the coloured whey wine increased rapidly at the beginning of the fermentation up to day 3 (figure 2) and could be due to the high rate of fermentation (alcohol production) which is affected by the initial sugar concentration (Sharma et al., 2018). Later, the increase observed was slow and between days 7 and 8, the alcohol content did not change showing that fermentation was stopped. This result is in line with that of Okemini and Dilim (2017) who observed similar trend during tiger nut wine production. Also, the studies from Maragatham and Panneerselvam (2011) revealed an increasing trend of alcohol content in papaya wine during fermentation. The low increase of alcohol content with increase in time should be explained by the fact that, as the alcohol content increased, it exerted and inhibitory effect on fermentation process (Joshi et al., 2011; Joshi et al., 2017). This should be also associated with the low sugar content of the medium.
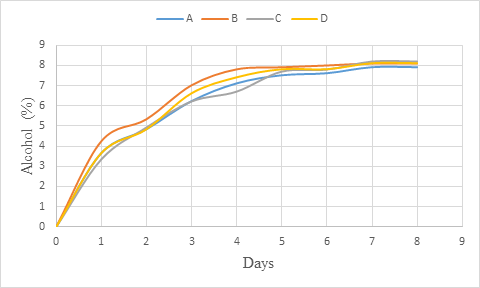
Figure 2: Alcohol (%) change during primary fermentation of beet roots and carrots coloured wine
pH
During the primary fermentation, there was a rapid decrease of pH values of all the wine samples within 02 days and the decrease was low up to day 4 (figure 3). From day 4, the increasing was observed till the end of fermentation (day 7). In general, the pH dropped during primary fermentation and could be attributed to increase in the production of organic acids as the fermentation progressed (Ogu and Mgbebu, 2011; Maragatham and Panneerselvam, 2011). This results is similar with that of Maragatham and Panneerselvam (2011) on papaya wine. However, Okemini and Dilim (2017) and Okeke et al. (2015) observed rather a complete reduction trend during primary fermentation respectively with tiger nuts wine and mixed fruit wines using yeast isolated from palm wine. In general, the pH depends on the composition of the medium which for wine, is in relation with the metabolic activity of the yeasts. The decrease in the acidity during fermentation could be due to its utilisation by the yeast for production of carbon dioxide and water (Maragatham and Panneerselvam, 2011). Also, the yeast activity is in relation with the environment such as sugar composition and concentration of acetic acid (Fleet, 2003; Chilaka et al., 2010; Duarte et al., 2010).
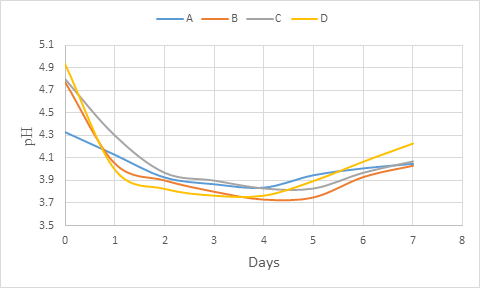
Figure 3: pH change during primary fermentation of beet roots and carrots coloured wine
Titratable acidity
An increasing of titratable acidity was observed with all the wine samples during primary fermentation (figure 4). However, the titratable acidity (% citric acid) increased at the beginning of the primary fermentation and remained almost at the same level towards the end. Okemini and Dilim (2017) rather obtained a full increasing trend during primary fermentation (06 days) of tiger nuts wine. The increase in titrable acidity during primary fermentation should probably be as a result of increase in the production of organic acids. Maragatham and Panneerselvam (2011) revealed an increase of titratable acidity during primary fermentation which decreased slightly towards ageing. They also observed a gradual decrease in the acidity during fermentation storage. The reduction of titratable acidity observed during fermentation was ascribed to the utilisation of organic acids by the yeast for production of carbon dioxide and water. This could justify the light increase of titratable acidity obtained during primary fermentation.
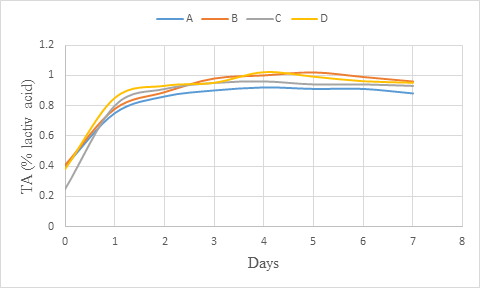
Figure 4: Titratable acidity (% citric acid) change during primary fermentation of beet roots and carrots coloured wine
3.2. Physicochemical parameters of beet roots and carrots coloured whey wine
pH
The pH values of wine samples varied between 4.23±0.12 and 4.3±0.17 (table 1). Also, all wine samples presented similar (p˃0.05) pH values. This pH values are close to those obtained by okeke et al (2015) for the production of mixed fruit wines. However, they seem to be lower compared to values obtained by Okemini and Dilim (2017), Satav and Archana (2017) and Sharma et al. (2018). According to Okemini and Dilim (2017), the high acidity observed during their studies is more of volatile acidity than the residual acidity which is a good phenomenon. The low acidity (high pH) observed should be as result of decline of volatile acids throughout different pasteurisation stages which occurred during production. In fact, Ogodo et al (2015) suggested that volatile acids can easily be removed from the body system of wine through perspiration.
Table 1: Physicochemical properties of beet roots and carrots coloured whey wine
|
Parameters |
Samples |
|||
|
A |
B |
C |
D |
|
|
pH |
4.27±0.23a |
4.23±0.12a |
4.26±0.06a |
4.3±0.17a |
|
Titratable acidity (% citric acid) |
0.83±0.03a |
0.90±0.03a |
0.88±0.05a |
0.93±0.03a |
|
Dry matter (%) |
6.19±0.01ab |
6.62±0.02a |
6.27±0.01ab |
6.11±0.35b |
|
Ash content (%) |
0.72±0.02a |
0.65±0.09 ab |
0.53±0.07b |
0.54±0.14b |
|
Sugar content (°Brix) |
11±1.32a |
10.67±1.15a |
10.67±0.29a |
11±1a |
|
Alcohol (%) |
7.89±0.90 a |
8.09±1.23a |
8.19±0.17a |
8.09±0.68a |
(a,b): Values with the same letter in the same row are not significantly different (p˃0.05); Sample A: 100% of whey, 0% of beet roots juice and 0% of carrots juice; Sample B: 90% of whey, 10% of beet roots juice and 0% of carrots juice; Sample C: 90% of whey, 5% of beet roots juice and 5% of carrots juice; Sample D: 90% of whey, 0% of beet roots juice and 10% of carrots juice
Titratable acidity
The titratable acidity did not indicate significant (p˃0.05) change amongst the wine samples and the values ranged from 0.83±0.03% to 0.93±0.03%. The values obtained are between 0.5% and 1.0%, the total acidity of final wine as reported by Chilaka et al. (2010) and are close to values revealed by findings from Joshi et al. (2009) and Satav and Archana (2017). However, they appear to be lower than value reported by Iyang et al. (2015) and higher than those from Okemini and Dilim (2017) and Sharma et al. (2018). The titratable acidity of wine is in relation with the production of organic acids and their utilisation by yeasts during fermentation. It also depends on volatile acids content (Okemini and Dilim, 2017).
Dry matter content
The whey wine samples indicated the dry matter content varying from 6.11±0.35% (sample D) to 6.62±0.02% (sample B). A significant difference (p˂0.05) was observed only between these two values. The values reported by Satav and Archana (2017) on banana wine are very low comparatively to values of the present study and could be explained by chaptalisation. In fact they did chaptalisation at 19 °Brix while in the present finding it was done at 24-25 °Brix, indicating high table sugar quantity used and, the quantity of sugar added could positively affect the dry matter of the final product. Sharma et al. (2018) revealed that a TSS of the wine is correlated to the TSS at the beginning of the fermentation process.
Ash content
The sample A (uncoloured whey wine) had the highest ash content (0.72±0.02%) and this value was significantly (p˂0.05) different to the values presented by samples C and D (respectively 0.53±0.07% and 0.54±0.14%) which were the lowest. It seemed like addition of carrots juice (sample C and D) led to the reduction of ash content of the wine and may be due to its low ash content (0.66 ± 0.12%) comparatively to whey and beet roots juice ash contents (1.2 ±0.19% and 0.72 ±0.015%, respectively).
Sugar content
The wine samples showed similar (p˃0.05) values of sugar content which were ranged between 10 °Brix and 11 °Brix. This could be attributed to their comparable values of sugar content at the beginning of the primary fermentation which were between 24.33 ± 2.84 °Brix (samples A and B) and 24.67 ± 2.08 °Brix (sample D). This result is in accordance with that of Sharma et al. (2018) who obtained The highest (8.9 °Bx) TSS with wine prepared in presence of 26 °Bx of sugar and the lowest (7.5 °Bx) with that prepared in presence of 24 °Bx of sugar.
Alcohol content
The alcohol content varied from 7.89±0.90% (sample A) to 8.19±0.17% (sample C) and were comparable (p˃0.05). The addition of colourings (beet roots and carrots juices) induced an increasing of alcohol content of whey wine although there was no significance (p˃0.05) difference amongst samples. The values of alcohol obtained appear to be higher comparatively to values reported by Satav and Archana (2017) and this could be due to the initial sugar content or TTS. They did chaptalisation at 19 °Brix which is lower than 24-25 °Brix used in the present study. Sharma et al. (2018) revealed that the rate of fermentation is affected by the initial sugar concentration and decreased with the total soluble solids (TSS). Joshi et al. (2009) and Okemini and Dilim (2017) obtained high alcohol content (respectively 12% and 15.8%). The high alcohol content could be related with the high concentration of fermentable sugars and the yeast (S. cerevisiae) is a known high performance species (Okemini and Dilim, 2017). Also, the type of sugar present in the medium affect the fermentation by S. cerevisae which are strains mostly glucophilic in nature so the fructose utilization is inhibited more than glucose in the presence of high ethanol content (Berthels et al., 2004). In addition, the fermentation (alcohol production) is affected by several other factors including the medium composition and nitrogen assimilation (Amerine et al., 1980; Joshi et al., 2011).
3.3. Microbial properties of beet roots and carrots coloured whey wine
All the whey wine samples showed very low level of contamination from total bacterial count (˂100 cfu/ml), total coliforms count (˂10 cfu/ml) and E. coli count (˂10 cfu/ml) (table 2). This could be due to the effect of pasteurisation which destroys pathogenic or unwated microorganisms and the action of potassium metabisulfite which is an antibacterial agent. Samples B and C with respectively 1.55±0.23 (x102 cfu/ml) and 1.55±0.32 (x102 cfu/ml) indicated low yeasts and moulds count value across all samples, which were significantly (p˂0.05) different to 2.12±0.33 (x102 cfu/ml) obtained by sample A. However, sample D with 1.67±0.35 (x102 cfu/ml) showed similar (p˃0.05) yeasts and moulds count with other samples. All the whey wine samples revealed low level of yeast content which could also be associated to the effect of pasteurisation and the presence of metabisulfite which avoid growth of wild yeast.
Table 2: Microbial properties of beet roots and carrots coloured whey wine
|
Samples |
TBC (x 102 cfu/ml) |
TCC (x 101 cfu/ml) |
ECC (x 101 cfu/ml) |
Y&M count (x 102 cfu/ml) |
|
A |
< 1 |
< 1 |
< 1 |
2.12±0.23a |
|
B |
< 1 |
< 1 |
< 1 |
1.55±0.23b |
|
C |
< 1 |
< 1 |
< 1 |
1.55±0.32b |
|
D |
< 1 |
< 1 |
< 1 |
1.67±0.35ab |
(a,b): Values with the same letter in the same row are not significantly different (p˃0.05); Sample A: 100% of whey, 0% of beet roots juice and 0% of carrots juice; Sample B: 90% of whey, 10% of beet roots juice and 0% of carrots juice; Sample C: 90% of whey, 5% of beet roots juice and 5% of carrots juice; Sample D: 90% of whey, 0% of beet roots juice and 10% of carrots juice; Sample E: Commercial red wine; TBC: Total Bacteria Count; TCC: Total Coliforms Count; ECC: E. coli Count; Y&M: Yeasts and Moulds.
3.3. Sensory evaluation
All the whey wine samples had similar (p˃0.05) appreciation level in terms of colour, clarity, aroma, taste and alcohol burn (table 3) according to the panel members. The commercial wine (sample E) was significantly (p˂0.05) the best in terms of colour, smell/aroma and taste. Furthermore, about clarity and alcohol burn, it also presented the highest score which was comparable (p˃0.05) to that of sample D (coloured whey wine with 10% of carrots juice).
Table 3: sensory evaluation scores of beet roots and carrots coloured whey wine
|
Parameters |
A |
B |
C |
D |
E |
|
Colour |
3.38±1.20b |
3.53±1.21b |
3.31±1.01b |
3.81±0.8b |
4.81±0.4a |
|
Clarity |
3.5±1.15b |
3.27±1.20b |
3.69±1.1b |
4.13±0.81ab |
4.69±0.48a |
|
Aroma |
3.38±0.89b |
3.67±0.77b |
3.38±0.72b |
3.44±1.03b |
4.75±0.58a |
|
Taste |
3.31±1.0b |
3.27±0.9b |
3.5±0.63b |
3.69±0.7b |
3.81±0.50a |
|
Alcohol burn |
3.13±1.0b |
3.13±1.0b |
3.13±0,7b |
3.75±0.9ab |
4.8±0.98a |
|
Aftertaste |
3.19±0.75b |
3±0.97b |
3.0±60.77b |
3.81±0.83a |
3.19±0.91b |
|
Overall Acceptability |
3.31±1.01b |
3.33±0.70b |
3.56±1.03ab |
4±1.03a |
4±0.63a |
(a,b): Values with the same letter in the same row are not significantly different (p˃0.05); Sample A: 100% of whey, 0% of beet roots juice and 0% of carrots juice; Sample B: 90% of whey, 10% of beet roots juice and 0% of carrots juice; Sample C: 90% of whey, 5% of beet roots juice and 5% of carrots juice; Sample D: 90% of whey, 0% of beet roots juice and 10% of carrots juice; Sample E: Commercial red wine.
Concerning aftertaste, sample D (coloured whey wine with 10% of carrots juice) was significantly (p˂0.05) the most preferred amongst all the wine samples tested. Sample D and sample E obtained the same score with respect to overall acceptability. This result was not significantly (p˃0.05) different with that of sample C (whey wine sample coloured with 5% carrots juice and 5% beet roots juice) but significantly (p˂0.05) different with scores obtained by sample A (uncouloured whey wine) and B (whey wine sample coloured with 10% beet roots juice).
All the whey wines were appreciated at a greater level and the increasing of carrots juice as colouring led to the increasing of its sensory properties in general.
Colouring whey wine with carrots juices or/and beet roots juice has no effect on pH, titratable acidity, dry matter and sugar content of whey wine. However, there is decrease of ash content at 10% carrots juice and by using a mixture of carrots juice and beet roots juice at 5% each. Beet roots and carrots juice as food dyes, induce a reduction on yeasts and moulds count of whey wine. Whey wine is well accepted and colouring using especially carrots juice at 10% (V/V) could be as a strategy for improvement of its acceptability. However, many factors such as clarification will be for a great interest in improving its quality.
Ahmad T, Cawood M, Iqbal Q, Ariño A, Batool A, Tariq RMS, Azam M, Akhtar S (2019) Phytochemicals in Daucus carota and their health Benefits: Review Article. Foods. 8:424; Doi:10.3390/foods8090424. PMid:31546950
View Article PubMed/NCBIAmerine MA, Kunkee KE, Ough CS, Singleton VL, Webb AD (1980) The Technology of Wine Making, 4th ed., AVI, Westport, CT.
AOAC (1990) Official methods of analysis. 15th ed Washington DC. Edr., Washington DC.
Barukčić I (2013) Optimizing efficiency of whey microfiltration and ultrafiltration by applying ceramic membranes - A dissertation. University of Zagreb, Zagreb, Croatia.
Barukčić I (2018) Whey as a Potential Functional Food-Properties, Processing and Future Perspective. 2(1):2p.
Berthels NJ, Cordero RR, Bauer FF, Thevelein JM, Pretorius IS (2004) Discrepancy in glucose and fructose utilization during fermentation by Saccharomyces cerevisiae wine yeast strains, FEMS Yeast Res. 4:683-689. PMid:15093771
View Article PubMed/NCBIChatterton DEW, Smithers G, Roupas P, Brodkorb A (2006) Bioactivity of β-lactoglobulin and α-lactalbumin-Technological implications for processing. Int. Dairy J. 16:1229-1240.
View ArticleChatzipaschali AA, Stamatis AG (2012) Biotechnological utilization with a focus on anaerobic treatment of cheese whey: current status and prospects. Energies 5:3492-3525.
View ArticleChavan RS, Shraddha RC, Kumar A, Nalawade T (2015) Whey Based Beverage: Its Functionality, Formulations, Health Benefits and Applications. J Food Process Technol. 6(10):8p.
View ArticleChilaka CA, Uchechukwu N, Obidiegwu JE, Akpor OB (2010) Evaluation of the efficiency of yeast isolates from palm wine in diverse fruit wine production. Afr J Food Sci. 4(12):764-774.
Darewicz M, Iwaniak A, Minkiewicz P (2014) Biologically active peptides derived from milk proteins. Med. Wet. 70:348-352.
Duarte WF, Dias DR, Oliveira MJ, Teixeira JA, Silva JD, Schwan RF (2010) Characterization of different fruit wines made from cocoa, cupuassu, gabiroba, jaboticaba and umbu. Food Sci Technol. 30:1-9.
Fleet GH (2003) Yeast interaction and wine flavour. Int J Food Microbiol. 86:11-22. 00245-9
View ArticleGokhale SV, Lele SS (2011) Optimization of convective dehydration of β- Vulgaris for color retention. Food Bioprocess Tech. DOI: 10.1007/s11947-010-0359-8
View ArticleHashimoto T, Nagayama T (2004) Chemical composition of ready to eat fresh carrot. J. Food Hyg. Soc. Japan. 39:324-328.
View ArticleInyang UE, Etuk KB (2015) Production and evaluation of Cola argentea wine: a value added product. Nigerian Journal of Agriculture, Food and Environment. 11(1):133-136.
Jacobson JL (2006) Introduction to wine laboratory practices and procedures. Springer Science & Business Media, New York, pp 164-166, 269-271.
Joshi VK, Attri D, Singh TK, and Abrol GS (2011) Fruit wines: Production technology, in Joshi VK (ed) Handbook of Enology: Principles Practices and Recent Innovations, 3rd edn. Asia Tech, New Delhi, pp1177-1221.
Joshi VK, Sharma S, Devi MP (2009) Influence of different yeast strains on fermentation behaviour, physico-chemical and sensory qualities of plum wine. Indian J. Nat. Prod. Res. 8(4):445-451.
Joshi, V. K., Sharma, S., and Thakur, A. D. (2017) Current Developments in Pandey A, Du G, Sanroman MA, Soccol CR, Dussap CG (eds) Biotechnology and Bioengineering, in Wines-White, Red, Sparkling, Fortified, and Cider. Elsevier, Amsterdam, pp353-401.
Królczyk JB, Dawidziuk T, Janiszewska-Turak E, Sołowiej B (2016) Use of Whey and Whey Preparations in the Food Industry - a Review. Pol. J. Food Nutr. Sci. 66(3):157-165.
View ArticleKumar R, Sangwan RB, Mann B (2008) Separation and application of bioactive whey proteins. Technological Advances in the utilization of dairy by-products, 22nd Short Course. Available at: content/uploads/ 2012/05/Byproducts-2008.pdf
View ArticleLeja M, Kaminska I, Kramer M, Maksylewicz-Kaul A, Kammerer D, Carle R, Baranski R (2013) The Content of Phenolic Compounds and Radical Scavenging Activity Varies with Carrot Origin and Root Color. Plant. Foods Hum. Nutr. 68:163-170. PMid:23613033
View Article PubMed/NCBIMaragatham C and Paneerselvam A (2008) Wine production from banana peel. Indian journal of Applied Microbiology. 9(1):1-3.
Maragatham C, Panneerselvam A (2011) Standardization technology of papaya wine making and quality changes in papaya wine as influenced by different sources of inoculums and pectolytic enzyme. Advances in Applied Science Research. 2(3):37-46.
Mathlouthi M (2001) Water content, water activity, water structure and the stability of foodstuffs. Food Control. 12:409-417. 00032-9
View ArticleNeha P, Jain SK, Jain NK, Jain HK, Mittal HK (2018) Chemical and functional properties of Beetroot (Beta vulgaris L.) for product development: A review. International Journal of Chemical Studies. 6(3):3190-3194.
Ogodo AC, Ugbogu OC, Ugbogu AE, Ezeonu CS (2015) Production of mixed fruit (pawpaw, banana and watermelon) wine using Saccharomyces cerevisiae isolated from palm wine. SpringerPlus, 4:683. PMid:26576326
View Article PubMed/NCBIOgu EO, Mgbebu PO (2011) Foundation for African development through international biotechnology (FADIB), 19: 49-53.
Okeke BC, Agu KC, Uba PO, Awah NS, Anaukwu CG, Archibong EJ, Uwanta LI, Ezeneche JN, Ezenwa CU, Orji MU (2015) Wine production from mixed fruits (Pineapple and Watermelon) using high alcohol tolerant yeast isolated from palm wine. Universal Journal of Microbiology Research. 3(4):41-45.
View ArticleOkemini OF, Dilim I-OC (2017) Physico-Chemical Properties and Sensory Evaluation of Wine Produced from Tiger Nut (Cyperus esculentus). International Journal of ChemTech Research. 10(12):155-164.
Satav PD, Pethe AS (2017) Influence of different yeast strains on physicochemical characteristics of banana wine. Bioscience Discovery. 8(4):712-715
Sharma S, Thakur AD, Sharma S, Atanassova M (2018). Effect of different yeast species on the production of pumpkin based wine. J. Inst. Brew. 7p.
View ArticleTratnik Lj, Božanić R (2012) Mlijeko i mliječni proizvodi (Milk and dairy products). Hrvatska mljekarska udruga, Croatian Dairy Association, Zagreb, Croatia.
Umar G, Kaur S, Gurumayum S, Rasane PE (2015) Effect of hot water blanching time and drying temperature on the thin layer drying kinetics of and anthocyanin degradation in black carrot (Daucus carota L.) Shreds. Food Technol. Biotechnol. 53, 324-330. PMid:27904364
View Article PubMed/NCBIWesołowska-Trojanowska M, Targoński Z (2014) The whey utilization in biotechnological processes. Nauki Inżynierskie i Technologie. 1(12):102-119 (in Polish; English abstract).
