Andreu A.B
Telephone: +54 9 (0223) 154231464
E-mail: abandreu@mdp.edu.ar
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 2 ISSUE: 2
Page No: 205-217
Martínez Maria Julia1, Barbini Luciana2, Andreu Adriana Balbina1*.
1 Instituto Investigaciones Biológicas, Universidad Nacional de Mar del Plata, Deán Funes 3350, Mar del Plata, Argentina.
2 Departamento de Química, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata, Deán Funes 3350, Mar del Plata, Argentina.
Jana Sic Zlabur(jszlabur@agr.hr)
Ireneusz Kapusta(ikapusta@ur.edu.pl)
Joseph H Y Galani(josephgalani@gmail.com)
J Simal-G%C3%A1ndara(jsimal@uvigo.es)
Martinez Maria Julia, Barbini Luciana, Andreu Adriana Balbina, Chemical characterization of polyphenol extracts from Andean and industrial Solanum tuberosum tubers and their cytotoxic activity on human hepatocarcinoma cells(2017)SDRP Journal of Food Science & Technology 2(2)
Background: Polyphenols are plant metabolites that have been largely studied for their beneficial effect on human health. Potato is one of the most important crops worldwide and is a relevant source of human dietary nutrients and antioxidants. Particularly, pigmented potatoes contain the highest levels of polyphenolic compounds. Hepatocellular carcinoma is one of the most frequent types of cancers worldwide and despite the existence of treatments; it is yet associated with a high mortality rate. Thus, new drugs are needed, and polyphenols are a potential source of anti-hepatocellular carcinoma compounds. The objectives of this study were to determine the content of different groups of polyphenols (phenolic acids, anthocyanins and flavan-3-ols) in five potato polyphenolic extracts, and to study their antioxidant and cytotoxic activities on a human hepatocellular carcinoma cell line.
Methods: four Andean varieties and one industrial potato variety were selected for this study. Polyphenolic quantification and composition were determined by spectrophotometric assays and HPLC-DAD analysis. The antioxidant and cytotoxic activities were evaluated through DPPH and MTS assays respectively.
Results showed that pigmented varieties possessed higher levels of the analyzed phenolic compounds. HPLC analysis showed that chlorogenic acid was the main phenolic acid in all the potato polyphenolic extracts. Also, pigmented potatoes presented higher levels of antioxidant activity compared to non-pigmented ones, showing a positive correlation with the total phenolic content. Finally, treatment with three of the studied potato polyphenolic extracts reduced the viability of Hep3B. Furthermore, one extract from a non-pigmented variety affected cell viability to a similar extent as extracts from pigmented potatoes, suggesting that other compounds, besides anthocyanins, may be responsible of the cytotoxic effect of this polyphenolic extracts.
Conclusion: These results suggested that polyphenolic compounds present in the Andean potato varieties could be used as a potential source of anti-hepatocarcinoma drugs.
Keywords: potato, phenolic acids, anthocyanins, flavan-3-ols, antioxidant activity, cytotoxicity.
The potential health benefits of antioxidant compounds have been broadly reported. Furthermore, several epidemiological studies showed that consuming foods with high levels of antioxidants, like polyphenols, might correlate with lower risk of developing some diseases such as cardiovascular diseases, diabetes or cancer [1-5]. Polyphenols are a group of plant metabolites whose main role is to protect the plant from different types of abiotic and biotic stresses, such as: UV radiation, wounds, ROS, and herbivores [6, 7]. In addition to their relevance in plants, the effects of these compounds on human health have been extensively studied. Different in vitro and in vivo assays have described polyphenols as antioxidant, anti-inflammatory, anti-microbial and anti-cancer compounds [8-10]. Particularly, in vitro results showed that phenolic acids, like chlorogenic acid (CGA) or anthocyanins, like pelargonidin, exerted different biological activities in various cellular models [11-14].
Potato (Solanum tuberosum) is one of the most important crops worldwide, and is a significant source of carbohydrates, minerals and vitamins for the human diet [15, 16]. Also, due to its high intake levels, potato represents one of the main sources of dietary antioxidants such as, carotenoids and polyphenols [17]. Compared to non-pigmented potatoes, pigmented varieties contain higher levels of polyphenols, mainly because of the presence of both anthocyanins and phenolic acids [18-20]. Several studies reported CGA as the main phenolic acid in both pigmented and non-pigmented potato varieties [21-23]. Moreover, pigmented and non-pigmented potatoes present a similar profile of phenolic acids but, pigmented potato varieties also present antochyanins like pelargonidin and malvidin [24] and, compared to non-pigmented, pigmented potato varieties exhibit higher levels of antioxidant capacity [23, 25-28].
Hepatocellular carcinoma (HCC) is one of the most frequently occurring tumors worldwide and is associated with high mortality [29, 30]. Different etiological factors are related to the development of HCC, including chronic hepatitis B or C virus infections, mycotoxin consumption and alcoholic cirrhosis [29]. Despite the existence of treatments, patients diagnosed with late HCC still have a poor prognosis [31, 32]. Thus, the development of new anti-HCC drugs is essential, and natural compounds like polyphenols, are a promising source of potential molecules for cancer treatment. Particularly, anti-HCC activity has been described for polyphenolic extracts (PEs) from various commonly consumed vegetables or herbs [33-35]. In the case of potato polyphenolic extracts (PPEs) their cytotoxic activity has been demonstrated in vitro in different tumoral cell lines, including HCC [21, 36-40]. The objectives of this work were to characterize and quantify the composition of polyphenolic extracts from potatoes with different pigments (S. tuberosum L ssp. tuberosum and S. tuberosum L ssp. andigena), and to evaluate their antioxidant and cytotoxic activities against human HCC cells in vitro.
Plant material
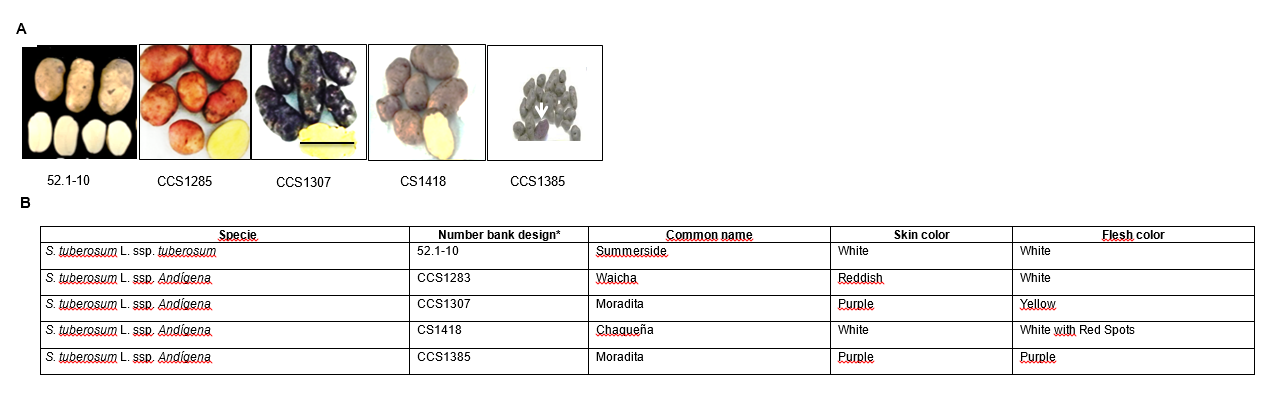
Four S. tuberosum L ssp. andigena potatoes varieties (CCS1283, CCS1307, CS1418 and CCS1385), here after referred to as Andean, were grown in a field located in Yavi Department (22° 6' 4" S, 65° 35' 44" O, 3377 MAMSL), Jujuy, Argentina, during the 2012/2013 season. One S. tuberosum L ssp. tuberosum variety (52.1-10) here after referred to as the industrial variety was grown in an experimental field located in Balcarce (37° 49' 9.65" S, 58° 13' 11" W, 130 MAMSL), Buenos Aires, Argentina, during the 2012/2013 season of McCain Argentina S.A. All potato varieties and their pigmentation and morphological characteristics are presented in Figure 1. All varieties were planted in random plots and harvested at the end of their respective cycles. The tubers were transported to the laboratory where they were washed, peeled and potato flesh from the different varieties were frozen in liquid nitrogen and stored at -80 °C for further analysis.
Preparation of potato polyphenolic extracts
Two grams of potato tuber flesh were homogenized with liquid nitrogen, and extracted with 40 mL 100 % methanol (HPLC grade, Pharmco-aaper) at 4 °C overnight, in darkness with constant agitation. Only for the purple variety, CCS1385, the extracts were prepared from the whole tubers due to their small size. Then, extracts were centrifuged at 6000 rpm for 20 min at 4 °C, concentrated using a rotary evaporator (Senco) and resuspended in 1 mL of methanol 30 % (v/v). After centrifugation at 13000 rpm for 15 min at 4 °C the supernatant was filtered through a 0.22 µm filter. Potato polyphenolic extracts (PPEs) were stored at -20 °C until analysis.
Determination of total phenolic content
The total phenolic content was determined using the Folin - Ciocalteu colorimetric method, as previously described [41]. Briefly, 20 µL of PPE were diluted to a final volume of 0.5 mL with methanol (HPLC-grade). Then 7.5 mL of water was added, mixed with 0.5 mL of Folin - Ciocalteu reagent (Merck), diluted in water (1:7), and after 3 min of reaction, 1 mL of 0.5 M Na2CO3 was added and allowed to react for 10 min. Finally, absorbance at 725 nm was measured in a visible light spectrophotometer (Hitachi 156 U-1900). Distilled water was used as a blank. Chlorogenic acid (CGA, Sigma-Aldrich) was used as a standard, and total phenolic content was expressed as µg of CGA equivalents per 1 g of potato tuber fresh weight (µg of CGA equiv. / gfw). Each sample was measured in triplicate in four independent experiments.
Determination of total flavan-3-ols
Total flavan-3-ols were calculated as previously described [42]. Briefly, 100 µL of PPE were diluted to a final volume of 200 µL and mixed with 1 mL of 4-(Dimethylamino) - cinnamaldehyde (DMCA, Sigma), 30/70 (v/v). Finally, absorbance at 640 nm was measured. Catechin (Sigma) was used as standard, and the total flavan-3-ols quantity was expressed as µg of catechin equivalents per 1 g of potato tuber fresh weight (µg of catechin equiv. / gfw). Each sample was measured in triplicate in four independent experiments.
Determination of total monomeric anthocyanin content
Total anthocyanin content was calculated using the pH-differential method [43]. Two dilutions of the sample were prepared: 100 µL of PPE were diluted with 2000 µL of 0.025 M KCl buffer (pH 1) and another 100 µL of PPE were diluted 2000 µL of 0.4 M CH3CO2Na3 (pH 4.5). After 15 min of reaction, absorbance at 500 nm and 700 nm, respectively was measured in a visible light spectrophotometer. The difference in absorbance (A) at different pH values and wavelengths was calculated according the equation below:
A = (A500-A700) pH1- (A500-A700) pH4.5
Anthocyanin concentration (AC) of the PPE was calculated in terms of cyaniding – 3 - glucoside, using the following formula:
AC (mg/L) = (A x MW x DF x 1000) / (e x 1)
Where, MW is cyanidin-3-glucoside (C-3-G) molecular weight of 449.2 g / mol; e, is the extinction coefficient of 26900 L / cm / mol; and DF, is the dilution factor. Anthocyanin content was reported as µg of C-3-G per 1 g of potato tuber fresh weight (µg C-3-G equiv. / gfw). Each sample was measured in triplicate in four independent experiments.
Determination of antioxidant activity
The total hydrophilic antioxidant activity was measured using the DPPH (2, 2 – diphenyl - 1 -picrylhydrazyl, Sigma) assay, as previously described [44]. Briefly, 10 µL of PPE were diluted to a final volume 150 µL with methanol (HPLC-grade). Then, 4 mg of DPPH were diluted in 100 mL of methanol to obtain a working solution with an absorbance at 515 nm of 1-1.1. Diluted PPE was mixed with 2.85 mL of DPPH and incubated 24 h at room temperature in the dark. Finally, absorbance at 515 nm was measured in a visible light spectrophotometer. Methanol (HPLC-grade) was used as a blank, and trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid, Sigma) was used for the standard curve. Antioxidant activity was expressed as µg of trolox equivalents per 1 g of potato tuber fresh weight (µg trolox equiv. / gfw). Each sample was measured in triplicate in four independent experiments.
Characterization and quantification of phenolic acids, flavan-3-ols and anthocyanidins by HPLC-DAD
Quantification of phenolic acids, flavan-3-ols and anthocyanidins was carried out with a high performance liquid chromatography (HPLC) system (Shimadzu, Prominence) equipped with a diode array detector (DAD). To analyze phenolic acids and flavan-3-ols, 20 µL of the sample (previously filtered through a 0.45 µm PVDF membrane, Millipore), were injected using a flow rate of 1 mL/min, onto a C-18 Phenomenex Luna column 153 (250 x 4.6 mm i.d.; 5 μm particle size). The mobile phase was: (A) acidified distilled water (pH 2.3) and (B): CH3CN. The gradient used was: 0-20 min, linear gradient of B 20% to 100%; 20-25 min, B was decreased back to 20% and 25-30 min conditions were kept constant.
For characterizing anthocyanidins, only PPEs from the pigmented varieties (CS1418, CCS1385) were used. The samples were hydrolyzed with a final concentration of 2 M HCl at 100° C for 1 h. Anthocyanin detection was also carried out with an octadecylsilane C - 18 column (250 L x 4.6, 5 µm particle size). A flow rate of 0.8 mL/min was used and sample injection volume was 20 µL. The mobile phase was: (A) 4% H3PO4 buffer, (B) CH3CN. The gradient used was: 0-25 min, B was increased from 15% to 25%; 25-30 min, B was increased to 27%; 30.5-33 min B was returned to 15%.
The phenolic acids [CGA, caffeic acid (CA), ferulic acid (FA) and coumaric acid (CoA), Sigma], anthocyanins (pelargonidin, peonidin, petunidin, malvidin and delphinidin, Sigma) and flavan-3-oles [catechin and epicatechin, Sigma] were charazterized and quantified by comparing retention times and spectra of the different standards.
Human hepatocarcinoma cell line
Hep3B cells (American Type Tissue Collection, ATCC), derived from human hepatocellular carcinoma, were grown in minimal Eagle’s medium (MEM, Gibco), supplemented with 100 mL / L fetal bovine serum (FBS, Natocor), 2 mmol / L glutamine (Gibco), 1.5 g/L sodium bicarbonate, 1 mmol / L nonessential amino acids (Gibco), and 1 mmol / L sodium pyruvate (Gibco). For experiments, FBS was reduced to 10 mL / L. The cells were cultured at 37 °C in a humidified atmosphere, containing 5 % CO2.
Determination of cell cytotoxicity
To analyze the cytotoxic activity of the PPEs, Hep3B cells were seeded in 96 multiwell plates and incubated at 37° C, 5% CO2 for 24 h. Cells were treated with different concentrations (25, 50, 100, 200 and 400 µg / mL) of the PPEs for 24 h. Controls with 30 % methanol and non-treated cells were also included. After incubation, cytotoxicity was measured by an MTT assay (3 - (4, 5 -dimethiylthiazol – 2 – yl ) 5 - ( 3 – carboxymethoxyphenyl ) – 2 - tetrazolium, Sigma) and absorbance was read at 570 nm. The viability percentage was calculated as % = (Absorbance of treated cells/ Absorbance of control (30 % methanol) cells) x 100. The 50% cytotoxic concentration (CC50) was calculated for each potato variety.
Statistical
All experiments were carried out four times, and expressed as the mean ± standard error of the mean (SEM). Phenolic acids, flavan-3-ols, and anthocyanins quantification, HPLC analysis and cytotoxic activity experiments of the PPEs were analyzed using parametric one-way analysis of variance (ANOVA), followed by Bonferroni’s multiple comparison test. Correlations among TPc, total anthocyanin content, flavan-3-ols, CGA, CA, FA, CoA, and antioxidant activity (DPPH) were calculated following Pearson’s correlation method. All statistical analyses were performed using GraphPad Prism 6.
Quantification of total phenolic acids, monomeric anthocyanins, and flavan-3-ols contents
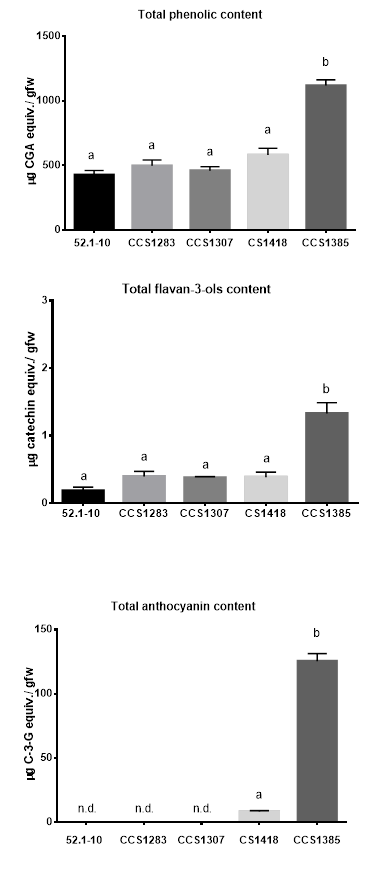
First, the total amount of three groups of polyphenols (phenolic acids, flavan-3-ols and anthocyanins) in the PPEs of the different varieties was quantified. Total phenolic compounds from four Andean varieties (CCS1283, CCS1307, CS1418 and CCS1385) and the industrial variety (52.1-10) were quantified using the Folin - Ciocalteu method. As shown in Figure 1, only CS1418 and CCS1385 varieties exhibit pigmented flesh, being white with red spots or purple, respectively. The quantification resulted in a total phenolic acid content ranging from 427.22 ± 68.04 to 1118.15 ± 90.33 µg of CGA equiv. / gfw. CS1418 and all white fleshed varieties showed similar values of this group of compounds, with no significant differences between Andean varieties (CS1418, CCS1283 and CCS1307) and the industrial variety (52.1-10). The purple variety CCS1385 contained the highest levels of phenolic acids, 1118.15 ± 90.33 µg of CGA equiv. / gfw (Figure 2A).
Then, the total flavan-3-ol levels were quantified in all the PPEs. Coinciding with phenolic acid content, the purple variety CCS1385 presented the highest amounts of flavan-3-ols: 1.33 µg catechin equiv./gfw. Again, none of the other PPEs from the other four varieties (Andean or industrial) presented significant differences in flavan-3-ol levels (Figure 2B).
Finally, total anthocyanin content was measured in all PPEs by the differential pH method. No anthocyanins were detected in white or yellow fleshed varieties (52.1-10, CCS 1283, and CCS1307). The purple variety CCS 1385 showed the highest levels of total anthocyanin content: 125.43 ± 10.16 µg C-3-G equiv. / gfw (Figure 2C).
Characterization and quantification of phenolic acids, flavan-3-ols and anthocyanidins content by HPLC-DAD
To determine the content of the phenolic acids: chlorogenic acid (CGA), caffeic acid (CA), ferulic acid (FA), and coumaric acid (CoA), the five PPEs were analyzed by HPLC-DAD. Flavan-3-ols catechin (CT) and epicatechin (ECT) were also quantified. Table 1 shows the phenolic acid profiles found in the different PPEs. CGA represented the main phenolic acid in all the PPEs, showing significantly higher levels in the pigmented varieties. CA (only absent in CCS1307), CoA (absent in 52.1-10 and CC1418) and FA appeared in lower proportions, than CGA, and in similar quantities in all the PPEs. CT and ECT were not detected in any of the PPEs by this method.
Based on the results of total anthocyanins content obtained by the differential pH method, only the two pigmented varieties, CS1418 and CCS1385, were analyzed by HPLC. Table 2 shows the anthocyanidin profiles found for both cultivars. In agreement with the monomeric anthocyanin quantification, PPE from CCS1385 showed the highest levels of anthocyanidins. Also, the anthocyanidin profile CCS1385 was more diverse than that of CS1418. Particularly, pelargonidin was the most abundant anthocyanin found in CS1418, and in a lower proportion peonidin and cyanidin. Instead, CCS1385 presented a completely different profile, with malvidin as the main anthocyanin, followed by peonidin and in similar quantities pelargonidin, delphinidin, and cyanidin.
Determination of antioxidant activity by the DPPH assay
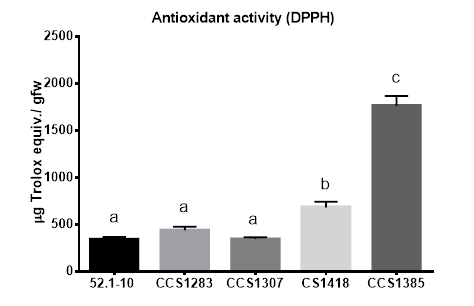
The antioxidant activity of the five PPEs was measured by the DPPH assay. This activity ranged from 344.71 ± 47.09 to 1765.73 ± 207.17 µg Trolox equiv. / gfw. Similarly to what was observed in the polyphenol quantification, both PPEs from pigmented varieties (CCS1385 and CS1418) showed high antioxidant activity, exhibiting 2 to 6 fold greater values than PPEs from non-pigmented varieties. The PPE from the purple variety (CCS1385) presented the highest antioxidant activity: 1765.73 ± 207.17 µg trolox equiv. / gfw. All PPEs from white or yellow fleshed varieties (52.1-10, CCS1283 and CCS1307) presented lower and similar values, showing no significant differences between them (Figure 3).
Correlation between different groups of polyphenols and antioxidant activity
Correlation between total phenolic compounds, flavan-3-ols, CGA, CA, FA, CoA and the antioxidant activity (DPPH) of the five PPEs was analyzed. Table 3 shows the R2 and p values for the correlation analyses. A positive and significant relationship was found between total phenolic compounds and antioxidant activity. Also, the analysis of the main phenolic acids presented in all varieties demonstrated that CGA and CA exhibited a significant correlation with the antioxidant activity (DPPH).
Cytotoxic activity of PPEs on human hepatocarcinoma cells
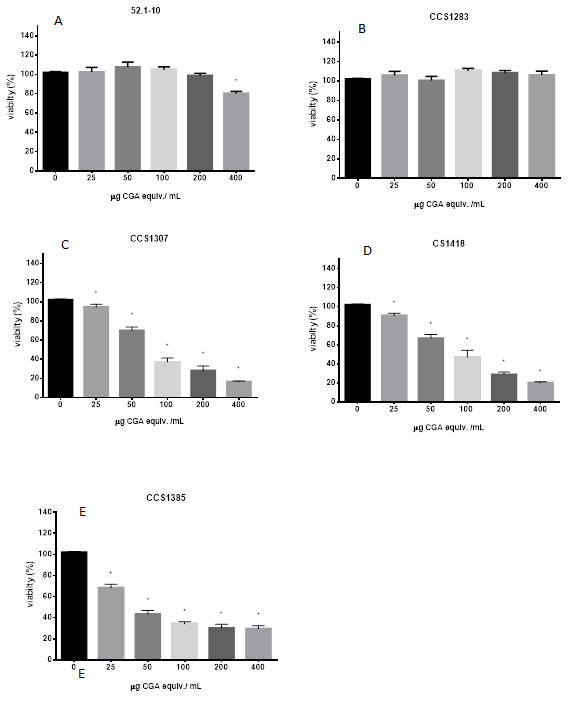
The cytotoxic activity of the five PPEs was evaluated on a human hepatocellular carcinoma cell line, Hep3B. Cells were treated with different concentrations of PPEs (0, 25, 50, 100, 200, and 400 µg CGA equiv./mL) for 24 h, and cytotoxicity was measured by the MTT assay. Figure 4 illustrates the viability rate of Hep3B cells treated with the PPEs. From the five studied PPEs, three of them, (CCS1307, CS1418, and CCS1385) resulted in a significant reduction of Hep3B cell viability in a concentration dependent manner after 24 h of treatment (Figure 4C, D and E). Cells treated with the maximum concentration of the 52.1-10 variety PPE showed low but significant cytotoxicity after 24 h treatment (Figure 4A). CCS1283 showed no differences in viability, compared to the control at any of the concentrations tested (Figure 4B). All the experiments were validated by the absence of cytotoxic effects in cells incubated with 30% methanol (extraction solvent), compared to non-treated cells. Similar results were observed on another human hepatocarcinoma cell line Huh-7 (data not shown). The cytotoxic concentration 50 (CC50) was calculated for the three cytotoxic PPEs; representing a low CC50 value a higher cytotoxic activity. CCS1385 presented the lowest CC50 = 37.28 µg CGA equiv. / mL, followed by CS1418 = 54.55 µg CGA equiv. / mL and CCS1307 = 66.71 µg CGA equiv. / mL. According to these results, the purple variety CCS1385, exhibited the highest cytotoxic activity by reducing cell viability in human malignant hepatocytes (Hep3B cell line), followed by the white fleshed variety CS1418 and the yellow fleshed variety CCS1307.
Polyphenols are a broad group of plant molecules, which main function is to protection from different types of stresses. Beside their relevance in plants, these compounds have been extensively studied for their beneficial effects on human health. Polyphenols can be incorporated to the diet by ingesting different fruits and vegetables, with potato being an important source of these compounds due to its vast consumption.
The results of this work showed that the content of total phenolics, total anthocyanins, and flavan-3-ols varied among the five studied S. tuberosum varieties (4 andigena and 1 tuberosum). Furthermore, the obtained data is in agreement with results of previous studies from this laboratory and other research groups [23, 25-27], finding that pigmented variety contained the highest levels of the different groups of polyphenolic compounds. A direct association between total phenolic content and the pigmentation of the tuber has been established in several reports; being the quantity of polyphenols higher in purple or red varieties than in yellow or white ones [28, 45, 46]. Similarly, flavan-3-ols and anthocyanin contents have been previously reported to be higher in purple tubers [19, 22, 23, 27].
Different factors can affect the polyphenolic content in the potato tuber, including genotype and type of tissue (peel or flesh), different growing location, method of extraction or sample preparation [47, 48]. Related to the mentioned factors, the non-pigmented varieties 52.1-10 (S. tuberosum ssp. tuberosum- industrial) and CCS1283 and CCS1307 (S. tuberosum ssp. andigena- Andean), presented similar quantities of compounds, suggesting that neither the genotype nor the growing location had a great effect on the polyphenol levels of these varieties. Peels and flesh of potato tubers might differ not only in quantity but also in the diversity of polyphenolic compounds present in them [22, 23, 45, 49]. Also, phenolic content in purple-fleshed varieties could be between 6 to 8 fold greater than in white or yellow fleshed ones; and this could be explained by the presence of both anthocyanins and phenolic acids [27]. This might explain the results observed for the purple variety CCS1385, which showed the highest levels of total phenolic, total anthocyanin, and flavan-3-ols content, probably due to the combination of peel and flesh used in these PPEs.
In accordance with previous studies from this and other groups [22, 23, 40, 45, 50]; HPLC analysis showed that CGA is the main phenolic acid present in the five studied PPEs, representing between 70-90 % of the total phenolic acids in EPPs. Also, pigmented varieties presented higher quantities of CGA, with CCS1385 containing the highest levels. The relationship between CGA content and tuber pigmentation has been well documented [28, 51]; with 8 to 22 fold higher CGA content observed in pigmented varieties. Moreover, the HPLC results showed that CA, CoA, and FA appear in a minimum proportion in relation to CGA in all the PPEs. In agreement with our results, CA was described as the second most abundant phenolic acid present in potato cultivars, showing higher levels in the pigmented clones than in non-pigmented clones [27, 49]. In contrast, previous studies in this lab showed that neither CT nor ECT could be detected in the PPEs [23]. This difference between the spectrophotometric quantification and the HPLC analysis might be due to the fact that only two compounds of this group were analyzed by HPLC. Finally, in agreement with previous studies [45, 52] the HPLC analysis performed for anthocyanins showed pelargonidin as the main anthocyanidin in red varieties and petunidin and malvidin as the main anthocyanin in the purple ones.
Antioxidant activity was evaluated by the DPPH method for all the PPEs. The results showed that pigmented varieties have higher antioxidant capacity, exhibiting 2 to 4 fold higher levels than those observed in non-pigmented ones. These results suggest that not only the phenolic content but also the presence of anthocyanin contribute to the antioxidant activity of PPEs. Also, the results showed a positive significant correlation between total phenolic, CGA and CA content and the antioxidant activity. This correlation between phenolic composition and the antioxidant activity was previously reported [21]. Furthermore, it has been reported that the antioxidant potential of pigmented cultivars can be 2 to 8 fold higher than that of non-pigmented ones, which can be explained by the presence of anthocyanins along with phenolic acids [21, 28]. The present study only included two pigmented varieties, making it impossible to calculate the statistical correlation between anthocyanins and antioxidant activity.
Plant polyphenol activity has been largely studied against several types of human cancers such as colon, prostate, and breast [53-55]. Particularly, numerous publications have reported the effects of PE from different fruits, vegetables and beverages against HCC [21, 56-58] but also, for many indigenous herbs used in traditional medicine [59-62]. Furthermore, the obtained results demonstrated that PPEs significantly reduced cell viability in a human HCC cell line, although the mechanisms by which PPEs exert their effect need to be determined. Cytotoxic and pro-apoptotic effects against Hep3B of different polyphenolic extracts from different sources like Iponema batatas Lam., green tea polyphenols and Vitex negundo, have been previously reported [63-65]. Furthermore, not only total phenolic extracts but also anthocyanidins and anthocyanin fractions from different plants have been studied for their anti-HCC activity [66, 67]. It is important to mention that the Hep3B cell line (analyzed in this work) is p53 defective, suggesting that PPEs may induce cell death by a p53 independent pathway.
The results of this work are the first that demonstrate the cytotoxic effect of PPEs in Hep3B cells. From the five studied PPEs, three of them reduced cell viability, with the purple variety CCS1385 exhibiting the strongest anti-HCC activity, followed by the white with red spots variety CS1418, and the non-pigmented variety CCS1307. In accordance with previous studies, the purple potatoes presented higher levels of phenolic compounds and antioxidant activity that correlated with higher anticancer effect [27]. The relevant cytotoxic activity of the potato varieties detected in Hep3B cells, justifies further analysis in other human malignant hepatocyte cell lines. Also, the mechanisms underlying PPE-induced hepatocyte cell death should be characterized in a future study.
There are numerous reports about effects of the PPE or their anthocyanin fraction from pigmented varieties against different types of cancer in vitro [38, 40, 68]. For example, the anthocyanin fraction from S. tuberosum L. var. Vitelotte is cytotoxic in cervical, breast, and prostate cancer cell lines [69]. Recently, it was described that anthocyanin from purple-fleshed potatoes significantly suppressed the proliferation of colon cancer stem cells with or without p53 expression [36]. However, it is noteworthy that treatment with CCS1307, a non-pigmented cultivar, resulted in cytotoxicity against the Hep3B cell line in a similar extent as pigmented varieties (CS1418 and CCS1385). Although cytotoxic activity has been mainly described for PPEs from pigmented varieties, there are some previous reports that showed anti-cancer effects of PPEs from white or yellow tubers [21, 48]. Furthermore, the cytotoxic effect of CCS1307 could not be explained based on spectrophotometric or HPLC characterization, as it presented similar quantities and phenolic acid profiles as the other two non-pigmented varieties. Taken together, these results indicate that other factors besides the presence of anthocyanins might be involved in the cytotoxic activity against Hep3B cells. These characteristics make CCS1307 an interesting variety to continue analyzing in order to find potential novel compounds with anti-HCC activity.
In conclusion, the phenolic acids were the main group of phenolic compounds in all the studied varieties, with CGA being the main compound in all the PPEs. Generally, pigmented potatoes presented higher levels of the different compounds than non-pigmented ones. A similar trend was observed for the antioxidant activity. Finally, three of the studied PPEs presented relevant anti-HCC activity, one of them being from a non-pigmented variety, suggesting that this activity might be driven by other compounds besides anthocyanins. Further studies are needed to determine which are the active compounds present in PPEs and to characterize the molecular pathways involved in the cytotoxic activity of PPEs on human malignant hepatocytes. Finally, these results demonstrate the biological activity of PPEs, and suggest their potential use as a source for new anti-HCC molecules.
We thank Dr. Daniel Caldiz (McCain Foods Limited) and MSc. Carolina de Lasa (McCain Argentina S.A.) for providing 52.1-10 tubers; MSc. Andrea Clausen and MSc. Ariana Digilio (Germplasm Bank, National Institute for Agricultural Technology, Balcarce, Argentina) for providing Andean tubers. We thank Lic. Patricia Suárez and Lic. Daniela Villamonte (Institute for Biological Research, Mar del Plata, Argentina) for technical assistantce in Andean tuber crop and HPLC-DAD measurements, respectiviey. We thank Ann Marie Greene for reading and editing the manuscript.
This work was financially supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (PICT 2010 Nº 511 and PICT 2014 Nº 1434); Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 2015 Nº 762) and Universidad Nacional de Mar del Plata (UNMdP) EXA 627//13 and EXA 720/15)
L.B. and A.B.A. are career members from CONICET. M.J.M. is a PhD fellow from CONICET, Argentina, directed by A.B.A and codirected by L.B.
|
Phenolic acid |
52.1-10 |
CCS 1283 |
CCS 1307 |
CC 1418 |
CCS 1385 |
|
CGA |
21.55 ± 2.86 a (71.29) |
17.44 ± 5.62 a (70.32) |
26.89 ± 8.52 a (77.54) |
143.27 ± 13.79b (96.01) |
390.44 ± 65.66 c (94.64) |
|
CA |
3.44 ± 0.75 a (11.38) |
2.09 ± 0.59 a (8.43) |
N/D |
3.52 ± 1.24 a (2.36) |
13.61 ± 1.33 b (3.3) |
|
CoA |
3.8 ± 0.38 a (12.57) |
4.33 ± 1.45 a (17.46) |
4.37 ± 0.48 a (12.6) |
N/D |
6.69 ± 2.34 a (1.61) |
|
FA |
1.44 ± 0.16 a (4.63) |
0.94 ± 0.29 a (3.79) |
1.42 ± 0.56 a (4.09) |
2.44 ± 1.09 a (1.64) |
1.8 ± 0.58 a (0.44) |
|
CT |
N/D |
N/D |
N/D |
N/D |
N/D |
|
ECT |
N/D |
N/D |
N/D |
N/D |
N/D |
|
TOTAL |
30.23 ± 4.15 |
24.8 ± 7.95 |
34.68 ± 9.56 |
149.23 ± 16.12 |
412.54 ± 69.97 |
Bjarklund, G. and Chirumbolo, S., Role of oxidative stress and anti-oxidants in daily nutrition and human health. Which suggestion may came from current literature?, Nutrition, 2016, 1-11.
Geleijnse, J.M. and Hollman, P.C., Flavonoids and cardiovascular health: which compounds, what mechanisms?, The American journal of clinical nutrition, 88 (2008), 12-13. PMid:18614717
PubMed/NCBIKing, J. C., and Slavin, J.L., White Potatoes, Human Health, and Dietary Guidance, 2013.
Medina-Rem?n, A., Tresserra-Rimbau, A., Pons, A., Tur, J.A., Martorell, M., Ros, E., et al., Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial, Nutrition, Metabolism and Cardiovascular Diseases, 25 (2015), 60-67 PMid:25315667
View Article PubMed/NCBITresserra-Rimbau, A., Guasch-Ferre, M., Salas-Salvado, J., Toledo, E., Corella, D., Castaner, O., et al., Intake of Total Polyphenols and Some Classes of Polyphenols Is Inversely Associated with Diabetes in Elderly People at High Cardiovascular Disease Risk , Journal of Nutrition, 146 (2016), 767-77. PMid:26962181
View Article PubMed/NCBIStagos, D., Amoutzias, G.D., Matakos, A., Spyrou, A., Tsatsakis, A.M. and Kouretas, D., Chemoprevention of liver cancer by plant polyphenols , Food and Chemical Toxicology, 50 (2012), 2155-70. PMid:22521445
View Article PubMed/NCBIYang, C.S., Landau, J.M., Huang, M.T. and Newmark, H.L., Inhibition of carcinogenesis by dietary polyphenolic compounds , Annual Review of Nutrition, 21 (2001), 381-406. PMid:11375442
View Article PubMed/NCBIDaglia, M., Polyphenols as antimicrobial agents , Current Opinion in Biotechnology, 23 (2012), 174-81. PMid:21925860
View Article PubMed/NCBIKim, H.S., Quon, M.J. and Kim, J., New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate , Redox Biology, 2 (2014), 187-95. PMid:24494192
View Article PubMed/NCBILee, Y.H., Kwak, J., Choi, H.K., Choi, K.C., Kim, S., Lee, J., et al., EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity, International Journal of Molecular Medicine, 30 (2012), 69-74. PMid:22505206
PubMed/NCBIBandyopadhyay, G., Biswas, T. , Roy, K.C., Mandal, S., Mandal, C., Pal, B.C., Chlorogenic acid inhibits Bcr-Abl tyrosine kinase and triggers p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemic cells , Blood, 104 (2004), 2514-22. PMid:15226183
View Article PubMed/NCBIShih, P.H., Yeh, C.T. and Yen, G.C., Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. , Food and chemical toxicology?: an international journal published for the British Industrial Biological Research Association, 43 (2005), 1557-66. PMid:15964118
View Article PubMed/NCBISwaminathan, K., Patrick M. and Downard K.M., Substituent effects on the binding of natural product anthocyanidin inhibitors to influenza neuraminidase with mass spectrometry , Analytica Chimica Acta, 828 (2014), 61-69. PMid:24845816
View Article PubMed/NCBIWang, G. F., Li P.S., Yu D. R., Qun F. L., Hou F. L., Ru J. Z., et al., Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro , Antiviral Research, 83 (2009), 186-90. PMid:19463857
View Article PubMed/NCBIFriedman, M., Chemistry, Biochemistry, and Dietary Role of Potato Polyphenols. A Review , 1997.
Zaheer, K. and Akhtar M.H., Potato Production, Usage, and Nutrition?A Review , Critical Reviews in Food Science and Nutrition, 56 (2016), 711-21. PMid:24925679
View Article PubMed/NCBICamire, M. E., Kubow S., and Donnelly, D.J., Potatoes and Human Health, Advances in Potato Chemistry and Technology: Second Edition, 2009, 685-704
Ezekiel, R., Singh N., Sharma S., and Kaur A., Beneficial phytochemicals in potato - a review , Food Research International, 50 (2011), 487-96.
View ArticleReyes, L.F., Miller, J.C.and Cisneros-Zevallos L., Antioxidant Capacity , Anthocyanins and Total Phenolics in Purple- and Red-Fleshed Potato (Solanum tuberosum L .) Genotypes , American Journal of Potato Research, 82 (2005), 271-77.
View ArticleVisvanathan, R., Jayathilake, C., Chaminda, B. and Liyanage, R., Health-beneficial properties of potato and compounds of interest, J Sci Food Agric, 2015.
Wang, Q., Chen, Q., He, M., Mir, P., Su, J. and Yang, Q., Inhibitory Effect of Antioxidant Extracts From Various Potatoes on the Proliferation of Human Colon and Liver Cancer Cells , Nutrition and Cancer, 63 (2011), 1044-52. PMid:21888504
View Article PubMed/NCBIVali-as, Mat?as Ariel, Mar?a Luciana Lanteri, Arjen Ten Have, y Adriana Balbina Andreu, Chlorogenic Acid Biosynthesis Appears Linked with Suberin Production in Potato Tuber (Solanum tuberosum) , Journal of Agricultural and Food Chemistry, 63 (2015), 4902-13. PMid:25921651
View Article PubMed/NCBIVali-as, M. Ar., Lanteri, M. L., Ten Have, A. and Andreu, A. B., Chlorogenic acid, anthocyanin and flavan-3-ol biosynthesis in flesh and skin of Andean potato tubers (Solanum tuberosum subsp. andigena) . Food Chem. (2017) 15;229:837-846. PMid:28372251
View Article PubMed/NCBITejeda, L., Alvarado, J. A., Debiec, M., Penarrieta, J. M. , Cardenas, O., Alvarez, M. T., et al., Relating genes in the biosynthesis of the polyphenol composition of Andean colored potato collection. , Food Science & Nutrition (Hoboken, NJ, United States), 2 (2014), 46-57.
View ArticleAlbishi, T., John, J. A., Al-Khalifa, A. S. and Shahidi, F., Phenolic content and antioxidant activities of selected potato varieties and their processing by-products , Journal of Functional Foods, 5 (2013).
View ArticleHale, A. L., Reddivari, L., Nzaramba, M. N., Bamberg, J. B. and Miller, J. C., Interspecific variability for antioxidant activity and phenolic content among Solanum species , American Journal of Potato Research, 85 (2008), 332-41.
View ArticleMadiwale, G.P., Reddivari, L. , Holm, D.G. and Vanamala, J., Storage elevates phenolic content and antioxidant activity but suppresses antiproliferative and pro-apoptotic properties of colored-flesh potatoes against human colon cancer cell lines , Journal of Agricultural and Food Chemistry, 59 (2011), 8155-66. PMid:21736387
View Article PubMed/NCBIStushnoff, C., Holm, D., Thompson, M. D. , Jiang, W., Thompson, H. J., Joyce, N. I., et al., Antioxidant properties of cultivars and selections from the Colorado potato breeding program , American Journal of Potato Research, 85 (2008), 267-76.
View ArticleFeitelson, M. A., Sun, B. , Tufan, N.L.S., Liu, J., Pan, J. and Lian, Z., Genetic mechanisms of hepatocarcinogenesis. , Oncogene, 21 (2002), 2593-2604. PMid:11971194
View Article PubMed/NCBIOkuda, K., Hepatocellular carcinoma , 32 (2000), 225-31.
Rasool, M., Rashid, S., Arooj, M., Ansari, S.A., Khan, K.M., Malik, A., et al., New possibilities in hepatocellular carcinoma treatment, Anticancer Research, 34 (2014), 1563-72. PMid:24692683
PubMed/NCBISong, M. J., and Bae, S.H., Newer treatments for advanced hepatocellular carcinoma , Korean Journal of Internal Medicine, 29 (2014), 149-55. PMid:24648795
View Article PubMed/NCBIPark, H.S, Park, K. I., Lee, D. H., Kang, S. R., Nagappan, A., Kim, J. A., et al., Polyphenolic extract isolated from Korean Lonicera japonica Thunb. induce G2/M cell cycle arrest and apoptosis in HepG2 Cells: Involvements of PI3K/Akt and MAPKs , Food and Chemical Toxicology, 50 (2012), 2407-16. PMid:22561682
View Article PubMed/NCBIWang, H. C., Chung, P. J., Wu, C. H., Lan, K. P., Yang, M. Y. and Wang, C. J., Solanum nigrum L. polyphenolic extract inhibits hepatocarcinoma cell growth by inducing G2/M phase arrest and apoptosis , Journal of the Science of Food and Agriculture, 91 (2011), 178-85. PMid:20853273
View Article PubMed/NCBIYang, X. R., Wang, Y.Y., La, K.K., Peng, L., Song, X. H., Shi, X.G., et al., Inhibitory effects of cocoa tea (Camellia ptilophylla) in human hepatocellular carcinoma HepG2 in vitro and in vivo through apoptosis , Journal of Nutritional Biochemistry, 23 (2011), 1051-57. PMid:22018604
View Article PubMed/NCBICharepalli, V., Reddivari, L., Radhakrishnan, S., Vadde, R., Agarwal, R., and Vanamala, J. K. P., Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells , Journal of Nutritional Biochemistry, 26 (2015), 1641-49. PMid:26383537
View Article PubMed/NCBIFriedman, M., Lee, K.R., Kim, H. J., Lee, I.S., and Kozukue, N., Anticarcinogenic effects of glycoalkaloids from potatoes against human cervical, liver, lymphoma, and stomach cancer cells , Journal of Agricultural and Food Chemistry, 53 (2005), 6162-69. PMid:16029012
View Article PubMed/NCBIHayashi, K., Hibasami, H., Murakami, T., Terahara, N., Mori, M., and Tsukui, A., Induction of apoptosis in cultured human stomach cancer cells by potato anthocyanins and its inhibitory effects on growth of stomach cancer in mice , Food Science and Technology Research, 12 (2006), 22-26.
View ArticleOmbra, M. N., Fratianni, F., Granese, T., Cardinale, F., Cozzolino, A., and Nazzaro, F., In vitro antioxidant, antimicrobial and anti-proliferative activities of purple potato extracts ( Solanum tuberosum cv Vitelotte noire) following simulated gastro-intestinal digestion , Natural Product Research, 29 (2014), 1087-91. PMid:25420792
View Article PubMed/NCBIReddivari, L., Vanamala, J., Chintharlapalli, S., Safe, S.H., and Miller, J. C., Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways , Carcinogenesis, 28 (2007), 2227-35. PMid:17522067
View Article PubMed/NCBICampos, D., Noratto, G., Chirinos, R., Arbizu, C., Roca, W. and Cisneros-Zevallos, L., Antioxidant capacity and secondary metabolites in four species of Andean tuber crops: native potato (Solanum sp.), mashua (Tropaeolum tuberosum Ruiz & Pav?n), Oca (Oxalis tuberosa Molina) and ulluco (Ullucus tuberosus Caldas) , Journal of the Science of Food and Agriculture, 86 (2006), 1481-88.
View ArticleChirinos, R., Campos, D., Arbizu, C., Rogez, H., Rees, J.F., Larondelle, Y., et al., Effect of genotype, maturity stage and post-harvest storage on phenolic compounds, carotenoid content and antioxidant capacity, of Andean mashua tubers (Tropaeolum tuberosum Ruiz & Pav?n) , Journal of the Science of Food and Agriculture, 87 (2007), 437-46.
View ArticleGiusti, M.M. and Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy, en Current Protocols in Food Analytical Chemistry (Hoboken, NJ, USA: John Wiley & Sons, Inc., 2001).
View ArticleReddivari, L., Hale, A. L.and Miller, J. C. Determination of phenolic content, composition and their contribution to antioxidant activity in specialty potato selections. Am. J. Potato Res. 2007, 84, 275?282.
View ArticleJi, X., Rivers, L., Zielinski, Z., M. Xu, E. MacDougall, J. Stephen, et al., Quantitative analysis of phenolic components and glycoalkaloids from 20 potato clones and in vitro evaluation of antioxidant, cholesterol uptake, and neuroprotective activities , Food Chemistry, 133 (2012), 1177-87.
View ArticleLee, S. H., Oh, S.H., Hwang, I.G., Kim, H. Y., Woo, K.S., Woo, S.H., et al., Antioxidant contents and antioxidant activities of white and colored potatoes (Solanum tuberosum L.) , Preventive Nutrition and Food Science, 21 (2016), 110-16. PMid:27390727
View Article PubMed/NCBIMadiwale, G. P., Reddivari, L., Stone, M., Holm, D.G. and Vanamala, J., Combined effects of storage and processing on the composition and anti-cancer properties of color-fleshed potatoes in vitro , Journal of agricultural and food chemistry, Just Accep (2012), A-I.
Zuber, T., Holm, D., Byrne, P., Ducreux, L., Taylor, M., Kaiser, M ., et al., Optimization of in vitro inhibition of HT-29 colon cancer cell cultures by Solanum tuberosum L. extracts., Food & function, 6 (2014), 72-83. PMid:25338312
PubMed/NCBIDeu?er, H., Guignard, C., Hoffmann, L.and Evers, D., Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg , Food Chemistry, 135 (2012), 2814-24. PMid:22980877
View Article PubMed/NCBIDao, L, and Friedman, M., Chlorogenic Acid Content of Fresh and Processed Potatoes Determined by Ultraviolet Spectrophotometry , Journal of Agricultural and Food Chemistry, 40 (1992), 2152-56.
View ArticleNavarre, D.A., Pillai, S.S., Shakya, R., and Holden, M.J., HPLC profiling of phenolics in diverse potato genotypes , Food Chemistry, 127 (2011), 34-41.
View ArticleBrown, C.R., Culley, D., Yang, C.P., Durst, R.and Wrolstad, R., Variation of Anthocyanin and Carotenoid Contents and Associated Antioxidant Values in Potato Breeding Lines , Journal of the American Society for Horticultural Science, 130 (2005), 174-80.
Nzaramba, M.N., Reddivari, L., Bamberg, J.B. and Miller, J.C., Antiproliferative activity and cytotoxicity of Solanum jamesii tuber extracts on human colon and prostate cancer cells in vitro. , Journal of agricultural and food chemistry, 57 (2009), 8308-15. PMid:19711917
View Article PubMed/NCBISahpazidou, D., Geromichalos, G.D., Stagos, D., Apostolou, A., Haroutounian, S.A., Tsatsakis A.M., et al., Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells , Toxicology Letters, 230 (2014), 218-24. PMid:24508987
View Article PubMed/NCBIThakur, V.S., Gupta, K. and Gupta, S., Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases , Carcinogenesis, 33 (2012), 377-84. PMid:22114073
View Article PubMed/NCBIChu, Y.F., Sun, J., Wu, X.Z., and Liu, R.H., Antioxidant and antiproliferative activities of common vegetables , Journal of agricultural and food chemistry, 50 (2002), 6910-16. PMid:12405796
View Article PubMed/NCBIRamos, S., Alia, M., Bravo, L. and Goya, L., Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (HepG2) , Journal of Agricultural and Food Chemistry, 53 (2005), 1271-80. PMid:15713052
View Article PubMed/NCBISun, J., Chu, Y.F., Wu, X. and Liu, R.H., Antioxidant and Antiproliferative Activities of Common Fruits , 2002
Naowaratwattana, W., De-eknamkul, W. and Gonzalez De Mejia, E., Phenolic-Containing Organic Extracts of Mulberry (Morus alba L.) Leaves Inhibit HepG2 Hepatoma Cells Through G2/M Phase Arrest, Induction of Apoptosis, and Inhibition of Topoisomerase IIa Activity, J Med Food, 13(5), 1045-1056. PMid:20828312
View Article PubMed/NCBISakulnarmrat, K., Fenech, M., Thomas, P. and Konczak, I. Cytoprotective and pro-apoptotic activities of native Australian herbs polyphenolic-rich extracts , Food Chemistry, 136 (2013), 9-17. PMid:23017386
View Article PubMed/NCBISawadogo, W.R., Schumacher, M., Teiten, M.H., Dicato, M. and Diederich, M., Traditional West African pharmacopeia, plants and derived compounds for cancer therapy , Biochemical Pharmacology, 84 (2012), 1225-40. PMid:22846603
View Article PubMed/NCBIYang, M.Y., Hsu, L.S., Peng, C.H., Shi, Y.S., Wu, C.H. and Wang, C.J., Polyphenol-rich extracts from solanum nigrum attenuated PKC alpha-mediated migration and invasion of hepatocellular carcinoma cells , Journal of Agricultural and Food Chemistry, 58 (2010), 5806-14. PMid:20349911
View Article PubMed/NCBIGuha, G., Rajkumar, V. and Ashok Kumar, R., Polyphenolic constituents of methanolic and aqueous extracts of Vitex negundo render protection to Hep3B cells against oxidative cytotoxicity , Food and Chemical Toxicology, 48 (2010), 2133-38. PMid:20472016
View Article PubMed/NCBILin, W., and Tongyi, S., Role of Bax/Bcl-2 family members in green tea polyphenol induced necroptosis of p53-deficient Hep3B cells , Tumor Biology, 35 (2014), 8065-75. PMid:24839007
View Article PubMed/NCBIL?, S., Lin, C. and Xu, P. Screening of the anti-tumor active fraction from Ipomoea batatas Lam. (cv.simon) leaves., Journal of Central South University. Medical sciences, 40 (2015), 499-503.
Shin, D.Y., Ryu, C.H., Lee, W.S., Kim, D.C., Kim, S.H., Hah, Y.S., et al., Induction of apoptosis and inhibition of invasion in human hepatoma cells by anthocyanins from meoru , Annals of the New York Academy of Sciences, 1171 (2009), 137-48. PMid:19723048
View Article PubMed/NCBIYeh, C.T. and Yen, G.C., Induction of apoptosis by the Anthocyanidins through regulation of Bcl-2 gene and activation of c-Jun N-terminal kinase cascade in hepatoma cells. , Journal of agricultural and food chemistry, 53 (2005), 1740-49. PMid:15740068
View Article PubMed/NCBIFeng, R., Wang, S.Y., Shi, Y.H., Fan, J. and Yin, X.M., Delphinidin induces necrosis in hepatocellular carcinoma cells in the presence of 3-methyladenine, an autophagy inhibitor , Journal of Agricultural and Food Chemistry, 58 (2010), 3957-64. PMid:20025272
View Article PubMed/NCBIBontempo, P., Carafa, V., Grassi, R., Basile, A., Tenore, G., Formisano, C., et al., Antioxidant, antimicrobial and anti-proliferative activities of Solanum tuberosum L. var. Vitelotte. , Food and chemical toxicology?: an international journal published for the British Industrial Biological Research Association, 55 (2013), 304-12. PMid:23313609
View Article PubMed/NCBI