CORRESPONDENCE AUTHOR: Dr. Sadhana Ravishankar
Address: 1117 E. Lowell Street, Tucson, Arizona 85721; Tel: United States (520)-626-1499; FAX: United States (520)-621-6366;
E-mail: sadhravi@email.arizona.edu
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 1 ISSUE: 2
Page No: 51-59
CORRESPONDENCE AUTHOR: Dr. Sadhana Ravishankar
Address: 1117 E. Lowell Street, Tucson, Arizona 85721; Tel: United States (520)-626-1499; FAX: United States (520)-621-6366;
E-mail: sadhravi@email.arizona.edu
Xeeroy Rada1, Jennifer Todd-Searle1, Mendel Friedman2, Jitendra Patel3, Divya Jaroni4 and Sadhana Ravishankar1 *
1 School of Animal and Comparative Biomedical Sciences, University of Arizona, 1117 E. Lowell Street, Tucson, Arizona 85721, USA
2 USDA-ARS, Western Regional Research Center, Albany, California 94710, USA
3 USDA-ARS, Beltsville Agricultural Research Center, Beltsville, Maryland 20705, USA
4 Department of Animal Science, Oklahoma State University, Stillwater, OK 74074, USA
Andrei Baiceanu(Baiceanu.Andrei@umfcluj.ro)
Prof. Raj K. Lal(rk.lal@cimap.res.in)
Sadhana Ravishankar, Combining essential oils and olive extract for control of multi-drug resistant Salmonella enterica on organic leafy greens(2016)SDRP Journal of Food Science & Technology 1(2)
Organic fresh produce sales have been increasing in recent years and ensuring safety of produce is important. We investigated the combined antimicrobial effects of plant essential oils and olive extract against Salmonella on organic leafy greens. Organic baby spinach, mature spinach, romaine lettuce, and iceberg lettuce were inoculated with antibiotic-resistant Salmonella Newport and dip-treated in phosphate buffered saline, 3.0% hydrogen peroxide, 0.1% cinnamon leaf oil and 3.0% olive extract or 0.1% oregano oil and 3.0% olive extract combination treatments. Leaves were sampled on days 0, 1, and 3 for enumeration. Treatment with both antimicrobials induced reductions in Salmonella population of up to 3.5-4 logs and 3-4.4 logs CFU/g on baby spinach and romaine lettuce, respectively. Salmonella population was reduced by 3 logs CFU/g on mature spinach. Cinnamon leaf oil and olive extract treatment yielded about 3.0 logs, while oregano oil and olive extract treatment yielded about 3.5 logs CFU/g reduction on iceberg lettuce. Compared to previously reported treatments with individual antimicrobials, the combination treatments had greater antimicrobial effect. The results showed that combination treatments involving essential oils and olive extract are a potential option for use as produce washes.
Nontyphoidal Salmonella species are associated with foodborne illnesses, causing approximately 11% of domestically acquired cases, 35% of hospitalizations, and 28% of deaths (CDC 2011). Salmonella serotypes isolated from human infections include Typhimurium, Enteritidis, and Newport. S. Newport has become increasingly frequent since 1995 (CDC 2013). In 2010, S. Newport was attributed to a massive, multistate outbreak associated with raw alfalfa sprouts which led to product recalls (CDC 2010). S. Typhimurium and S. Newport caused an outbreak owing to contaminated cantaloupes that resulted in 261 cases and 3 deaths in 2012 (CDC 2012).
Between 1988 and 2007, nearly 40% of foodborne outbreaks resulting from contaminated produce consumption were caused by Salmonella species (Greig and Ravel 2009). The risk of pathogen transmission from contaminated produce is greater because most produce is consumed raw with very little processing or terminal treatment. Safety considerations become even more important because of the increased demand by consumers for organic produce (Williams and Hammitt 2001). An increase in demand suggests the possibility of increased consumption of organic produce, which might increase the risk due to bacterial contamination of raw organic produce. Organic food sales in the United States are estimated to be $27 billion in 2012, with fresh produce as the top-selling organic category (Osteen et al. 2012). To qualify as organic, fruits and vegetables must be grown and harvested on farms that have not used synthetic pesticides, herbicides, and/or fertilizer (Forman and Silverstein 2012). Foodborne outbreaks associated with Salmonella, an increase in organic consumption, and the shortage of effective treatment options in the organic industry make controlling the spread of Salmonella on organic fresh produce a challenging problem.
Essential oils and extracts derived from plants have been useful as antimicrobial agents to reduce pathogens on contaminated produce (Du et al. 2009). Studies using carvacrol, the major component of oregano oil, have shown that it has antimicrobial activity against Gram positive and Gram negative bacteria (Friedman et al. 2002; Veldhuizen et al. 2007). Moore-Neibel et al. (2013) found that oregano oil was effective against S. Newport on organic leafy greens and Ravishankar et al. (2010) found that cinnamaldehyde, the antimicrobial component in cinnamon leaf oil, and carvacrol, inactivated Salmonella serovars in vitro and S. Newport on celery and oysters.
In the processing of fresh produce from harvesting to consumption, few treatments with natural antimicrobials are available for organic produce to prevent the spread of foodborne pathogens. Essential oils and plant extracts have been evaluated for their antimicrobial properties in order to provide an alternative treatment option for reducing the number of pathogens on fresh fruits and vegetables. The present study compares the antimicrobial efficacy of cinnamon and oregano essential oils in combination with a commercial olive extract against antibiotic-resistant Salmonella Newport on organic leafy greens. Because the combination of oregano with thyme at a lower concentration was reported to be more effective than the essential oils used separately at higher concentrations (Gutierrez et al. 2008), and the MIC value reported for the individual phenolic compounds was reduced up to 75% when applied as binary combinations (Gutierrez-Fernandez et al. 2013), it was of interest to find out whether the combinations evaluated in the present study would show similar beneficial antimicrobial effects.
Oregano oil, olive extract, and cinnamon leaf oil are all Generally Recognized as Safe (GRAS) for human consumption as approved by the USDA – National Organic Program and can be used as organic antimicrobials (Periago and Moezelaar 2001; Tzortzakis 2009; USDA-NOP 2011). The objective of this study was therefore, to assess the effectiveness of cinnamon leaf oil and olive extract as well as oregano oil and olive extract combinations as a wash treatment against S. Newport on organic leafy greens.
Bacterial Culture and Media
A multi-drug resistant Salmonella enterica serovar Newport LAJ160311 (JJPX01.0014 PulseNet PFGE profile) provided by Dr. Lynn Joens, University of Arizona, Tucson, Arizona was used in this study. This strain is resistant to amoxicillin-clavulanic acid, ampicillin, cefoxitin, chloramphenicol, streptomycin, and tetracycline (Ravishankar et al. 2010). Stock culture of the organism was maintained in cryovials (MicrobankTM Austin, TX, USA.) at –80 °C and activated by transferring 100 µL into tryptic soy broth with 0.6% yeast extract (TSBYE; Difco, Becton Dickinson, Sparks, MD, USA.). The bacterial cultures were maintained in TSBYE at 4°C with weekly transfers. Overnight cultures were made for each experiment by inoculating tryptic soy broth (TSB, Difco, Becton Dickinson, Sparks, MD, USA) and incubating at 37°C for 18–22 hours. The population of overnight culture was adjusted to 6 log CFU/ml in buffered peptone water (BPW, Difco, Becton Dickinson) for all of the experiments, which was confirmed by plating.
Antimicrobials and Food Products
The cinnamon essential oil (100% pure cinnamon leaf oil) was obtained from Olive Nation (Charleston, MA, USA). The oregano essential oil, from pure Origanum vulgare, was obtained from Lhasa Karnak Company (Berkeley, CA, USA). Commercial Hidrox™-12 olive extract powder (consisting of 12% olive phenolics), prepared by freeze-drying the juice of organic California-grown olives was a gift of the manufacturer (CreAgri Inc., Hayward, CA, USA). Hydrogen peroxide (3%) was purchased from the local grocery store and used as control.
Dip solutions of 0.1% cinnamon leaf oil and 3.0% olive extract combination and 0.1% oregano oil and 3.0% olive extract combination were prepared in sterile phosphate buffered saline (PBS, pH 7) and thoroughly mixed using a stomacher (Seward, London, UK) at normal speed (230 paddle speed/min) for 30 seconds prior to use. Organic leafy greens, including baby spinach, mature spinach, romaine lettuce, and iceberg lettuce, were purchased from a local organic grocery store on the morning of experimental trials.
Antimicrobial Activities of Cinnamon Essential Oil and Olive Extract Combination and Oregano Essential Oil and Olive Extract Combination Against S. Newport on Organic Leafy Greens
All leafy greens were washed with deionized water three times and separated into 10 g samples. Following this, the samples were placed under UV light (254 nm) in a biohood for 30 minutes to help reduce existing background microflora. The samples were then dip inoculated for 2 min in the BPW solution containing 6 log CFU/ml of S. Newport, and dried for 30 minutes in the biohood for bacterial attachment to the leafy greens. Samples were then washed in one of the following: a) cinnamon leaf oil (0.1% vol./vol.) and olive extract (3% wt./vol.) combination treatment, b) oregano oil (0.1% vol./vol.) and olive extract (3% wt./vol.) combination treatment, c) PBS, or d) hydrogen peroxide (3%) solution for 2 min with gentle agitation. The samples were placed in stomacher bags and refrigerated at 4°C. Sampling was performed on days 0, 1, and 3. Ninety ml of BPW was placed in the stomacher bags containing 10 g samples. Samples were mixed using a stomacher for 1 min, serially diluted, and plated on xylose lysine desoxycholate agar (XLD, Difco, Becton Dickinson). Enumeration of S. Newport was performed after 24 h incubation at 37°C. Each experiment was repeated three times for each leafy green.
Statistical analysis
A randomized complete block design with three replicates per treatment was used. Colony counts recorded at each sampling time were converted to log CFU/g. Data were analyzed by two-way analysis of variance using Proc Mixed (SAS 9.3, SAS institute, Cary, NC) for interaction effects of treatment and sampling period. Means were compared with Sidak adjusted P values (< 0.05).
Reductions of S. Newport on Samples Treated with the Cinnamon Essential Oil and Olive Extract Combination
The Salmonella population in inoculated untreated control (positive control) samples of baby spinach, mature spinach, and iceberg lettuce remained at about 5.0 log CFU/g throughout days 0, 1, and 3 of storage. Salmonella populations in inoculated untreated control samples of romaine lettuce were initially 4.75 log CFU/g and decreased to 3.5 log CFU/g by day 3.
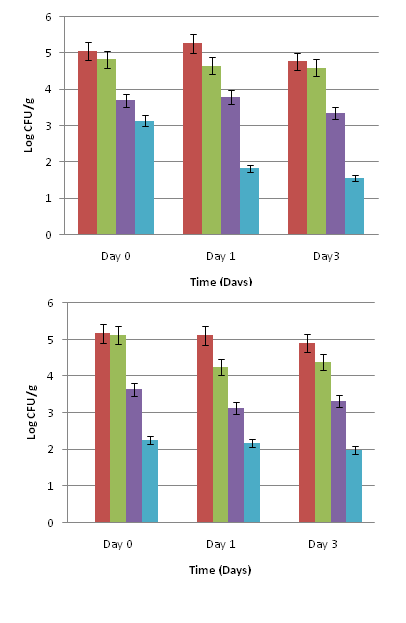
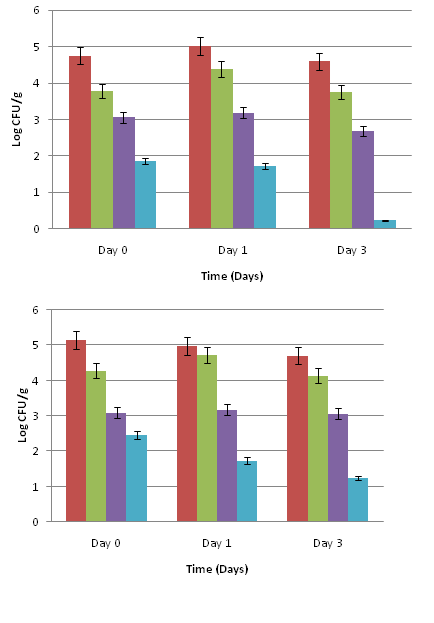
Salmonella on baby spinach leaves (Figure 1 A) exhibited a significant 1.92 log CFU/g reduction when exposed to 0.1% cinnamon leaf oil and 3.0% olive extract combination treatment on day 0 (P<0.05) when compared to the inoculated untreated control. By days 1 and 3, there was a significant 3.0 to 3.5 log CFU/g reduction in Salmonella population after treatment with 0.1% cinnamon leaf oil and 3 % olive extract combinations (P<0.05). The Salmonella population on leaves treated with the PBS control consistently remained at about 4.5 log CFU/g on days 0, 1, and 3. Baby spinach exposed to the hydrogen peroxide treatment showed about 1.5 log CFU/g reduction in Salmonella population on day 0 (P<0.05), which remained consistent on days 1 and 3. Mature spinach leaves (Figure 1 B) treated with 0.1% cinnamon leaf oil and 3.0% olive extract had a significant 2.91 log CFU/g reduction in Salmonella population on day 0, which remained consistent up to day 3 when compared to the inoculated untreated control (P<0.05). Salmonella on leaves exposed to the PBS control remained at about 4 to 5 log CFU/g on days 0, 1, and 3. The hydrogen peroxide treatment showed about 1.5 to 2.0 log CFU/g reductions in Salmonella population on days 0, 1, and 3.
For romaine lettuce leaves (Figure 2 A), there was a significant initial reduction of 2.31 log CFU/g in Salmonella population which further reduced by days 1 and 3, when exposed to the 0.1% cinnamon leaf oil and 3.0% olive extract combination treatment compared to the inoculated untreated control (P<0.05). Salmonella was reduced by about 0.5 log CFU/g in PBS control and 2.0 log CFU/g with hydrogen peroxide and remained unchanged on days 0, 1, and 3.
On iceberg lettuce leaves (Figure 2 B), there was a significant 2.75 to 3.06 log CFU/g reduction in Salmonella population on days 0, 1, and 3 after treatment with the 0.1% cinnamon leaf oil and 3.0% olive extract combination (P<0.05). The reductions in Salmonella on leaves treated with the PBS control and hydrogen peroxide were about 0.5 and 2.0 log CFU/g, respectively. No further reduction was observed during subsequent storage for 3 days.
Reductions of S. Newport on Samples Treated with the Oregano Essential Oil and Olive Extract Combination
The Salmonella population in inoculated untreated control samples of baby spinach, mature spinach, and iceberg lettuce remained at about 5.0 log CFU/g throughout days 0,1,and 3. For romaine lettuce, Salmonella population in the inoculated untreated control was about 4.5 to 5.0 log CFU/g throughout the 3 days of storage.
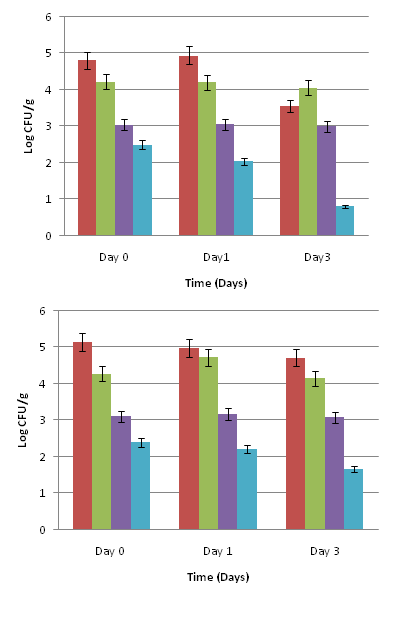
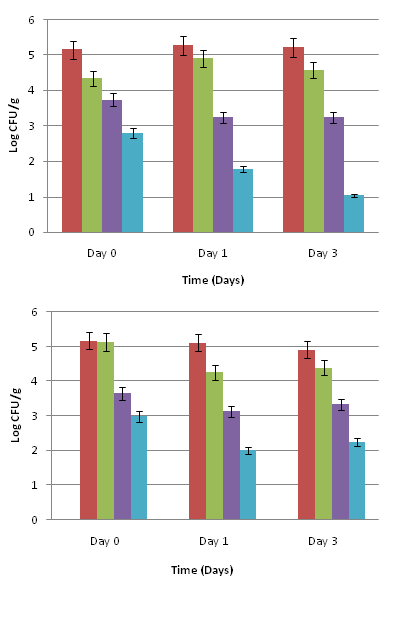
For baby spinach leaves (Figure 3 A), there was an initial significant reduction of 2.35 log CFU/g in Salmonella population when exposed to the 0.1% oregano oil and 3.0% olive extract combination treatment in comparison to the inoculated untreated control (P<0.05). Salmonella was further reduced by 3.49 log CFU/g (P<0.05) and 4.17 log CFU/g (P<0.05) on days 1 and 3, respectively. Baby spinach leaves treated with the PBS control had about 0.5 log CFU/g reduction on days 0, 1, and 3. The hydrogen peroxide treatment yielded about 1.5-2.0 log CFU/g reduction.
On mature spinach leaves, a 2.21 log CFU/g reduction in Salmonella population (P<0.05) was observed when exposed to 0.1% oregano oil and 3.0% olive extract combination treatment on day 0 (Figure 3 B). By days 1 and 3, there was a 3.11 and 2.67 log CFU/g reduction, respectively in Salmonella population (P<0.05). The PBS control yielded almost no reduction on day 0, and about a 0.5 log CFU/g reduction in Salmonella population by day 3. Mature spinach exposed to the hydrogen peroxide treatment showed about 1.53 log CFU/g reduction on day 0, and 1.99 and 1.58 log CFU/g reductions in Salmonella population on day 1 and day 3, respectively.
On romaine lettuce leaves (Figure 4 A), there was a 2.89 to 4.36 log CFU/g reduction in Salmonella population on days 0 to 3, after treatment with the 0.1% oregano oil and 3.0% olive extract combination (P<0.05). Leaves treated with the PBS control showed 0.5 to 0.8 log CFU/g reduction in Salmonella population during 3 days of storage. Romaine lettuce treated with the hydrogen peroxide had about 1.92 log CFU/g reduction in Salmonella population by day 3.
Iceberg lettuce leaves (Figure 4 B) exposed to 0.1% oregano oil and 3.0% olive extract had about 2.69 log CFU/g reduction in Salmonella population on day 0 when compared to the inoculated untreated control (P<0.05). There was about a 3.24 to 3.47 log CFU/g reduction on days 1 and 3. The PBS control had a 0.57 log reduction in Salmonella population by day 3. The hydrogen peroxide treatment showed an initial 2.04 log CFU/g reduction in Salmonella population on day 0, which decreased slightly by days 1 and 3.
The consumption of fresh fruits and vegetables has increased in the United States over the past two decades (Pollack 2001). With the increase in produce consumption, there has also been an increase in the number of foodborne illnesses in the United States (Sivapalasingam et al. 2004). Documented foodborne outbreaks are associated with the contamination of fresh produce, which may occur in the field, during initial processing, or during food preparation in the kitchen (Lynch et al. 2009). The high risk of foodborne contamination during the farm to fork continuum makes the understanding of growth and survival of such pathogens extremely important. We believe that the reason why the combination of essential oils and the olive extract were expected to show increased activity is because they act by different mechanisms; the oils disrupt cell membranes and the phenolic compounds in the olive extract probably act mainly by antioxidative effects.
Effectiveness of Combination Treatments
Both the cinnamon leaf oil and olive extract combination and oregano oil and olive extract combination treatments used in this study were effective in reducing a single strain of antibiotic-resistant S. Newport on all leafy greens. The combination treatments were more effective when compared to cinnamon leaf oil, oregano oil, and olive extract used individually described in our previous studies. Cinnamon leaf oil at 0.1%, oregano oil at 0.1%, and olive extract at 3% resulted in less than 1.0 log, about 1 log and 2.3 log CFU/g reduction, respectively, when compared to the PBS control for organic leafy greens after 3 days of storage (Todd et al. 2013; Moore-Neibel et al. 2013; Moore et al. 2011). With the 0.1% cinnamon leaf oil and 3.0% olive extract combination used in the present study, there was an initial 1.9 – 2.9 log CFU/g reduction on all leafy greens, which increased to 2.9 – 3.9 log CFU/g by day 3. In the present study, a 2.2 – 2.8 log CFU/g reduction was seen on day 0 for the oregano oil and olive extract combination which increased to about 4.0 log CFU/g after 3 days of treatment.
Although higher concentrations of cinnamon leaf oil (0.3% or higher), oregano oil (0.3% or higher), and olive extract (5%) yielded ≥3 logs reduction of S. Newport (Todd et al. 2013; Moore-Neibel et al. 2013; Moore et al. 2011), the present study demonstrates the use of lower concentrations of essential oils (0.1%) and plant extracts (3%) to obtain significant reductions similar to those found previously. Using lower concentrations of plant antimicrobials may be better for maintaining the sensory properties of the treated leafy greens, an aspect that needs to be further tested.
Combination Treatments and Storage Time Dependence
The application of both combination treatments demonstrated a reduction of S. Newport in all leafy greens during storage. Lettuce inoculated with different S. Baildon initial inoculum levels was sampled on days 0, 2, 5, and 8 after storage at 4°C, and the pathogen was recovered for up to 12 days regardless of inoculum levels (Weissinger et al. 2000). In the present study, storage time at 4°C did not significantly change the population of Salmonella for baby spinach, mature spinach, and iceberg lettuce which remained at about 5 log CFU/g throughout the 3 days of storage for the control. Romaine lettuce had, however, a slight decrease in Salmonella population after 3 days of storage. The reduction was the greatest for romaine lettuce for both combination treatments after 3 days of storage at 4°C.
The population of S. Newport in the inoculated untreated control remained consistent over the 3 day storage. The PBS control showed no further reduction, whereas the combination treatments showed further reductions during storage from day 0 to day 3. Therefore, the antimicrobial activity of the combination treatments continued throughout storage. These results are consistent with those from the previous studies involving cinnamon leaf oil, oregano oil, and olive extract (Todd et al. 2013; Moore-Neibel et al. 2013; Moore et al. 2011), which supports the finding that plant antimicrobials continue to exhibit antimicrobial activity over a period of time. The 1.0 – 2.0 log CFU/g reduction observed with 3.0% hydrogen peroxide is also consistent with the data from Moore et al. (2011) indicating that hydrogen peroxide did not have residual activity during storage.
Industrial Applications of Plant Antimicrobials
An estimated 3000 essential oils are known, of which only about 10% are important for the flavors and fragrances market (van de Braak and Leijten 1994). The recent trend in “green” consumerism has led to an interest in the antimicrobial properties of these substances (de Silva 1996; Nychas 1995). Although many of these essential oils are classified as GRAS (Kabara 1991), their use is limited due to sensory considerations, because effective minimum inhibitory concentrations (MIC) of essential oils exceed organoleptically acceptable levels (Lambert et al. 2001).
In the organic food industry, hydrogen peroxide is commonly used as a sanitizer (Moore et al. 2011). The present study demonstrates that the inactivation of S. Newport by hydrogen peroxide is not storage time dependent, while the combination treatments yielded an immediate reduction that increased over time. Thus, using hydrogen peroxide as a sanitizer may not be optimal for application to organic leafy greens in comparison to plant antimicrobials.
Currently, there are many advantages for the use of plant antimicrobials. Essential oils and plant extracts can extend the shelf-life of unprocessed and processed foods by reducing microbial growth rate, and can contribute to the self-defense of plants against infectious organisms (Beuchat and Golden 1989; Deans and Ritchie 1987; Kim et al. 2001). Antifungal activity has been found in cinnamon leaf oil that prevents the hyphal growth and reduces the spore production of Colletotrichum coccodes, Cladosporium herbarum, Aspergillus niger, Botrytis cinerea, and Rhizopus stolonifer (Tzortzakis 2009). Essential oils and spices are already utilized as flavor enhancers, and cinnamon is already being added to milk in Latin America and Spain (Cava et al. 2007). Edible films, such as apple films containing carvacrol and cinnamaldehyde, have been effective against C. jejuni, S. Enteritidis, and E. coli O157:H7 on chicken and L. monocytogenes on ham and bologna (Mild et al. 2011; Ravishankar et al. 2009; Ravishankar et al. 2012).
The combination treatments evaluated in this study were more effective when compared to the previously investigated individual treatments. A maximum of 3.0-4.4 log CFU/g reductions were observed on the four types of organic leafy greens. Utilizing two plant antimicrobials at lower concentrations could help maintain acceptable sensory attributes of leafy greens, an aspect that merits further investigation. Although the combination treatments demonstrated strong antimicrobial properties, further studies on different types of foods against a broad spectrum of foodborne pathogens should be conducted to support the generality of our findings. In addition, sensory analyses of leafy greens treated with lower concentrations of antimicrobial combinations will aid in their potential applications in the produce industry.
This study was funded by United States Department of Agriculture –National Institute of Food and Agriculture-Organic Research and Extension Initiative Competitive Grant No. 2010-51300-21760. We thank Carol E. Levin and Libin Zhu for facilitating the preparation, formatting and submission of the manuscript.
Beuchat, L.R., Golden, D.A. 1989. Antimicrobials occurring naturally in foods. Food Technol. 43, 134-142.
Cava, R., Nowak, E., Taboada, A., Marin-Iniesta, F. 2007. Antimicrobial activity of clove and cinnamon essential oils against Listeria monocytogenes in pasteurized milk. J. Food Prot. 70, 2757-2763. PMid:18095427
View Article PubMed/NCBICDC. 2010. Salmonella. Multistate outbreak of human Salmonella Newport infections linked to raw alfalfa sprouts (final update). Accessed: Aug 10, 2013.
View ArticleCDC. 2011. Estimates of Foodborne Illness in the United States. CDC 2011 Estimates: Findings. Accessed: Aug 10, 2013.
View ArticleCDC. 2012. Salmonella. Multistate outbreak of Salmonella Typhimurium and Salmonella Newport infections linked to cantaloupe (final update). Accessed: Aug 10, 2013.
View ArticleCDC. 2013. Salmonella. Technical Information. Accessed: Aug 10, 2013.
View ArticleDe Silva, K.T. (ed.) 1996. A manual on the essential oil industry. United Nations Industrial Development Organization (UNIDO), Vienna.
DEANS, S.G., RITCHIE, G. 1987. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 5, 165-180. doi: 10.1016/0168-1605(87)90034-1 90034-1
View ArticleDU, W.X., OLSEN, C.W., AVENA-BUSTILLOS, R.J., MCHUGH, T.H., LEVIN, C.E., FRIEDMAN, M. 2009. Effects of allspice, cinnamon, and clove bud essential oils in edible apple films on physical properties and antimicrobial activities. J. Food Sci. 74, M372-M378. doi: 10.1111/j.1750-3841.2009.01282.x
View ArticleFORMAN, J., SILVERSTEIN, J. 2012. Organic foods: health and environmental advantages and disadvantages. Pediatrics. 130, e1406-1415. doi: 10.1542/peds.2012-2579
View ArticleFriedman, M., Henika, P.R., Mandrell, R.E. 2002. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 65, 1545-1560. PMid:12380738
View Article PubMed/NCBIGREIG, J.D., RAVEL, A. 2009. Analysis of foodborne outbreak data reported internationally for source attribution. Int. J. Food Microbiol. 130, 77-87. doi: 10.1016/j.ijfoodmicro.2008.12.031
View ArticleGUTIERREZ-FERNANDEZ, J., GARCIA-ARMESTO, M.R., ALVAREZ-ALONSO, R., DEL VALLE, P., DE ARRIAGA, D., RUA, J. 2013. Antimicrobial activity of binary combinations of natural and synthetic phenolic antioxidants against Enterococcus faecalis. J. Dairy Sci. 96, 4912-4920. doi: 10.3168/jds.2013-6643
View ArticleGUTIERREZ, J., BARRY-RYAN, C., BOURKE, P. 2008. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 124, 91-97. doi: 10.1016/j.ijfoodmicro.2008.02.028
View ArticleKabara, J.J. 1991. Phenols and chelators. In: Food Preservatives, (N.J. Russell, G.W. Gould, eds.) pp. 200-214, Blackie, London.
Kim, H.Y., Lee, Y.J., Hong, K.H., Kwon, Y.K., Sim, K.C., Lee, J.Y., Cho, H.Y., Kim, I.S., Han, S.B., Lee, C.W., Shin, I.S., Cho, J.S. 2001. Isolation of antimicrobial substances from natural products and their preservative effect. Food Sci. Biotechnol. 10, 59-71.
Lambert, R.J.W., Skandamis, P.N., Coote, P.J., Nychas, G.-J.E. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91, 453-462. PMid:11556910
View Article PubMed/NCBILYNCH, M.F., TAUXE, R.V., HEDBERG, C.W. 2009. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 137, 307-315. doi: 10.1017/S0950268808001969
View ArticleMILD, R.M., JOENS, L.A., FRIEDMAN, M., OLSEN, C.W., MCHUGH, T.H., LAW, B., RAVISHANKAR, S. 2011. Antimicrobial edible apple films inactivate antibiotic resistant and susceptible Campylobacter jejuni strains on chicken breast. J. Food Sci. 76, M163-M168. doi: 10.1111/j.1750-3841.2011.02065.x
View ArticleMOORE-NEIBEL, K., GERBER, C., PATEL, J., FRIEDMAN, M., JARONI, D., RAVISHANKAR, S. 2013. Antimicrobial activity of oregano oil against antibiotic-resistant Salmonella enterica on organic leafy greens at varying exposure times and storage temperatures. Food Microbiol. 34, 123-129. doi: 10.1016/J.Fm.2012.12.001
View ArticleMOORE, K.L., PATEL, J., JARONI, D., FRIEDMAN, M., RAVISHANKAR, S. 2011. Antimicrobial activity of apple, hibiscus, olive, and hydrogen peroxide formulations against Salmonella enterica on organic leafy greens. J. Food Prot. 74, 1676-1683. doi: 10.4315/0362-028X.JFP-11-174
View ArticleNychas, G.J. 1995. Natural antimicrobials from plants. In: New Methods of Food Preservation, (G.W. Gould, ed.) pp. 58-89, Blackie Academic Professional, London.
View ArticleOSTEEN, C., GOTTLIEB, J., VASAVADA, U., AILLERY, M., BALL, E., BECKMAN, J., BORCHERS, A., CLAASSEN, R., DAY-RUBENSTEIN, K., EBEL, R., FERNANDEZ-CORNEJO, J., GREENE, C., HEISEY, P., HELLERSTEIN, D., HOPPE, R., HUANG, W.-Y., KUETHE, T., LIVINGSTON, M., NICKERSON, C., RIBAUDO, M., SCHAIBLE, G., WANG, S.L. 2012. Agricultural Resources and Environmental Indicators, 2012. Economic Information Bulletin No. (EIB-98). pp. 55.
View ArticlePeriago, P.M., Moezelaar, R. 2001. Combined effect of nisin and carvacrol at different pH and temperature levels on the viability of different strains of Bacillus cereus. Int. J. Food Microbiol. 68, 141-148. 00461-5
View ArticlePOLLACK, S.L. 2001. Consumer demand for fruit and vegetables: The U.S. example. pp. 49-54.
View ArticleRAVISHANKAR, S., JARONI, D., ZHU, L., OLSEN, C.W., MCHUGH, T.H., FRIEDMAN, M. 2012. Inactivation of Listeria monocytogenes on ham and bologna using pectin-based apple, carrot, and hibiscus edible films containing carvacrol and cinnamaldehyde. J. Food Sci. 77, M377-M382. doi: 10.1111/j.1750-3841.2012.02751.x
View ArticleRAVISHANKAR, S., ZHU, L., OLSEN, C.W., MCHUGH, T.H., FRIEDMAN, M. 2009. Edible apple film wraps containing plant antimicrobials inactivate foodborne pathogens on meat and poultry products. J. Food Sci. 74, M440-M445. doi: 10.1111/j.1750-3841.2009.01320.x
View ArticleRavishankar, S., Zhu, L., Reyna-Granados, J., Law, B., Joens, L., Friedman, M. 2010. Carvacrol and cinnamaldehyde inactivate antibiotic-resistant Salmonella enterica in buffer and on celery and oysters. J. Food Prot. 73, 234-240. PMid:20132667
View Article PubMed/NCBISivapalasingam, S., Friedman, C.R., Cohen, L., Tauxe, R.V. 2004. Fresh produce: A growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67, 2342-2353. PMid:15508656
View Article PubMed/NCBITODD, J., FRIEDMAN, M., PATEL, J., JARONI, D., RAVISHANKAR, S. 2013. The antimicrobial effects of cinnamon leaf oil against multi-drug resistant Salmonella Newport on organic leafy greens. Int. J. Food Microbiol. 166, 193-199. doi: 10.1016/j.ijfoodmicro.2013.06.021
View ArticleTZORTZAKIS, N.G. 2009. Impact of cinnamon oil-enrichment on microbial spoilage of fresh produce. Innov. Food Sci. Emerg. Technol. 10, 97-102. doi: 10.1016/j.ifset.2008.09.002
View ArticleUSDA-NOP. 2011. National Organic Program. Accessed: March 14, 2011.
View ArticleVan De Braak, S.A.A.J., Leijten, G.C.J.J. 1994. Essential oils and oleoresins: a survey in the Netherlands and other major markets in the European Union. CBI, Centre for the Promotion of Imports from Developing Countries, Rotterdam. PMid:7871698
PubMed/NCBIVeldhuizen, E.J., Creutzberg, T.O., Burt, S.A., Haagsman, H.P. 2007. Low temperature and binding to food components inhibit the antibacterial activity of carvacrol against Listeria monocytogenes in steak tartare. J. Food Prot. 70, 2127-2132. PMid:17900092
View Article PubMed/NCBIWeissinger, W.R., Chantarapanont, W., Beuchat, L.R. 2000. Survival and growth of Salmonella Baildon in shredded lettuce and diced tomatoes, and effectiveness of chlorinated water as a sanitizer. Int. J. Food Microbiol. 62, 123-131. 00415-3
View ArticleWilliams, P.R., Hammitt, J.K. 2001. Perceived risks of conventional and organic produce: pesticides, pathogens, and natural toxins. Risk Anal. 21, 319-330. PMid:11414540
View Article PubMed/NCBI