Dr. Renu Chadha,
Professor (Pharmaceutical Chemistry)
E-mail: renukchadha@rediffmail.com
Crystal engineering of fisetin: a step towards improved biopharmaceutical parameters
Corresponding Author
Affiliation
Yashika Bhalla, Renu Chadha*, Kunal Chadha and Maninder Karan
University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh-160014, India
Citation
Renu Chadha et al., Crystal engineering of fisetin: a step towards improved biopharmaceutical parameters(2019)SDRP Journal of Food Science & Technology 4(2)
Abstract
Background: In the present study, crystal engineering strategy is employed to facilitate the supramolecular synthesis of three new crystalline forms of a potential flavonoidal molecule, Fisetin whose efficacy is hampered due to its low solubility. Fisetin cocrystals with GRAS status coformers including glutaric acid, malic acid and theophylline are described herein. All the coformers complement the phytogen with respect to hydrogen bonding. .
Method: Mechanochemical grinding was utilized to prepare the cocrystals i.e., FGLU, FMAL and FTHY which were analyzed using DSC, FT-IR, PXRD and solid state NMR. The crystal structures determined using the PXRD pattern validated the existence of FGLU (triclinic crystal system (P1), FMAL (monoclinic P21/c) and FTHY(triclinic crystal system (P1). In each of the crystal structures, intermolecular hydrogen-bonding motif involving the hydroxyl group(OH) of fisetin with the carbonyl group and the phenolic group of the coformers was observed.The prepared cocrystals were further evaluated for their solubility, intrinsic dissolution and in vivo/in vitro profile.
Results: Solubility and dissolution studies of fisetin cocrystals were measured in aqueous buffer and demonstrated solubility improvement to be approximately 1.8-3.0 times higher as compared to the parent flavonoidal molecule, which subsequently led to improved pharmacokinetic parameters. Moreover, the antioxidant and antihemolytic effect of the cocrystals were found to be even high at low concentration when compared to fisetin molecule.
Conclusion:This report suggests cocrystallization as a viable approach to resolve the solubility and bioavailability issues that circumvent the use of a therapeutically potential isoflavone, fisetin.
Keywords: Fisetin, Cocrystals, DSC, FTIR, PXRD, Solid State NMR, Pharmacokinetics, Antioxidant, Antihaemolytic and accelerated stability studies.
Introduction
The key objective of a novel drug development process is to synthesis a drug molecule with optimal physiochemical and biopharmaceutical parameters [1]. Solubility alarmingly influences the therapeutic utility of an API as it governs the dissolution rate and pharmacokinetic profile following better absorption in the gastrointestinal tract (GIT).
In the recent years the field of crystal engineering related to pharmaceutical industry has poised to address these two key issues and has matured into a paradigm for the supramolecular synthesis of new compounds with desired properties. The possible ways in which crystal engineering can modulate the physiochemical properties of molecular solids are by generation of various polymorphs, cocrystals, solvates/hydrates and eutectics [2].
Cocrystals is a lucrative technique which has sparked the interest of many researchers in the crystal engineering field and is based on a concept of the supramolecular chemistry.Pharmaceutical cocrystals can be rationally designed to produce supermolecules with target structures and predetermined properties and has garnered the attention of pharmaceutical industry. The cocrystal comprises of a API and a GRAS status coformer which aims to bring two different components into one crystal lattice via intermolecular force: hydrogen bonding, π- π stacking, van der Waals forces, etc. [3]. Incorporation of a second constituents i.e., a coformer in cocrystals is being utilized for more than a decade for manipulating the physiochemical properties of an API and has been a humdrum within the pharmaceutical industry.
Many patents have been registeted in the name of pharmaceutical cocrystals owing to their unique design and synthesis which involves various elements of non-obviousness.The increased patent portfolio of cocrystallization has made its way into the regulatory guidelines laid by US FDA which marks as a great success in the field of crystal engineering [4]. This manuscript reports the cocrystals of potential nutraceutical molecule, fisetinwith various pharmaceutically acceptable coformers.
Fisetin (FIS), 3,3’,4’,7-Tetrahydroxyflavone, is a flavonol that is present in many fruits, vegetables i.e., grapes, cucumbers, strawberries, onion, persimmons [5]. Recently, FIS has been reported to exhibit antiallergic [6], cardioprotective [7],antioxidant [8], anticancer [9], antihydroid [10], anti-inflammatory activity [11] and antimutagenic [12]. However, its extremely low aqueous solubility (<1 mg/mL) [13] has hampered its therapeutic benefits.FIS is a rather weak acid with a pKa of 7.42 and an unstable constituent in a basic environment [14], an appropriate salt former (a strong base) would exaggerate FIS’ s instability whereas its cocrystals will improve the stability. So, cocrystallization will be a suitable method for improving the physicochemical properties of this bioactive molecule as it is concerned with the formation of a complex between neutral molecules [15]. Moreover, FIS has various competitive sites for hydrogen bonding i.e., donors and acceptors (four hydroxyls and one carbonyl), in its molecular skeleton, offering the possibility of cocrystal formation with the suitable conformers with complementary functional groups. In this study, several pharmaceutically acceptable coformers have been selected to prepare FIS cocrystals with an intension to improve its biopharmaceutical parameters.
Previous approaches for screening of solubilized forms of fisetin were based on empirical method like development of a nanoemulsion formation [16], liposomal formulation [17], complexation with a cyclophosphorus dimer[18] . As far as cocrystals are concerned three cocrystals of fisetin with nicotinamide, caffeine and isonicotinamide are reported however, the results are not supported by any biological evaluation[19,20]. In the present manuscript, an endeavor has been made to shed light on the development of few new cocrystals of fisetin(figure 1a) along with their pharmacokinetic analysis and evaluation for the improvement in biological activities including antioxidant activity and antihemolytiic activity have also been performed.The three nontoxic coformers, i.e., glutaric acid, theophylline, malic acid, utilized in this study have favorable aqueous solubility with intrinsic biological profile.
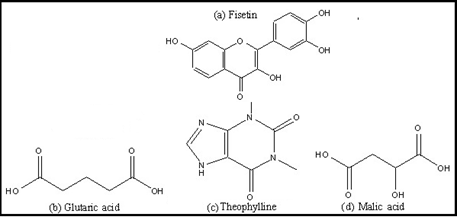
Figure 1. Chemical structures of FIS (a) and CCFs: THY (b), GLU (c), Mal (d).
Glutaric acid (figure 1b) is an FDA-approved API and is commonly used as a cocrysal coformer with high solubility[21].Theophylline (figure 1c) (acts as a phosphodiesterase inhibitor, adenosine receptor blocker, and histone deacetylase activator) is a methylxanthine derivative obtained from tea possess diuretic, smooth muscle relaxant, bronchial dilation, cardiac and central nervous system stimulant activities[22].The third coformer, Malic acid (figure 1d) is generally taken as a supplement and is an organic compound which is found in fruits(apple) and has been known for its therapeutic us to treat fibromyalgia and chronic fatigue.[23]
Materials & Methods
2.1 Materials
The procurement of Fisetin (> 97%, Alfa Aesar, England), glutaric acid (> 99%, Sigma Aldrich, USA), Malic acid (> 98%, HI-MEDIA, Mumbai, India), theophylline (> 98%, HI-MEDIA, Mumbai, India) and ethanol (> 99%, E.Merk Ltd, New Delhi, India) was done.
2.2 Designing of co-crystals
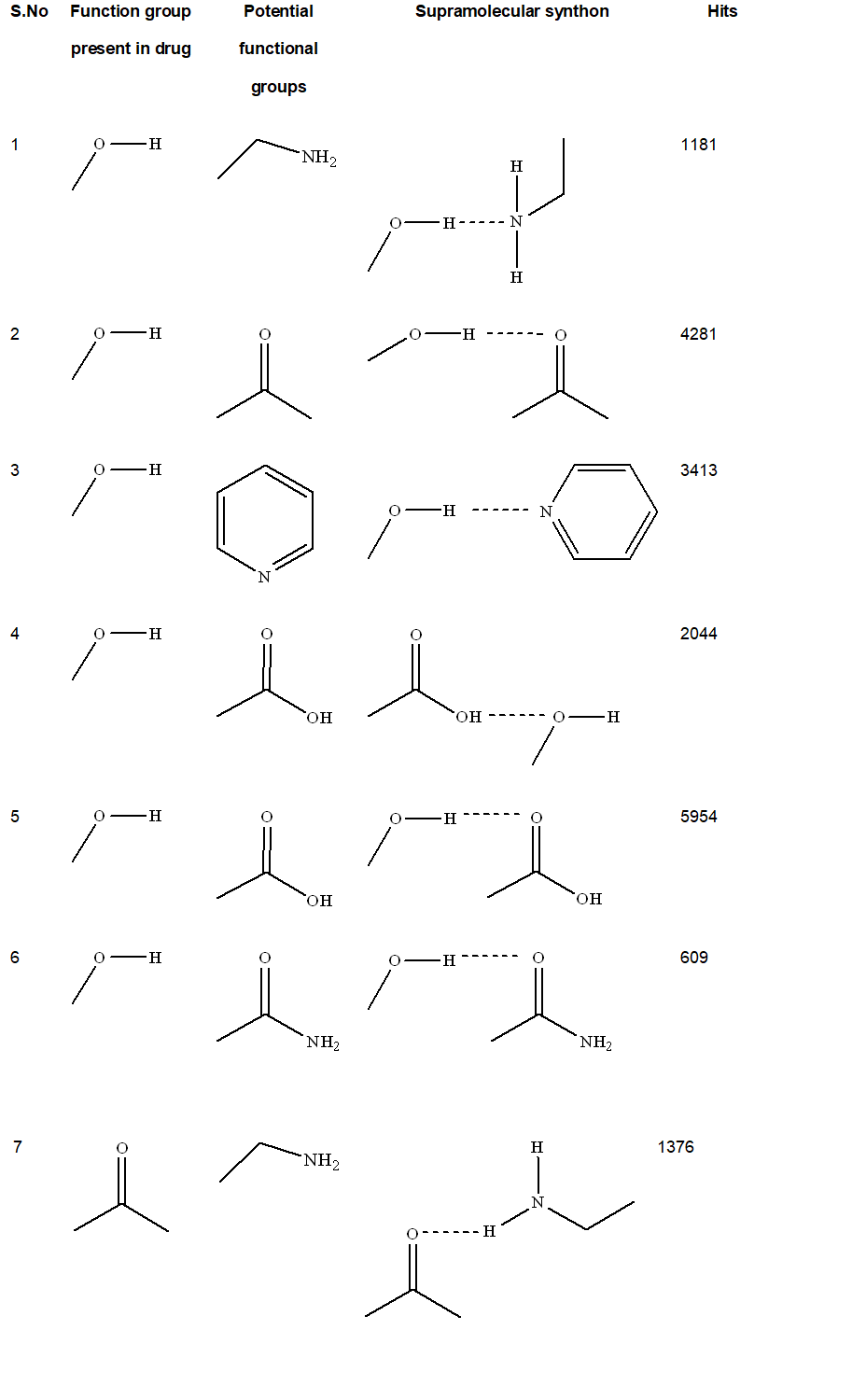
The first step for cocrystal synthesis is the design involving the analysis of the already existing associated crystal structures. This search is assisted by the Cambridge Structural Database (CSD) which is a reservoir of crystal structure. This search for already existing synthons of hydroxyl and carbonyl groups estimates the propensity of these groups present in fisetin molecule to form homo or hetero synthons with the functional complementary groups. The search was carried out using ConQuest software (version 1.7) which provides the data of pre-existing crystal structures in Cambridge Structural Database (CSD, version 5.36, 2014). A query for hydrogen bonding functional groups were prepared and explored for the hits. Based on the obtained result, suitable coformers were chosen to prepare the co-crystals.
2.3 Synthesis
The co-crystals of fisetinwere prepared with the coformers: glutaric acid(GLU), theophylline(THY), malic acid(MAL) using solvent assisted grinding method. Fisetin(286 mg) and the corresponding coformers i.e., glutaric acid 132 mg,theophylline 180 mg and malic acid 134 mg, were combined in a1:1 stochiometric ratio in a mortar. The mixture was ground with a pestle for one hour with drop-wise addition of ethanol (6ml) with a micropipette, at room temperature, until dry. This procedure was continued till a dry mixture was obtained which were further stored in desiccators.
2.4 Identification and Characterization
2.4.1 Differential scanning calorimetry (DSC)
DSC Q20 (TA Instruments, USA) was used to carry out DSC of all the samples. Samples were held in sealed aluminium pans under a dry nitrogen atmosphere (flow rate 50mL min-1) which were further scanned at rampling rate of 10 °C min−1. The data was then collected using TA Q series Advantage software (Universal analysis 2000).
2.4.2 Fourier Transform-Infra Red Spectroscopy (FTIR)
The FT-IR was performed in the KBr diffuse-reflectance mode (sample concentration 2 mg in 20 mg of KBr) using a Spectrum RX I FT-IR spectrometer (Perkin-Elmer, UK) in the range of 4000-400 cm-1.The data was analyzed using spectrum software.
2.4.3 Powder X- Ray Diffraction (PXRD)
X'Pert PRO diffractometer system (Panalytical, Netherlands) was utilized to analyze the PXRD patterns by means ofa Cu Kα radiation (1.54060 Å). The tube voltage and current was set at 45 kV and 40 mA respectively and the divergence slit and antiscattering slit were positioned at 0.48° while illumination on the 10-mm sample size. Analyzes of the PXRD pattern was done at 2θranging from 5° to 50° (step size of 0.017°). Pxrd pattern obtained experimentally was refined by X'Pert High Score software.
2.4.4 Solid State NMR (SSNMR)
Joel Resonance JNM-ECX400II Instrument from IISC, Bangalore, India was employed to obtain the solid state NMR of the prepared samples.The temperature was kept at 273 K with an acquisition time of 29.1s and polarization relaxation delay time was 5sec at 1024 complex data points.
2.4.5 Crystal structure determination
Material Studio® software by BIOVIA system was employedto determine the structuresof fisetinand its respective cocrystals. There were in all four stepsstarting with indexing of the peaks, pawley fitting of the created cell,structure solution and lastly therietveld refinement. The indexing was done using X-cell for the peaks positioned at 2θ value ranging from 5°-40° 2θ present in the PXRD pattern at position 5°-40° 2θ and further a crystal unit cell was obtained. The unit cell was then refined using Pawley refinement to optimize cell parameters and lattice constants (minimum Rwp). The molecular structures of FIS, GLU,THY and MAL were sketched using DMol3 module. The structures produced were geometrically optimized and werefurther imported inside the refined empty cell (having lowest Rwp) and all of them were subjected to simulated annealing algorithm (10 cycles, 21000000 iterations in each cycle) using Powder Solve module.
2.5 Equilibrium solubility studies
Equilibrium solubility study was performed at 37 ºCin water bath shaker (MSW-275)Macroscientific Works, Delhi) at 200 rpm. An excess amount samples (approx. 50 mg) in 10 ml of phosphate buffer of ph 6.8 were kept for shaking for 24 hours. The obtained samples were then strained viaa 0.45 μ membrane filter which werefurther quantitatively analysised for fisetinusing HPLC. At various time intervals (2 hrs and after 24 hrs) the samples were withdrawn. The FT-IR analysis was performed for the residual material.
2.6 Intrinsic dissolution studies
Intrinsic dissolution study was carried out using rotating disk dissolution test apparatus, DS 8000 (Lab India Analyticals) at 37°C in phosphate buffer pH 6.8 with 150 rpm for 4 hours. Pellets of the pure drug and the respected cocrystals were prepared using hydraulic pellet press, and the pellet of the sample was held in the dissolution apparatus holder which was further immersed in dissolution media. The aliquot buffer (5 ml) was replaced with fresh buffer at multiple time points (15, 30, 45, 60, 90, 120, 180 and 240 min) and strained through 0.45μm membrane filter. The quantitative analysis was carried out at 228 nm usingWaters Alliance HPLC system (Photodiode Array Detector).
2.7 High Performance Liquid Chromatography
Waters Alliance HPLC system which includes a Waters 2695 separation module, a Waters 2996 Photodiode Array Detector, and a 4.6 mm × 150 mm SunFire C18, 5 μm column (Waters Corporation, Milford, MA) was used for analysis. Stock solutions of the samples (fisetin and cocrystals) were prepared in phosphate buffer pH6.8 to get various concentrations of calibration standards (10, 20, 30, 40, 50, 60, 70, 80, 90, 100 µg/ml). 10 µl of all the samples was injected in the column and analyzed by isocratic mobile phase a mixture of methanol and 0.1% ortho phosphoric acid (62:38) pumped at a flow rate of 1.0 mL/min through the column at a temperature of 35 °C. The detector wavelength was set at 355 nm. Data acquisition and analysis were carried out using software Empower 2.0.The retention time of fisetin was determined to be 4.43 mins.
2.8 Biological Studies.
2.8.1 Antioxidant Activity
The scavenging activity for DPPH(2, 2’ diphenyl-1-picrylhydrazyl) free radicals of fisetin and its cocrystals was performedas per the procedure demonstrated by Blios (1958) with appropriate modifications [24]. By dissolving 3.94mg (0.1 mM) DPPH in 100 ml of 50:50 methanol and water stock solution was obtained. The working solution waspreapred by adding 1 ml stock solution in 1ml of cocrystal withdifferent concentrations (1, 2,3,4,5,6,7,8,10 µg/ml) and the volume was made upto 5ml using methanol.Further, absorbance was recorded at 517 nm utilizing the spectrophotometer(UV–Visible EZ201, Perkin Elmer, USA). Fisetinand its cocrystals were kept aside in a dark place for 45 mins so that they react with DPPH solution. Standard control used in this experiment was Ascorbic acid due to its known antioxidant activity. The obtained absorbance was converted into the percentage antioxidant activity utilizing the following equation:
%Radical scavenging activity = (Absorbance of control – Absorbance of sample) X100
(Absorbance of control)
2.8.2 Antihaemolytic activity
The antihemolytic activity was performed on rat red blood cells (RBC). The RBC’s were collected in centrifuge tubes having alsever solution in equal volume, this solution was further centrifuged at 3000 rpm at 4◦C for 10 mins. The packed blood cells then were rinsed usingisotonic(0.9% saline pH 7) buffer solution.These steps were repeated thrice.Ultimately the last cell volume (10% v/v) which was obtained was kept in isotonic buffer solution (pH 7). 500 μL of the final packed cells was added to 4.5 mL of hypotonic phosphate buffer saline solutions containing various concentrations of fisetin and its cocrystals (50, 100, 150 μg/mL in hypotonic PBS pH 7) incubated for 10 mins at room temperature and then and centrifuged for 15 min at 3000 rpm at 4oC. The extent of haemolysis by fisetinz and its cocrystals was evaluated by obtaining absorbance of the resulting supernatant at 540 nm by UV-Visible spectrophotometer (Perkin Elmer).
2.9 Pharmacokinetic studies in rat plasma
Male wistar rats were procured and used for the kinetic studies. Animals were accustomed with the regular feed/water ad libitum. The dose administered to the experimental rats was 50mg/kg. At various time intervals blood was withdrawn (approximately 0.2 ml) from the jugular vein cannula. The collected blood samples were kept in freezer until the HPLC analysis was performed.
Study protocol:
Overnight fasted rats were split in six groups (n=6) comprising of
Group I:Served as control (Received vehicle).
Group II: Rats were administered aqueous carboxymethylcellulose (CMC) (0.5%w/v)
Group III:Rats were given fisetin suspended in aqueous CMC (0.5%w/v)
Group IV:Rats received FTHY suspended in aqueous CMC (0.5%w/v)
Group V:Ratsreceived FGLU suspended in aqueous CMC (0.5%w/v)
Group VI: Rats received FMAL suspended in aqueous CMC (0.5%w/v)
Sampling of plasma: Blood samples collected from the retro-orbital capillary plexus of rats(all groups) at specified time gap (15, 60, 120, 180, 240, 360, 420 min) was centrifuged for 20 mins at 5000 rpm to discrete plasma from blood sample. Subsequently, the volume was made upto 1 mL using solvent and further the samples were analysed by HPLC for fisetin content.
HPLC analysis of fisetin in plasma: The calibration standards were made by spiking 100 µL of the fresh pure plasma from untreated rat with an appropriate amount of 1 mg/mL methanolic stock solution of fisetin. The calibration standards made were of 5, 10, 15 and 20 µg/mL concentrations. Further, the samples were injected into the system at an injection volume of 20 µl, with mobile phase consisting of o-phosphoric acid: methanol (20:80) at a flow rate of 1.2 ml/min.
2.10 Accelerated stability conditions
The cocrystals were further subjected to accelerated stability conditions (40°C/75% RH for the period of 3 months) and the samples were analysed by DSC and PXRD pattern.
Results
The solvent assisted grinding method had been employed to prepare the multicomponent crystals of fisetin, FIS-GLU, FIS-MAL and FIS-THY and were characterizedthermally, spectroscopically and structurally via, DSC, FT-IR, PXRD and SSNMR. Further, cocrystals were studied for their enhanced solubility and dissolution profile.Additionally the antioxidant activity and hemolytic acivity was also measured for the prepared cocrystals in comparison to that of the pure fisetin molecule.
3.1 Design and Synthesis of Co-crystals
Firstly before the synthesis of cocrystal a CSD search was performed depending on the functional groups(hydroxyl (OH) and carbonyl (C=O) present in FIS molecule. Search was carried out on each of the fragmented functional group following which the hits were obtained.The results obtained from the database showed high probability to prepare multicomponent forms with specific functional groups.
Based upon CSD search (Table I), it was observed that ceratin functional groups (carboxylic acid group, aromatic nitrogen and carbonyl group) are more prone to form hydrogen bond with hydroxyl group and carbonyl group of FIS. henceforth, various coformers including cytosine, piracetum, picolinic acid, nicotinic acid, histidine, theophylline, pyrogallol ,theobromine, isonicotinic acid, urea, acetamide, benzamide, piperazine, picolinamide, 4-hydroxy benzamide, pyridoxine were tried.Cocrystals were obtained with GLU,MAL ad THY, which were further characterized and evaluated.
Table 1.CSD statistics for potential functional groups
3.2 Characterization of Co-crystals
3.2.1 DSC
Single endothermic peaks were observed for FGLU (180.240C), FMAL (213.260C) and FTHY (284.450C) respectively. They are different from their parent constituents i.e.,coformers, GLU(101.230C), MAL(131.290C), THY(271.540C) and the pure drug FIS (346.260C).The thermograms are are given in Figure 2.
The appearance of sharp, peak with definite melting point in the DSC indicate that the new solid form prepared is crystalline in nature and is phase pure. Moreover, the appearance of a single endothermic eventpresent between the melting point of fisetin and respective coformers further point towards the existence of cocrystals over eutectics.
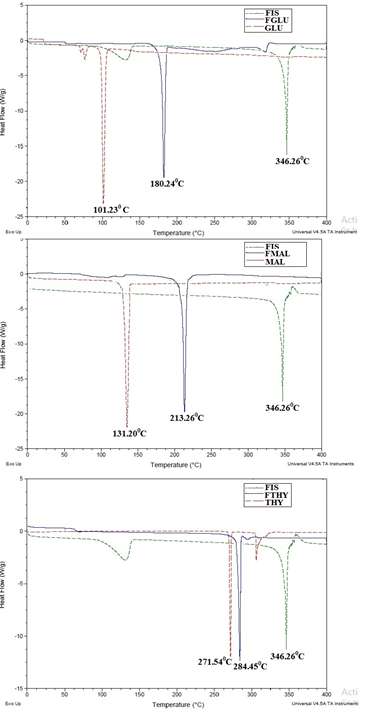
Figure 2. DSC of FIS, coformers, FGLU,FMAL and FTHY
3.2.2 PXRD
The formation of new crystalline phases as conferred by DSC was further confirmed by the PXRD pattern of the cocrystal (Figure 3) when compared to the parent molecule and the corresponding coformer.
PXRD is a reliable technique to obtain qualitative information regarding the formation of cocrystals .The existence of a distinct PXRD pattern of the resultant products conveys the generation of a new phase. The events pertaining to new peak appearance, disappearance of characterstic refelections and distortion in the original peaks provides information about certain interactions taking place within the fisetin and the respective coformers resulting in the cocrystals.
Some new peaks in FGLU, at 7.3°,11.0°,16.2° and 17.5° have appeared.A few characteristic peaks at 9.1°,9.8°,15.3°,19.6°,20.4°,20.9°,22.7° in PXRD of FIS and 21.9° in PXRD of GLU disappeared. The peaks at 21.2° in PXRD of GLU and 21.3° in PXRD of FIS have merged to 21.4° in PXRD of FGLU ,peaks positioned at 23.3° in PXRD of GLU and 23.8° in PXRD of FIS have produced a peak at 23.7° in PXRD of FGLU whereas, peak at 24.04° in PXRD of GLU and 24.1° in PXRD of FIS have merged to 24.2° in PXRD of FGLU. Besides, this peak at 26.0° in PXRD of GLU and peak at 25.5° in the PXRD of FIS has merged to give a peak at 26.2°.
Some new peaks at 5.6°,11.03°,17.5°,29.6° were observed in FMAL whereas few peaks at 9.8°,19.6°,20.4°,21.3°,31.4° of FIS disappeared. Besides this, peaks at 23.5° (23.6° in MAL merged with 23.8° in FIS), 25.9° (25.8° in MAL merged with 25.5° in FIS), 27.6° (27.4° in MAL merged with 27.3° in FIS) and 28.3° (28.15° in MAL merged with 28.0° in FIS) were observed in FMAL. Many peaks in the PXRD of both FIS and MAL were found to be shifted significantly from their initial positions .
Few new peaks have appeared at 6.3°, 14.4° and 16.06° 2θ in FTHY.Besides this, peaks positioned at 12.9°, 21.3°, 23.8° and 25.5° in FIS fused with 12.8°, 20.1°,23.6° and 26.7° in THY and resulted in peaks with 2θ 12.7°, 21.03°, 23.5° and 26.8° respectively in FTHY.
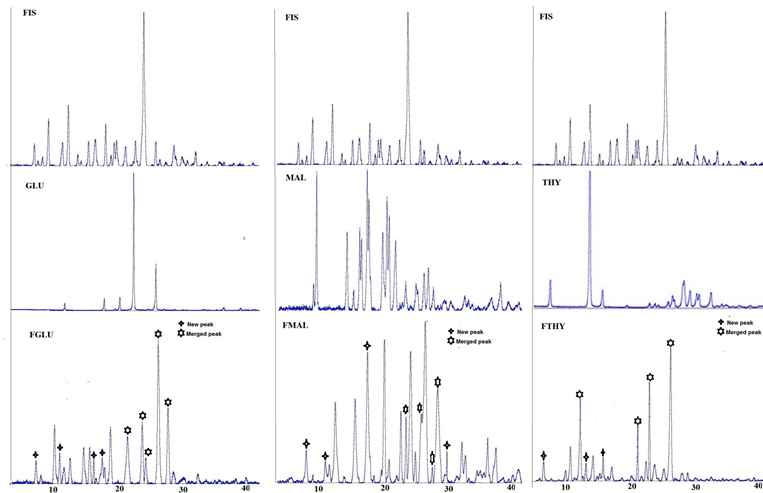
Figure 3. Diffraction patterns of FIS, all the CCF, FGLU, FMAL and FTHY
3.2.3 FT-IR
Infrared spectroscopic technique has been extensively used to identify the intermolecular interaction and hydrogen bond directed associations in the field of crystal engineering [25].
The shift in vibrationalbands ofFisetin as well as the respective coformers has been observed in the cocrystals FGLU,FMAL and FTHY(Figure 4).
There is a shift in the position of hydroxyl (-OH) group of fisetinat 3352 cm-1 as well as hydroxyl stretch (-OH) and carbonyl (C=O) stretch at 3046 cm-1 and 1695 cm-1 of glutaric acid to 3244 cm-1 , 2935cm-1 and 1710 cm-1 respectively in FGLU.
Whereas in the case of FMAL, the hydroxyl (-OH) stretch at 3352 cm-1 of fisetin and (-OH) stretch at 3446 cm-1 of MAL have appeared at 3345cm-1 and 3253 cm-1. The absence of peaks in the region of C=O stretch corresponding to the carboxylate salts(1750-1700 cm-1) shows that in FMAL and FGLU both the constituents i.e., the drug and the coformer are in the neutral state neglating the salt formation.
The hydrogen bond existence in FTHY can be manifested by the shift in the hydroxyl group (-OH) from 3352 cm-1 and carbonyl (C=O) group from 1683 cm-1 of FIS, and N-H stretching from 3131 cm-1 and carbonyl (C=O) stretch from 1667 cm-1 of THY to 3291 cm-1,1678 cm-1, 3129 cm-1 and 1645 cm-1 respectively.
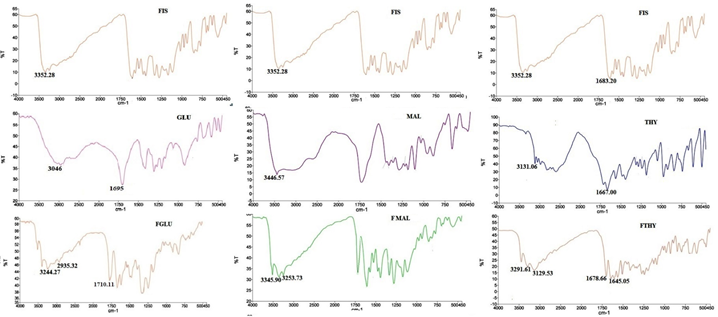
Figure 4. FTIR spectra of FIS, coformers, FGLU,FMAL and FTHY
3.2.4 SSNMR
SSNMR offers insight into the structural information which in collaboration with the vibrational spectroscopy, powder X-ray diffraction and crystal structural data can act as definite evidence for existence of cocrystals. The sensitivity of this analytical tool provides valuable information regarding the perturbations in the chemical environment attributing to the variation in the supramolecular interactions within the cocrystals.Significant differences were observed in the chemical shift of the carbon attached to the functional groups participating in the hydrogen bond between fisetin molecule and the respective coformers(Figure 5).
FGLU
The spectra of FGLU indicates the existence of a specific non-covalent interaction between the glutaric acid molecule and fisetin molecule. There is a shift in the peak position at C3, C4 and C7 of fisten from 137.1, 170.8 and 169.7ppm to 136.4, 172.9 and 165.8 ppm in the cocrystal alongwith, shift in peak position corresponding to C1 and C2 of glutaric acid from 181.9 , 34.1 ppm to 180.9 and 33.8 ppm. These chemical shifts is due to the involvement of the hydroxyl groups (C7 and C3) and carbonyl group (C4) of fisten and the hydroxyl and carboxylic group (C1 and C4) of glutaric acid in hydrogen bonding forming O…HO heterosynthon.
FMAL
Similarly, the peak positions at C3 (137.1), C4 (170.8) and C4’ (148.9) of fisetin have shifted to 135.9, 172.2, 147.3alongwith shift in the peak position of malic acid at 179.6, 69 and 41.8 ppm corresponding to C2, C3 and C4 to 180.4, 70.2, 42.1 ppm respectively. These variations infer that there is involvement of hydroxyl groups positioned at C7 and C4’ of fisetin and carbonyl group(C1 and C4) and hydroxyl group (C2) of glutaric acid.
FTHY
Perturbations due to hydrogen bonding at C3, C4 and C7 of fisetin corresponding to 137.1, 170.8 and 169.7ppm have are responsible for shift in their position to 136.8, 171.9, 164.2ppm respectively in FTHY.Whereas, the peak positioned at 150.9, 140.9 and 106.0 ppm corresponding to C2,C9 and C8 of theophylline have shifted to 151.8, 143.5, 107.3 ppm in FTHY.These variations in chemical shifts provide evidence for the existence of hydrogen bond between the hydroxyl group positioned at C3 of fisetin and carbonyl oxygen at C2 position of theophylline resulting in the formation of OH---O=C heterosynthon.
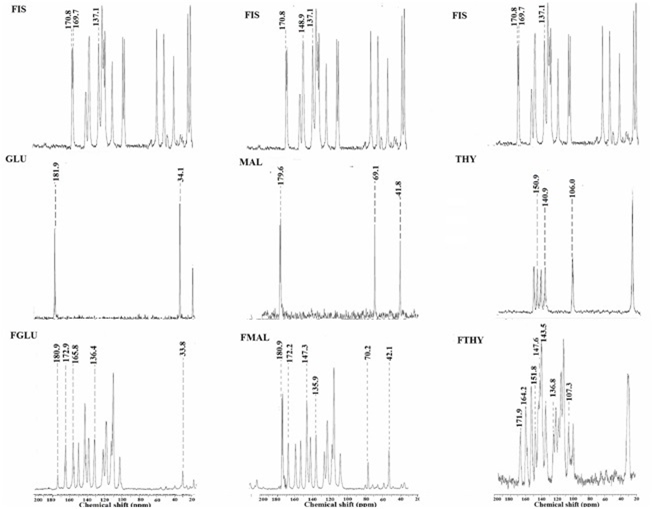
Figure 5: ssNMR patterns of FIS,coformers, FGLU,FMAL and FTHY
3.2.5 STRUCTURE DETERMINATION
As discussed in experimental section the crystal structure was determined from PXRD pattern utilizing BIOVIA software.Firstly the crystal structure of fisetin was determined as no report is available on its crystal structure.
Fisetin molecule crystallizes in P-1 space group of triclinic system. The fisetin molecules are linked via homosynthons between the hydroxylic group at C3 of F1 which donates its hydrogen to the oxygen of the hydroxylic group at C4’ of the adjacient F2 molecule forming a homosynthon (OH3…OH4’). Also, the hydrogen of the phenolic group at C7 of F3 donates its hydrogen to the oxygen of hydroxylic group at C3 of F1(homosynthon OH7…OH3) (Figure 6).
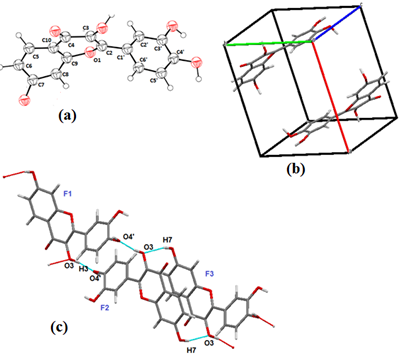
Figure 6. FIS (a) asymmetric unit, (b) Crystal packing pattern along b axis, (c) Hydrogen bonded interactions in FIS
The cocrystals are prepared from heteromic interactions by breaking the homomeric interactions amongst the FIS molecule.
Crystallographic data are summarized in Table 2 for the FIS cocrystals. The cocrystal (Fis-GLU) crystallizes in the P1— space group of triclinic system, with one FIS and one GLU molecule in the asymmetric unit(Figure 7 a).The FIS-MAL cocrystal crystallized in the P2/nspace group of the monoclinic system. The asymmetric unit contains one molecule each of FIS and MAL(Figure 8a) .The FIS-THY cocrystal crystallized in the P1¯ space group of the triclinic system, with one FIS molecule and one THY molecule in the asymmetric unit. (Figure 9a).
FGLU
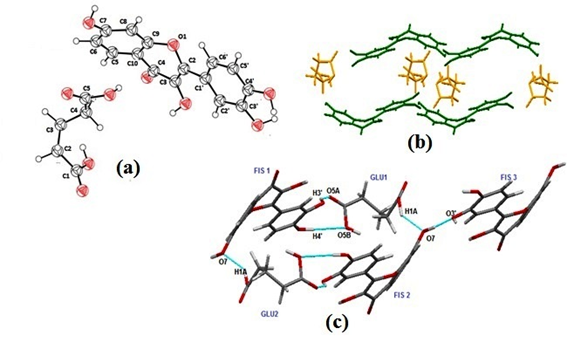
Structural analysis of FGLU reveals the presence of centrosymmetric tetramers assembled by GLU and FIS molecules, as illustrated in Figure 7c The homosynthon (H3’…O4) of fisetin breaks to form cocrystal with glutaric acid via heterosynthon (H3’…O5A).
In these assemblies, a ring motif is formed consisting of heteromolecular synthons in FIS1-GLU1 i.e., O4’–H4’…O5B (1.78 A,178u), wherein the hydroxyl group present at C4’ position of ring B of fisetin(FIS1) donates hydrogen to the oxygen of hydroxyl group positioned at C5 of glutaric acid(GLU1), O1A–H1A…O7 (2.20 A, 166u) in which the hydrogen of the hydroxyl group positioned at C1 of glutaric acid(GLU 1) donates its hydrogen to the oxygen of the hydroxyl group positioned at C7 of fisetin molecule(FIS 2). Besides this, heterosynthon O3’–H3’…O5A (2.04 A, 147u) exists between hydrogen of phenolic group at position C3’ of ring B (FIS 1) and carbonyl oxygen positioned at C5 of glutaric acid(GLU 1).Additionally homomeric interactions also exist within fisetin molecules FIS2-FIS3 connected to each other via O7-H7….O3’ hydrogen bond. Glutaric acid being a small aliphatic diacid molecule fits in the voids of the fisetin molecule. The packing pattern of FGLU is such that GLU molecules are oriented approximately perpendicular to the chain of FIS molecules (figure 7b).
FMAL cocrystal.
The crystal packing (Figure 8b) and hydrogen bonding in the FIS-MAL cocrystal are depicted in Figue 8c. In this cocrystal the homosynthon of fisetin(O3…H7) breaks to form heterosynthon(O3….H3) with MAL
The structural analysis shows that the fisetin (F1) molecule interact with two adjacent malic acid (M1 and M2) molecules. Oxygen of the hydroxyl group (O4’) of F1 is bonded to the hydrogen of hydroxyl group (H2) positioned at C2 of M2 forming a motif (H4’O4’…H2O2). Whereas, the hydrogen (H4’) of the hydroxyl group at C4’ of F1 is bond with the oxygen of carbonyl group at C4 of malic acid (M1) (04’H4’….O4=C4).
Additionally, F1 is also linked with M2 via hydrogen bonding that exists between the oxygen of the hydroxyl group at C3 position of F1 and the hydroxy hydrogen (O3H3) at C3 of M2. Besides, this the oxygen of the carbonyl group at C4 of fisetin (F1) accepts hydrogen from the hydroxyl group positioned at C3’ of another fisetin molecule (F2) leading to intermolecular interactions amongst the fisetin molecules.
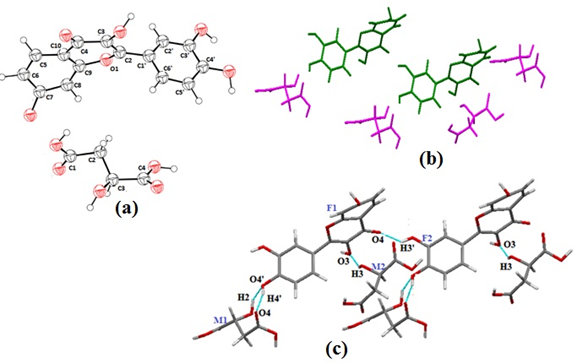
Figure 7. FGLU (a) asymmetric unit, (b) Crystal packing pattern where green and orange colour represent FIS and GLU molecules respectively, along the b axis, (c) Hydrogen bonded interactions in FGLU cocrystal
FTHY
The hydrogen bond between fisetin (FIS) and theophylline (THY) is formed between the hydroxyl hydrogen at C3 of FIS and the carbonyl oxygen atom at C2 of THY.Also the two theophylline molecules are held together in a cyclic motif (NH….O=C) by hydrogen bonding leading to aR22(8)homodimer.The homodimeric unit is linked with adjacent FIS molecules in sheets by O…H hydrogen bond between hydroxyl group located at C3 of FIS which acts as hydrogen bond donor and carbonyl oxygen at C2 (hydrogen bond acceptor) of THY via O3H3…O2 hydrogen bonds.The homodimers of THY are capped in centre with two sheets of flavanoid molecule on each end (Figure 9c). These are further connected via O…H hydrogen bond existing between oxygen of the carbonyl carbon (hydrogen bond acceptor) at C4 of F1 with hydrogen of hydroxyl group at C4 of F2 i.e., O4….H7O7 (F2).The oxygen atoms of phenol groups on the adjacent FIS molecules face each other in a close packed arrangement (Figure 9b), which stabilizes the 3-dimentional structure.The synthon(O4…H3’) originally present in fisetin lattice breaks to form heterosynthon(O3H3…O2) wih THY.
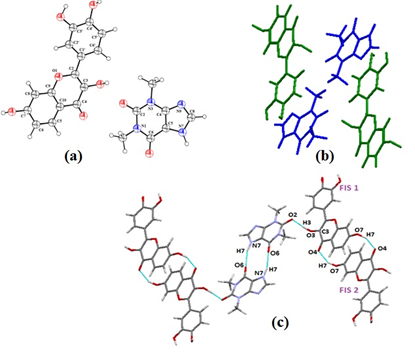
Figure 9. FTHY (a) asymmetric unit, (b) Crystal packing pattern where green and blue colour respresent FIS and THY molecules respectively, along b axis, (c) Hydrogen bonded interactions in FTHY cocrystal
Table 2. Crystallographic parameters
|
Crystallography Parameters |
FIS |
FGLU |
FMAL |
FTHY |
|
Chemical formula |
C15 H10 O6 |
C20 H18 O10 |
C19 H16 O11 |
C22 H18 N4 O8 |
|
Stoichiometry |
|
1:1 |
1:1 |
1:1 |
|
Temperature |
Room Temperature as specified 25ºC |
Room Temperature as specified 25ºC |
Room Temperature as specified 25ºC |
Room Temperature as specified 25ºC |
|
Crystal system |
Triclinic |
Triclinic |
Monoclinic |
Triclinic |
|
Cell volumes(Aº3) |
847.503 |
731.639 |
1279.62 |
853.524 |
|
Space group |
P-1 |
P-1 |
P2/n |
P-1 |
|
Cell lengths |
a 10.8694 b 9.3598 c 8.5290 |
a 11.6887 b 8.9868 c 7.4230 |
a 12.0193 b 10.4781 c 10.3186 |
a 12.4461 b 8.5843 c 8.0286 |
|
Cell angles(deg) |
α 94.7742 β 95.2038 γ 99.6883
|
α 86.9352 β 104.1696 γ 104.5818 |
α 90.0000 β 100.0403 γ 90.0000 |
α 94.1500 β 93.8458 γ 90.5247 |
|
Z |
2 |
2 |
4 |
2 |
|
2ɵ range |
5º 45º |
5º 45º |
5º 45º |
5º 45º |
|
Rwp(%) |
12.8 |
15.1 |
18.5 |
17.2 |
|
CCDC No. |
1884089 |
1884086 |
1884087 |
1884088 |
4. Equilibrium solubility and dissolution studies
The ability to solubilize a defined therapeutic dose of the API plays a very critical role in the context of drug development. The solubility profile of an API also influences the absorption process. Moreover, solubility together with permeability governs the bioavailability[26]. The thermodynamic solubility and kinetic solubility profile herein has been determined by performing equilibrium solubility and intrinsic dissolution. Equilibrium solubility indicates the solubility values whereas the intrinsic dissolution values reveal the dynamics of the whole solubility process and the difference in concentration of API during different time periods. Dissolution study represents a key link between in-vitro and in-vivo utilization of the API.[27]
The prepared cocrystals of fisetin display a spring and parachute phenomenon (Figure 10) since the two molecules (i.e., fisetin and respective coformer) dissociate into the individual constituents within the short span of time, owing to weak hydrogen bond holding the molecules together. The spring and parachute effect provides sufficient period of time for absorption of the cocrystals before their dissociation.The breakdown of the cocrystal into individual constituent was confirmed by the FTIR analysis of the residue. The residual material was analyzed after 2 and 24 hours of the solubility study to examine any change in the prepared co-crystals. The FT-IR analysis showed that all the three co-crystals existed as a single unit upto 2 hours. However, FT-IR spectra after 24 hours resembled that of the parent flavonoidal molecule.Thus, suggesting the breakdown of co-crystals.
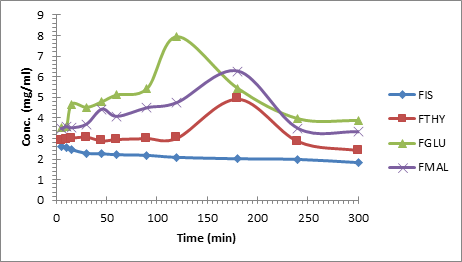
Figure 10. Dissolution profile of fisetin and its cocrystals
The improvent in the equilibrium solubility of fisetin was observed in its cocrystals which is found to be 3.03 times more for FGLU(7.93 mg/ml), 2.40 times more FMAL(6.27 mg/ml) and 1.88 times more for FTHY (4.92 mg/ml). The maximum concentration was attained for FGLU and FMAL at 120 min and for FTHY at 180 mins which started decreasing steadily to a value comparable to the pure fisetin molecule (2.61 mg/ml). The superior solubility and IDR pattern of fisetin cocrystals can be justified on the basis of the solubility profile and melting point of the coformers, the orientation of molecules in the crystal lattice and strength of supramolecular interactions. FGLU showed the maximum solubility which may be attributed to the fact that the molecules in FGLU are packed in bilayer pattern in crystal lattice while in FMAL the molecules are packed in multi-layers.Whereas, in FTHY the intra and intermolecular interactionsuggest strong crystal lattice resulting in less solubility in comparison to other cocrystals. Additionally, coformer glutaric acid has lowest melting point as compared to MAL and THY.
The better physicochemical properties of the new engineered cocrystals (FGLU,FMAL and FTHY) encouraged its further development, and thus further pharmacokinetic and pharmacodynamic studies were performed.
5. Accelerated Stability Study
The cocrystals of FIS were further subjected to accelerated stability conditions (40°C/75% RH for the period of 3 months) and the samples were analysed by DSC. The cocrystals were found to be stable also, no significant changes were seen in DSC thermogram (Table no. 3)
Table 3. Melting point of samples after 3 months
|
Samples |
Melting point |
Melting point after 3 months |
|
FGLU |
180.24ºC |
179.63ºC |
|
FMAL |
213.26ºC |
214.31º C |
|
FTHY |
284.45ºC |
283.25º C |
6. Bioavailability
Bioavailability of the prepared cocrystals was determined by measuring of “how much” and “how quickly” the active component of the cocrystal gets absorbed in the biological system. It has been evident from the literature that if upon cocrystallization there is an enhanced in vitro solubility then there is a probability of an increased Cmax as well. Therefore, bioavailability is a combination of solubility, dissolution and permeability profile of an API.[28]
In order to elucidate the pharmacokinetic characteristicsthe prepared fisetin cocrystals were administered in rats. The results showed that all the cocrystals had higher Cmax and AUC, and a shorter Tmax as compared to that of pure fisetin (figure 11). The highest concentration was achieved by FGLU which is approximately 3.7 times in 1.5 hours followed by FMAL 3.4 times in 1.5 mins and FTHY 2.6 times in 3 hours when compared to pure FIS(Table No.4).
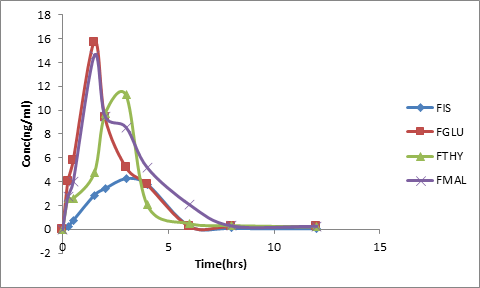
Figure 11. Pharmacokinetic profile
Table 4: Biopharmaceutical parameters of fisetin cocrystals
|
|
Cmax(ng/ml) |
Tmax(min) |
AUC(ng/ml min) |
|
FIS |
4.2 |
30 |
48840 |
|
FGLU |
15.7 |
15 |
181489 |
|
FMAL |
14.5 |
15 |
168447 |
|
FTHY |
11.2 |
30 |
130179 |
7. Biological evaluation
7.1 Antioxidant activity
The cocrystals were further evaluated for their antioxidant effect by determining the hydrogen donating capability of the fisetin in its cocrystals by performing the DPPH assay. The cocrystals showed a substantial melioration in scavenging the free radical (DPPH. radical). The free radical-scavenging activity was found to be maximum for FGLU > FMAL> FTHY (Figure 12). Improvement in scavenging activity was also found to be concentration dependent where the maximum activity was observed at 10 mcg/ml.
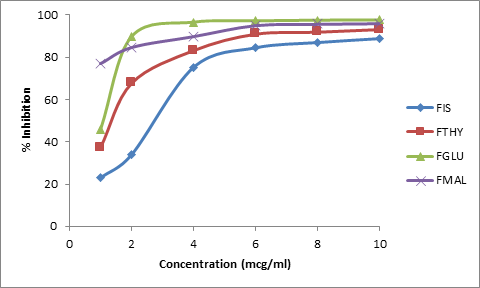
Figure 12. Antioxidant activity
7.2 Anti-hemolytic
The effect of cocrystallization on antihemolytic activity of fisetin was studied on rat RBC’s.Maximum inhibition of hemolysis was depicted by FGLU(88%) followed by FMAL(83%) whereas FTHY showed 77% antihemolytic activity as compared to the parent molecule which is 67% (Figure13).
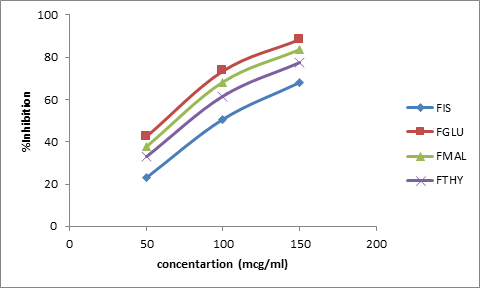
Figure 13: Antihemolytic activity
Conclusion
The present study demonstrates the design,synthesis and evaluation of pharmaceutical cocrystals of a poorly water soluble bioflavonoidal molecule, fisetin via crystal engineering approach. The article illustrates that the improvement in solubility, pharmacokinetic parameters and biological activity is dependent on the presence of hydrogen bond interactions amongst the coformers and the parent phytogen molecule. Finally, the study showcases the successful application of crystal engineering technique towards improving the clinical performance of fisetin.
Acknowledgement
The authors wish to thank Department of Science and Technology (DST), New Delhi (File number: SR/SI/OC-90/2012) and University Grants Commission (UGC-RFMS), New Delhi (F.5-94/2007(BSR).
ABBREVIATIONS
|
CSD Crystal structure database |
|
FIS Fisetin |
|
FGLU Fisetin-glutaric acid |
|
FMAL Fisetin-malic acid |
|
FTHY Fisetin-theophylline |
|
DSC Differential scanning calorimetry |
|
FTIR Fourier transform infrared |
|
KBr Potassium bromide |
|
PXRD Powder X-ray diffraction |
|
SSNMR Solid-state nuclear magnetic resonance |
|
HPLC High performance liquid chromatography |
|
DPPH 2, 2’ diphenyl-1-picrylhydrazyl |
|
GLU Glutaric acid |
|
MAL Malic acid |
|
THY Theophylline |
|
GRAS Generally regarded as safe |
|
IDR Intrinsic dissolution rate |
|
SAG Solvent assisted grinding |
|
API Active pharmaceutical ingredient |
|
RBC Red blood cells |
|
SCXRD Single crystal X-ray diffraction |
|
AUC Area under the curve |
References
Basavoju S, Boström D, Velaga SP. Indomethacin–saccharin cocrystal: design, synthesis and preliminary pharmaceutical characterization. Pharmaceutical research. 2008;25(3):530-41. PMid:17703346
View Article PubMed/NCBISathisaran I, Dalvi S. Engineering cocrystals of poorly water-soluble drugs to enhance dissolution in aqueous medium. Pharmaceutics. 2018;10(3):108. PMid:30065221
View Article PubMed/NCBIOdiase I, Nicholson CE, Ahmad R, Cooper J, Yufit DS, Cooper SJ. Three cocrystals and a cocrystal salt of pyrimidin‐2‐amine and glutaric acid. Acta Crystallographica Section C. 2015;71(4):276-83.
View ArticleThipparaboina R, Kumar D, Chavan RB, Shastri NR. Multidrug co-crystals: towards the development of effective therapeutic hybrids. Drug Discovery Today. 2016;21(3):481-90 PMid:26869329
View Article PubMed/NCBICheong H, Ryu S Y, Oak M H, Cheon SH, Yoo GS, Kim KM. Studies of structure activity relationship of flavonoids for the anti-allergic actions. Archives of pharmacal research. 1998;21(4):478-80. PMid:9875480
View Article PubMed/NCBITaubert D, Berkels R, Klaus W, Roesen R. Nitric oxide formation and corresponding relaxation of porcine coronary arteries induced by plant phenols: essential structural features. Journal of Cardiovascular Pharmacology. 2002;40(5):701-13. PMid:12409979
View Article PubMed/NCBIHanneken A, Lin F-F, Johnson J, Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress–induced death. Investigative ophthalmology & visual science. 2006;47(7):3164-77. PMid:16799064
View Article PubMed/NCBISung B, Pandey MK, Aggarwal BB. Fisetin, An Inhibitor of Cyclin-Dependent Kinase 6, Down-Regulates Nuclear Factor-κB-Regulated Cell Proliferation, Antiapoptotic and Metastatic Gene Products Through The Suppression of TAK-1 and RIP Regulated IκBα Kinase Activation. Molecular pharmacology. 2007.
View ArticleFerreira A, Lisboa P, Oliveira K, Lima L, Barros I, Carvalho D. Inhibition of thyroid type 1 deiodinase activity by flavonoids. Food and chemical toxicology. 2002;40(7):913-7. 00064-9
View ArticleHiga S, Hirano T, Kotani M, Matsumoto M, Fujita A, Suemura M. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. Journal of Allergy and Clinical Immunology. 2003;111(6):1299-306. PMid:12789233
View Article PubMed/NCBIPark KY, Jung GO, Lee KT, Choi J, Choi MY, Kim GT. Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. Journal of ethnopharmacology. 2004;90(1):73-9. PMid:14698512
View Article PubMed/NCBIMehta P, Pawar A, Mahadik K, Bothiraja C. Emerging novel drug delivery strategies for bioactive flavonol fisetin in biomedicine. Biomedicine & Pharmacotherapy. 2018;106:1282-91. PMid:30119198
View Article PubMed/NCBIGuzzo MR, Uemi M, Donate PM, Nikolaou S, Machado AEH, Okano LT. Study of the complexation of fisetin with cyclodextrins. The journal of physical chemistry A. 2006;110(36):10545-51. PMid:16956235
View Article PubMed/NCBISeguin J, Brullé L, Boyer R, Lu YM, Romano MR, Touil YS, et al. Liposomal encapsulation of the natural flavonoid fisetin improves bioavailability and antitumor efficacy. International journal of pharmaceutics. 2013;444(1-2):146-54. PMid:23380621
View Article PubMed/NCBIShan N, Zaworotko MJ. The role of cocrystals in pharmaceutical science. Drug discovery today. 2008;13(9-10):440-6. PMid:18468562
View Article PubMed/NCBIRagelle H, Crauste-Manciet S, Seguin J, Brossard D, Scherman D, Arnaud P, et al. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. International journal of pharmaceutics. 2012;427(2):452-9. PMid:22387278
View Article PubMed/NCBIMignet N, Seguin J, Romano MR, Brullé L, Touil YS, Scherman D, et al. Development of a liposomal formulation of the natural flavonoid fisetin. International journal of pharmaceutics. 2012;423(1):69-76. PMid:21571054
View Article PubMed/NCBIJeong D, Choi JM, Choi Y, Jeong K, Cho E, Jung S. Complexation of fisetin with novel cyclosophoroase dimer to improve solubility and bioavailability. Carbohydrate polymers. 2013;97(1):196-202. PMid:23769537
View Article PubMed/NCBISowa M, Ślepokura K, Matczak-Jon E. Cocrystals of fisetin, luteolin and genistein with pyridinecarboxamide coformers: crystal structures, analysis of intermolecular interactions, spectral and thermal characterization. CrystEngComm. 2013;15(38):7696-708.
View ArticleSowa M, Ślepokura K, Matczak-Jon E. Improving solubility of fisetin by cocrystallization. CrystEngComm. 2014;16(46):10592-601.
View ArticleMcNamara DP, Childs SL, Giordano J, Iarriccio A, Cassidy J, Shet MS, et al. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharmaceutical research. 2006;23(8):1888-97. PMid:16832611
View Article PubMed/NCBITilley SL. Methylxanthines in asthma. Methylxanthines: Springer; 2011. p. 439-56.
View ArticleAbraham GE, Flechas JD. Management of fibromyalgia: rationale for the use of magnesium and malic acid. Journal of Nutritional Medicine. 1992;3(1):49-59
View ArticleMensor LL, Menezes FS, Leitão GG, Reis AS, Santos TCd, Coube CS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy research. 2001;15(2):127-30. PMid:11268111
View Article PubMed/NCBIHeinz A, Strachan CJ, Gordon KC, Rades T. Analysis of solid‐state transformations of pharmaceutical compounds using vibrational spectroscopy. Journal of Pharmacy and Pharmacology. 2009;61(8):971-88. PMid:19703341
View Article PubMed/NCBIThakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodríguez-Hornedo N. Pharmaceutical cocrystals and poorly soluble drugs. International journal of pharmaceutics. 2013;453(1):101-25. PMid:23207015
View Article PubMed/NCBIGood DJ, Rodriguez-Hornedo N. Solubility advantage of pharmaceutical cocrystals. Crystal Growth and Design. 2009;9(5):2252-64.
View ArticlePindelska E, Sokal A, Kolodziejski W. Pharmaceutical cocrystals, salts and polymorphs: Advanced characterization techniques. Advanced drug delivery reviews. 2017;117:111-46. PMid:28931472
View Article PubMed/NCBI
