Praphathip Eamsobhana, E-mail address: praphathip.eam@mahidol.ac.th, Tel.: +66(0) 24196468
Darawan Wanachiwanawin, E-mail address: darawan.wan@mahidol.ac.th, Tel.: +66(0) 24196468
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 4
Page No: 748-752
Praphathip Eamsobhana, E-mail address: praphathip.eam@mahidol.ac.th, Tel.: +66(0) 24196468
Darawan Wanachiwanawin, E-mail address: darawan.wan@mahidol.ac.th, Tel.: +66(0) 24196468
Praphathip Eamsobhana1*, Anchana Prasartvit2, Hoi-Sen Yong3, Anchalee Tungtrongchitr1, Darawan Wanachiwanawin1*
1Department of Parasitology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand
2Department of Disease Control, Ministry of Public Health, Nonthaburi 11000, Thailand
3Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
Wanchai Maleewong(wanch_ma@kku.ac.th)
Yuan Gao(gaoyuangy89@126.com)
Dr Pooria Gill(pooriagill@yahoo.com)
Luis Manuel Madeira de Carvalho(madeiradecarvalho@fmv.ulisboa.pt)
P. Eamsobhana, A. Prasartvit, Hoi-Sen Yong, A. Tungtrongchitr, D. Wanachiwanawin, Evaluation of a user-friendly test device (AcQuickDx) for detection of specific antibodies to human angiostrongyliasis(2019) SDRP Journal of Food Science & Technology 4(4)
This study was conducted to evaluate the reliability of a recently developed AcQuickDx Test based on lateral flow immunochromatographic assay to detect the 31-kDa specific antibody against Angiostrongylus cantonensis for rapid serodiagnosis of angiostrongyliasis in patient-derived sera from the parasite endemic areas of northeast Thailand. The diagnostic performance of the assay was evaluated with a total of 184 serum samples from clinically diagnosed patients with eosinophilic meningitis due to A. cantinensis infection (n=98), and individuals at risk of infection with no evidence of eosinophilic meningitis (n=86). The serological AcQuickDx results were then compared with previous performance results based on dot-immunogold filtration assay (Ac-DIGFA) and dot-blot enzyme-linked immunosorbent assay (dot-blot ELISA) to determine its validity. The overall positivity rate of AcQuickDx in 98 clinically diagnosed cases from three highly endemic districts in Khon Kaen province was 39.79%. Among the 86 sera of subjects at risk of infection with A. cantonensis, 24.41% were positive by AcQuickDx. The positivity rates were comparable with the results obtained previously in both groups of defined serum samples: Ac-DIFGA, 39.79% and 24.41% respectively; and dot-ELISA, 37.75% and 23.25% respectively. This study demonstrated that AcQuickDx is equally sensitive and specific as Ac-DIGFA and dot-blot ELISA for clinically confirmed eosinophilic meningitis caused by A. cantonensis infection. In addition to rapidity and ease of performance, the dry-format lateral flow assay in AcQuickDx Test allows shipment and storage without requiring a cold chain and is thus more suitable for use in remote and resource-limited settings.
Keywords: Angiostrongylus cantonensis; angiostrongyliasis; food-borne zoonotic disease, lateral flow immunochromatographic assay; evaluation; 31-kDa antigen; rapid test
Eosinophilic meningitis caused by infection with Angiostrongylus cantonensis, the rat lungworm, is the most common food-borne zoonotic disease in Thailand and other tropical and subtropical regions of the world (Cross and Chen, 2007; Wang et al., 2012; Eamsobhana, 2015). The range of rat lungworm infection is expanding into more temperate regions due to increasing international travel that facilitates further spread of infected intermediate snail and definitive rodent hosts of A. cantonensis. (Eamsobhana, 2015; Baratt et al., 2016). Humans are usually infected with A. cantonensis through consumption of undercooked infected snails and the presentation of disease is often severe. In Thailand, detection of A. cantonensis-specific antibodies with domestically prepared enzyme-linked immunosorbent assay (ELISA) or immunoblotting have been used to support clinical diagnosis due to difficulty in recovering the parasite larvae from infected patients (Eamsobhana and Yong, 2009; Eamsobhana, 2015). In recent years, a more rapid flow-through, gold-based assay, Ac-DIGFA, had been developed and proven to be useful in the parasite endemic regions in Thailand (Eamsobhana et al., 2014; 2015). Although the use of visible gold-conjugated antibody in DIGFA instead of enzyme conjugates in ELISA makes the test rapid and simple, the colloidal gold detector reagent needs refrigeration and is therefore not suitable for large-scale field applications. Recently, an easier and rapid lateral flow immunochromatographic test format (AcQuickDx Test) with long shelf-life of the test device at ambient temperature has been successfully established (Eamsobhana et al., 2018). The present study tested archived serum samples from eosinophilic meningitis patients and individuals at risk of infection with A. cantonensis from three most endemic districts in Khon Kaen province to evaluate the usefulness of AcQuickDx Test for the detection of specific 31-kDa antibodies against A. cantonensis infection.
One hundred eighty-four archived serum samples, collected from three most endemic districts (Phu Wiang, Waeng Yai, and Phra Yuen) for A. cantonensis in Khon Kaen province (Table 1), were used in this study to evaluate AcQuickDx Test for detection of the 31-kDa specific antibody against A. cantonensis. They were from clinically diagnosed patients with eosinophilic meningitis (n=98), and individuals at risk of infection with A. cantonensis (n=86). The inclusion criteria for clinically diagnosed angiostrongyliasis cases were patients with fever, headache, stiff neck and painful paresthesia, more than 10% of eosinophils of the total cerebrospinal fluid (CSF) white blood cell count, and a history of eating raw freshwater snails, freshwater prawns, frogs, monitor lizards or raw vegetables within the past three months, while the individuals at risk of infection with A. cantonensis were cases who had a history of eating similar or the same uncooked snails dish along with the patients but with no evidence of eosinophilic meningitis and clinical symptoms. The serum samples were sent to the Department of Parasitology, Faculty of Medicine Siriraj Hospital, Bangkok, for specific antibody testing by a dot-blot ELISA to detect the specific antibody (31 kDa) against A. cantonensis (Chaiyaseth et al., 2002). This set of archived serum samples kept at -80oC had been retrieved and tested by Ac-DIGFA (Eamsobhana et al., 2015) and was re-tested in the present study using AcQuickDx Test to detect the 31-kDa specific antibody against A. cantonensis.
Purified 31-kDa antigen of A. cantonensis, prepared as described previously (Eamsobhana et al., 2001), was used in the immunochromatographic strip for the detection of specific antibody to A. cantonensis. The immunochromatographic test device, designated AcQuickDx (Eamsobhana et al., 2018), prepared as per the standard method by Serve Science Co., Ltd., Bangkok, Thailand, was assembled and housed in a protective plastic cassette, and stored in a desiccated sealed foil-package.
The test was performed as previously reported (Eamsobhana et al., 2018). In the detection test, the appearance of a red band at the T line and a red band at the C line within 15 min indicates a positive reaction; the absence of such a red band at the T line and appearance of a red band at the C line indicates a negative reaction (Figure 1). Each serum sample was tested twice to confirm the reproducibility of the results. Results were evaluated semi-quantitatively based on the color intensity (Figure 1). The results were compared with those previously obtained by dot-immunogold filtration assay (Ac-DIGFA) and enzyme-coupled dot-blot ELISA using the 31-kDa A. cantonensis antigen (Chaiyaseth et al., 2002; Eamsobhana et al., 2015).
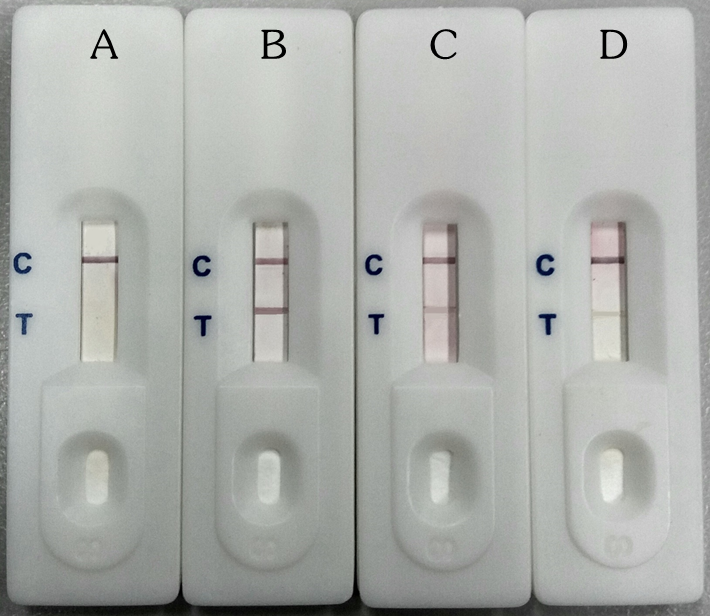
Figure 1. Interpretation of the results of AcQuickDx test for detection of A. cantonensis-specific antibody. A, negative (only 1 red band at the control (C) line area); B, C and D, positive (2 red bands in the read-out zone; 1 in the control (C) line and 1 in the test (T) line areas). The different positive reactions with AcQuickDx : strong (B), moderate (C), and weak (D).
The overall positivity rate of AcQuickDx Test for A. cantonensis infection in the sera from 98 clinically diagnosed cases with eosinophilic meningitis from three highly endemic districts in Khon Kaen province, i.e. Phu Wiang (n=39), Waeng Yai (n=26), and Phra Yuen (n=33), was 39.79% (39/98) (Table 1). Among the 86 sera of subjects at risk of infection with A. cantonensis – Phu Wiang (n=37), Waeng Yai (n=27), and Phra Yuen (n=22) – 24.41% (21/86) were positive by AcQuickDx Test (Table 1). The assay reproducibility proved good since identical positive results were obtained with two consecutive tests.
There was no significant difference between AcQuickDx, Ac-DIGFA and dot-blot ELISA on positive and negative rates of detection (Table 1). The positivity rates of AcQuickDx were comparable with the results obtained in both groups of defined serum samples with Ac-DIFGA (39.79% and 24.41% respectively) and dot-ELISA (37.75% and 23.25% respectively). Furthermore, the visual grading of AcQuickDx, Ac-DIGFA and dot-blot ELISA showed good correlation in both groups of defined sera (data not shown). The intensity of color bands reflected the antibody activity level of immune serum (Eamsobhana et al., 2014). Strong and weak bands indicate respectively high and low antibody level (Chaiyaseth et al., 2002; Eamsobhana et al., 2015).
Table 1. Comparison of AcQuickDx, Ac-DIGFA and dot-blot ELISA for the detection of specific 31-kDa antibody against A. cantonensis infection in sera from eosinophilic meningitis (EoM) patients and subjects at risk of infection (Data of Ac-DIGFA and dot-blot ELISA were from Eamsobhana et al., 2015)
|
Khon Kaen province (endemic district)
|
Positivity rate (%) |
||||||
|
EoM patient |
Individual at risk |
||||||
|
AcQuickDx |
Ac-DIGFA |
Dot-blot ELISA |
AcQuickDx |
Ac-DIGFA |
Dot-blot ELISA |
||
|
Phu Wiang |
46.15 (18/39) |
46.15 (18/39) |
43.58 (17/39) |
40.54 (15/37) |
40.54 (15/37) |
40.54 (15/37) |
|
|
Waeng Yai |
38.46 (10/26) |
42.30 (11/26) |
38.46 (10/26) |
14.81 (4/27) |
14.81 (4/27) |
11.11 (3/27) |
|
|
Phra Yuen |
30.30 (10/33) |
30.30 (10/33) |
30.30 (10/33) |
9.09 (2/22) |
9.09 (2/22) |
9.09 (2/22) |
|
|
Total |
39.79 (39/98) |
39.79 (39/98) |
37.75 (37/98) |
24.41 (21/86) |
24.41 (21/86) |
23.25 (20/86) |
|
Effective diagnostic is an essential tool in the control, elimination and eradication of eosinophilic meningitis caused by A. cantonensis, an emerging infectious disease now recognized as a serious global health issue (Graeff-Teixeira, et al., 2018). In this communication, AcQuickDx using parasite specific (31-kDa) antigen demonstrated good performance with archived serum specimens from eosinophilic meningitis patients in parasite endemic areas in Khon Kaen province, Thailand. Its diagnostic sensitivity to detect the specific 31-kDa antibody is consistent, showing agreement with those previously obtained by Ac-DIGFA and dot-blot ELISA for performance under field situations (Chaiyaseth et al., 2002; Eamsobhana et al., 2015).
Our dry-format lateral flow assay, AcQuickDx test device can be shipped and stored conveniently at ambient temperature with shelf life of at least one year (Eamsobhana et al., 2018). Although AcQuickDx and Ac-DIGFA can be performed anywhere by paramedical personnel without instrumentation or special training, drying the conjugate onto the pad improves the usefulness of AcQuickDx as a field-friendly test over Ac-DIGFA that requires a liquid conjugate. As the AcQuickDx test needs fewer number of steps to carry out the assay and does not require a cold chain, so it is more suitable in remote and resource-poor settings for mass screening survey to monitor the endemic status of A. cantonesis infection toward the surveillance and intervention control of the disease.
Additionally, other common food-borne parasitic helminthes (e.g. Gnathostoma spinigerum, Paragonimus heterotremus and Taenia solium metacestodes) that may invade the central nervous system and produce eosinophilic meningitis, also exist in Thailand. Since the performance of dry reagent assay indicated excellent field-robustness, the improvements/extensions of AcQuickDx (single test format) to also differentiate other clinically related parasites in a multiplex test format would broaden its applicability especially in angiostrongyliasis co-endemic areas in northeast and north Thailand, where cerebral gnathostomiasis and neurocysticercosis cases have been reported. Development of a dry-format lateral flow test, utilizing multiple antigens, either on the same lateral flow strip or a simple multiple channel device running multi-antigen strips from a single sample in parallel that can differentiate common parasitic causes of eosinophilic meningitis in Thailand will make such rapid device even more attractive.
In summary, the rapid, dry-format field applicable test, AcQuickDx is as sensitive as the rapid Ac-DIGFA and the dot-blot ELISA for confirming eosinophilic meningitis due to A. cantonensis, with advantages of low-tech and robust assay that allow storage and shipment of the test devices at ambient temperature. The AcQuickDx Test is thus very suitable for application in resource poor-settings in the field.
The authors thank the Director of the Siriraj Hospital, Faculty of Medicine Siriraj Hospital, Mahidol University for permission of using stored left-over clinical samples for simultaneous study, and Sudarat Boonyong for technical assistance. We express our special thanks to Dr. Passakorn Chaiyaseth and all the public health personnel in Khon Kaen province who conducted the survey and providing us serum samples for laboratory testing. We also thank the editor and the two anonymous reviewers whose comments led to a greatly improved manuscript. This work was supported in part by the Department of Disease Control, Ministry of Public Health, Thailand, and MoHE-HIR grant H-50001-00-000025 to HSY. This study was approved by the Ethics Committee of the Faculty of Medicine Siriraj Hospital, Mahidol University (Si369/2015).
Barratt J, Chan D, Sandaradura I, Malik R, Spielman D, Lee R, Marriott D, Harkness J, Ellis J, Stark D. 2016. Angiostrongylus cantonensis: A review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 143:1087-1118. PMid:27225800
View Article PubMed/NCBIChaiyaseth P, Poosikeaw P, Pooribuncha L, Phapirom S. 2002. Prevalent study of eosinophilic meningitis in Khon Kaen Province. Khon Kaen: Klungnana-vitaya Press.
Cross JH, Chen ER. Angiostrongyliasis. 2007. In: Black SJ, Seed JR, editors. Food-borne parasitic zoonoses. New York: Springer Science. p. 263-292.
View ArticleEamsobhana P, Yoolek A, Suvouttho S, Suvuttho S. 2001. Purification of a specific immunodiagnostic Parastrongylus cantonensis antigen by electroelution from SDS-polyacrylamide gels. Southeast Asian J Trop Med Public Health 32:308-313.
Eamsobhana P, Yong HS. 2009. Immunological diagnosis of human angiostrongyliasis cantonensis. Int J Infect Dis 13:425-431. PMid:19117782
View Article PubMed/NCBIEamsobhana P, Gan XX, Wanachiwanawin D, Ma A, Wang Y, Yong HS. 2014. Dot immunogold filtration assay (DIGFA) for the rapid detection of specific antibodies against the rat lungworm Angiostrongylus cantonensis (Nematoda: Metastrongyloidea) using purified 31-kDa antigen. J Helminthol 88:396-401. PMid:23710755
View Article PubMed/NCBIEamsobhana P. 2015. The Rat Lungworm Angiostrongylus cantonensis: Parasitology, Genetics and Molecular Phylogeny. Revised 2nd ed. Bangkok: Akson Graphic and Design Publishing House.
Eamsobhana P, Prasartvit A, Gan XX, Yong HS. 2015. Evaluation of dot immunogold filtration assay (DIGFA) for rapid serodiagnosis of eosinophilic meningitis due to Angiostrongylus cantonensis (Nematoda: Metastrongyloidea). Trop Biomed 32: 121-125.
Eamsobhana P, Tungtrongchitr A, Wanachiwanawin D, Yong HS. 2018. Immunochromatographic test for rapid serological diagnosis of human angiostrongyliasis. Int J Infect Dis 73:69-71. PMid:29908250
View Article PubMed/NCBIGraeff-Teixeira C, Morassutti AL, Jones MK. 2018. Diagnosing and understanding angiostrongyliasis, a zoonotic cause of meningitis. ACS Chem Neurosci 9:393-394. PMid:29411969
View Article PubMed/NCBIWang QP, Wu ZD, Wei J, Owen RL, Lun ZR. 2012. Human Angiostrongylus cantonensis: An update. Eur J Clin Microbiol Infect Dis 31:389-95. PMid:21725905
View Article PubMed/NCBI